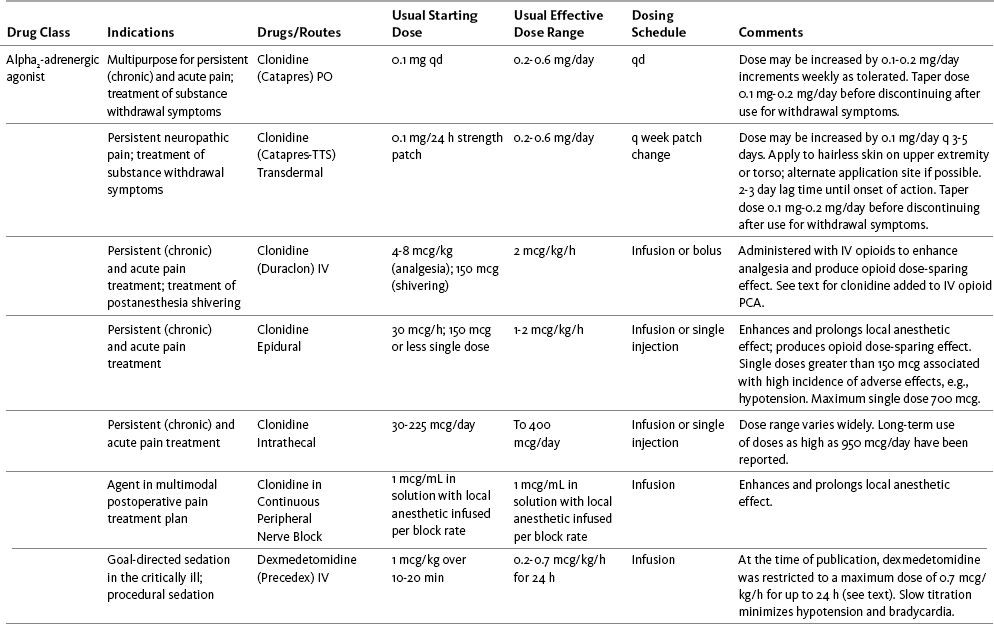
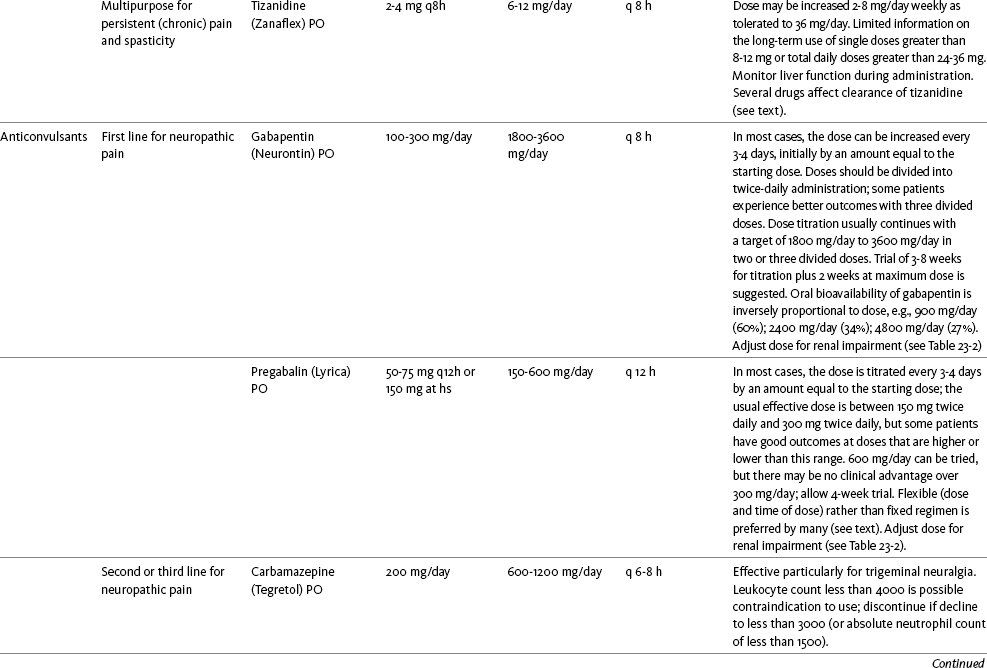
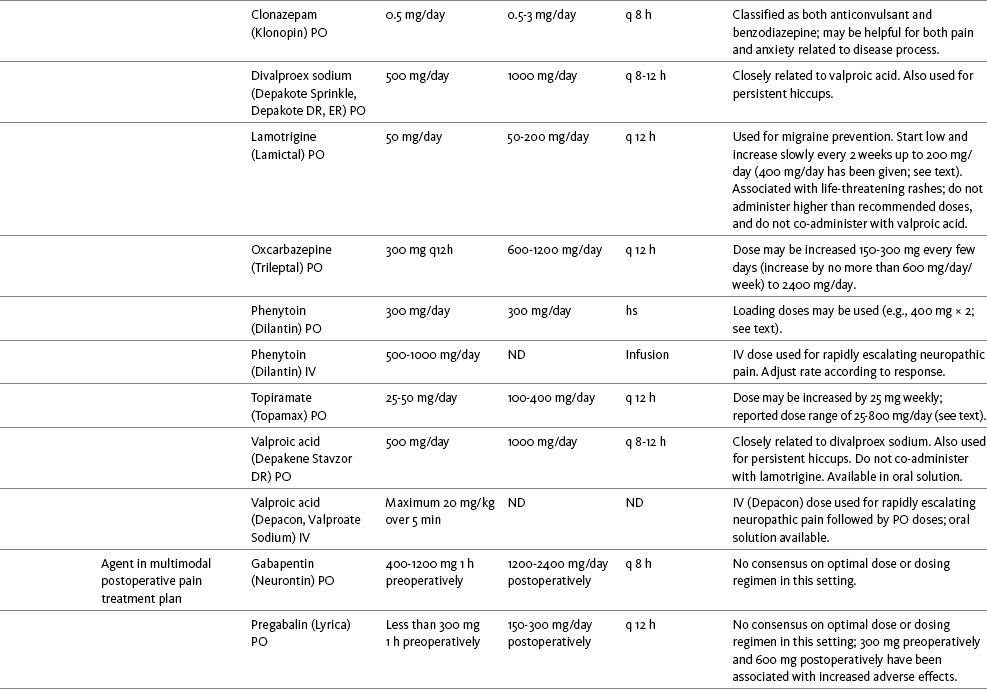
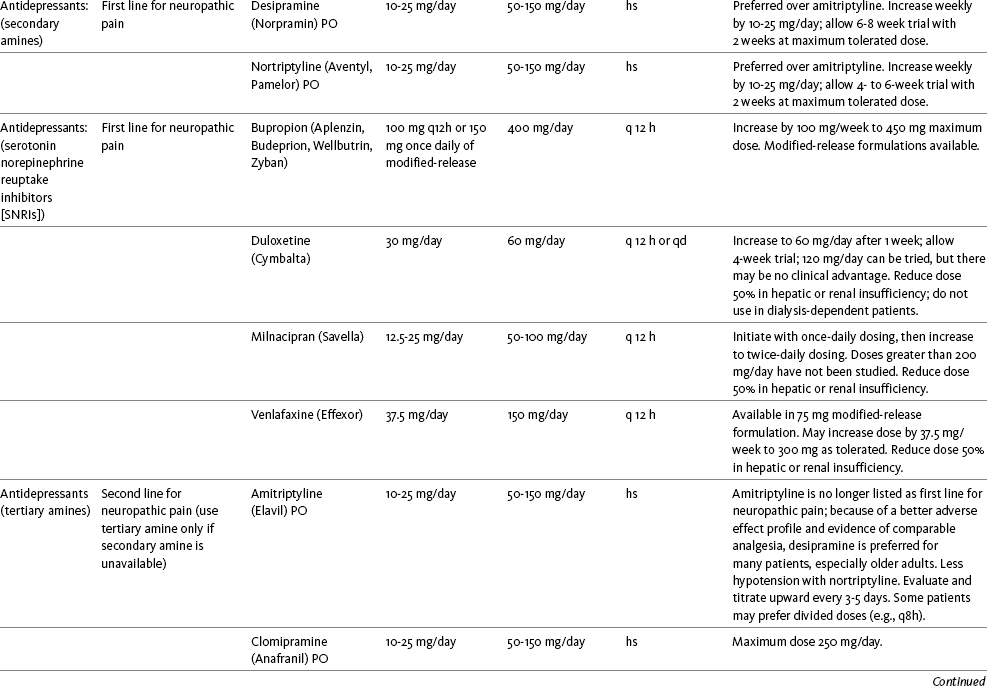
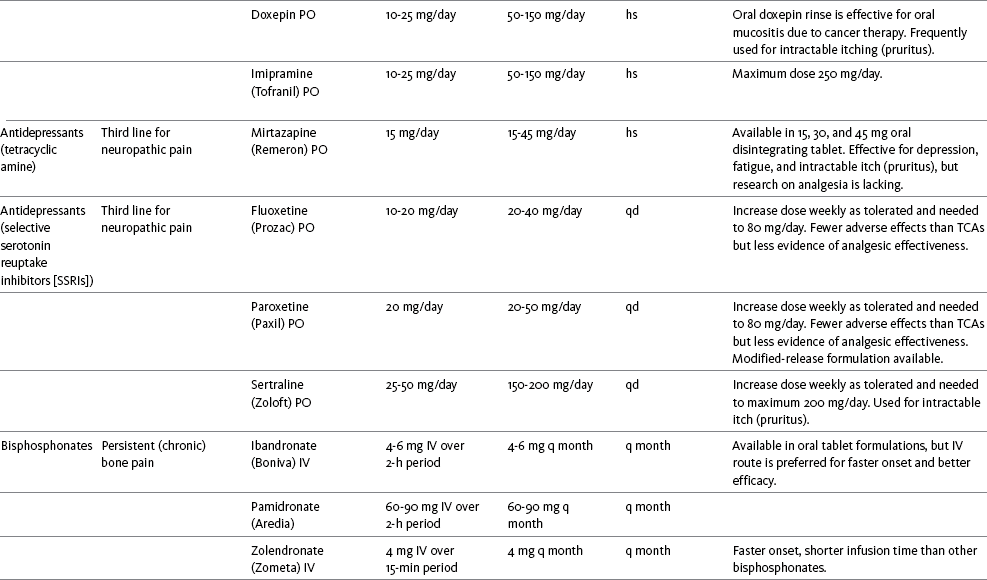
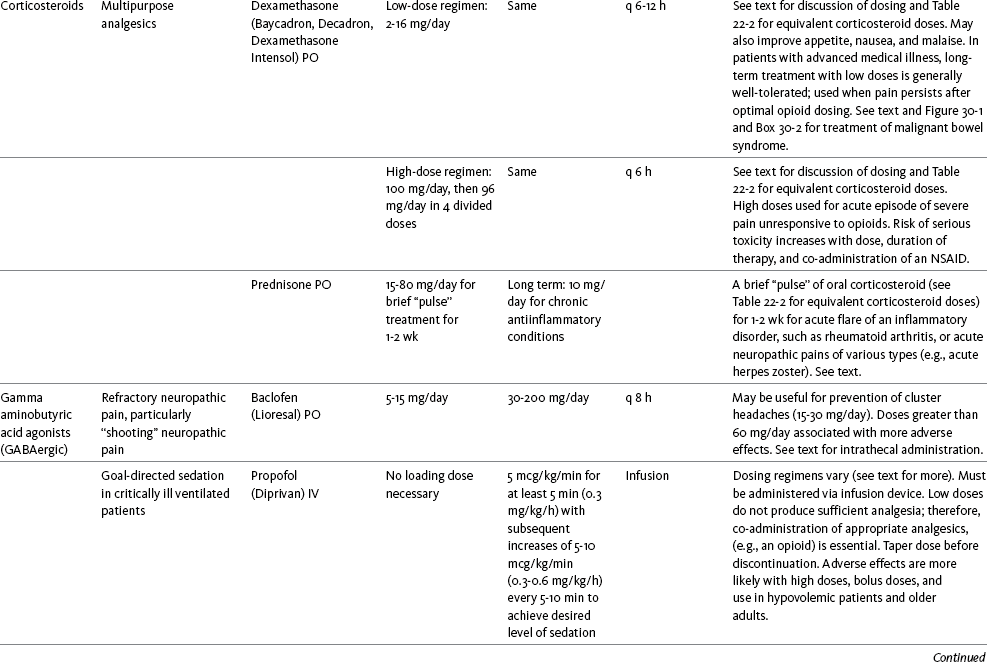
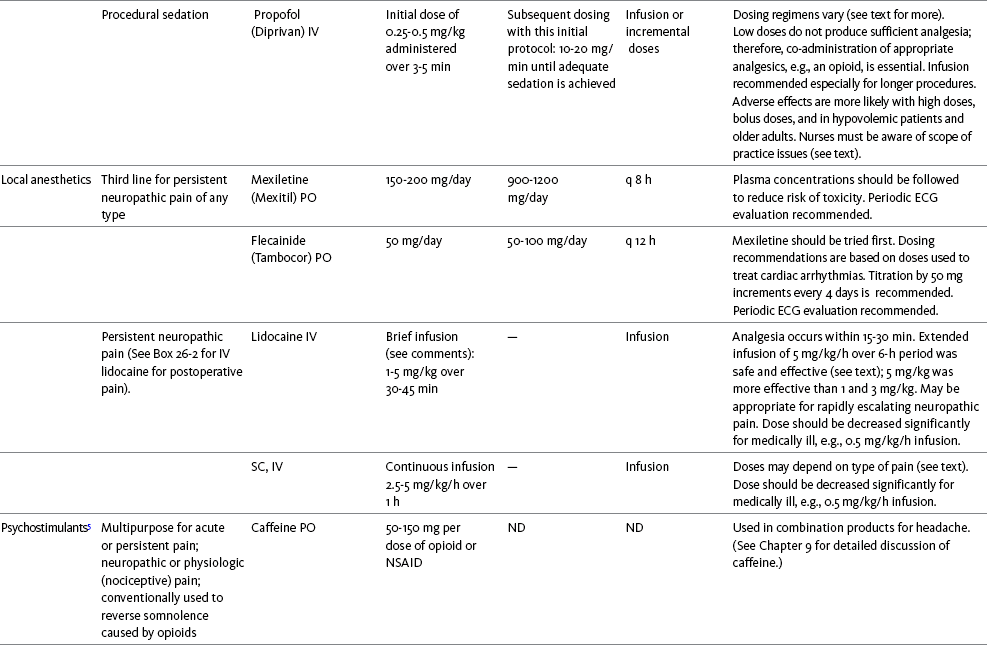
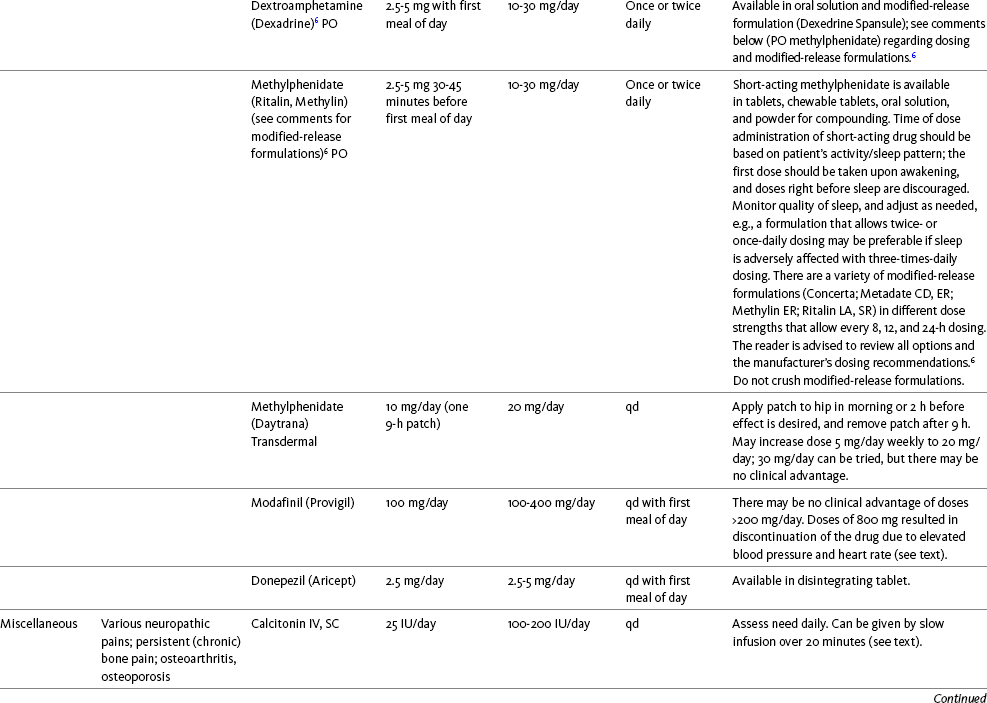
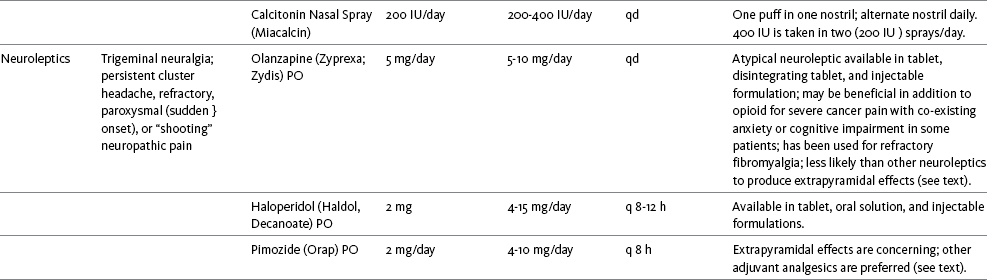
Chapter 31 There is substantial evidence that psychostimulants may be analgesic (Lussier, Portenoy, 2004). However, with the exception of caffeine, which is a constituent of many proprietary analgesic products, these drugs are generally used only when pain is accompanied by another symptom such as sedation or fatigue. Nevertheless, their effect on pain relief should be monitored to identify if and when a dose of analgesics can be lowered. Although there are no specific clinical guidelines available to direct therapy, clinicians often prescribe psychostimulants when it is difficult to titrate analgesics to pain relief or when patients report unresolved sleepiness from the sedating effects of analgesics. Opioid-induced sedation usually improves within 7 days of regular dosing; however, this varies, and some individuals will benefit from either short-term or long-term treatment with a psychostimulant (Harris, Kotob, 2006). From clinical experience, these drugs can improve concentration and physical activity. In addition, psychostimulants can be helpful with depression (Macleod, 1998; Menza, Kaufman, Castellanos, 2000) and fatigue (Fishbain, Cutler, Lewis, et al., 2004a). Table V-1, pp. 748-756, at end of Section V contains dosing recommendations for selected psychostimulants. Table V-1 Selected Adjuvant Analgesics1–4 IU, International unit; ND, no data; PO, oral; q, every; qd, every day; SC, subcutaneous 1Older adults are more sensitive than younger adults to adverse effects of many of the adjuvant agents; initiate therapy with lower starting doses, and titrate slowly. 2Dose escalation should be gradual and individualized based on assessment of both pain and adverse effects. 3Doses should be gradually tapered before discontinuation of most adjuvant analgesics. 4Take single doses or largest dose of sedating medication before longest sleep period. 5Take single or first dose of psychostimulant upon awakening or after longest sleep period. 6It is generally recommended that titration with a short-acting formulation be done before switching to a modified-release formulation of a drug. Switching may be done when the available dosage of the modified-release formulation corresponds with the titrated dose of the short-acting formulation (e.g., 8-hour dose of the modified-release formulation corresponds with the 8-hour titrated dose of the short-acting formulation). From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, pp. 748-756, St. Louis, Mosby. Data from Ackerman, L. L., Follett, K. A., & Rosenquist, R. W. (2003). Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage, 26(1), 668-677; Argoff, C. E., Backonja, M. M., Belgrade, M. J., et al. (2006). Consensus guidelines: Treatment planning and options. Diabetic peripheral neuropathic pain. Mayo Clin Proc, 81(Suppl 4), S12-S25; Backonja, M., & Glanzman, R. L. (2003). Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials, Clin Ther, 25(1), 81-104; Brush, D. R., & Kress, J. P. (2009). Sedation and analgesia for the mechanically ventilated patient. Clin Chest Med, 30(1), 131-141; Carrazana, E, & Mikoshiba, I. (2003). Rationale and evidence for the use of oxcarbazepine in neuropathic pain. J Pain Symptom Manage, 25(Suppl 5), S31-S35; Clinical Pharmacology Online. Gold Standard, Inc. Available at http://clinicalpharmacology.com. Accessed March 1, 2009; Dworkin, R. H., O’Connor, A. B., Backonja, M., et al.: Pharmacologic management of neuropathic pain: evidence-based recommendations, Pain, 132, 237-251, 2007; Eisenach, J. C., Hood, D. D., & Curry, R. (2000). Relative potency of epidural to intrathecal clonidine differs between acute thermal pain and capsaicin-induced allodynia. Pain, 84(1), 57-64; Elliott, J. A. (2009). α2-agonists. In H. S. Smith (Ed.), Current therapy in pain, Philadelphia, Saunders; Epstein, J. B., Epstein, J. D., Epstein, M. S., et al. (2006). Oral doxepin rinse: The analgesic effect and duration of pain reduction in patients with oral mucositis due to cancer therapy. Anesth Analg, 103(2), 465-470; Gilron, I. (2006). Review article: The role of anticonvulsant drugs in postoperative pain management: A bench-to-bed-side perspective. Can J Anesth, 53(6), 562-571; Guay, D. R. (2001). Adjunctive agents in the management of chronic pain. Pharmacotherapy, 21(9), 1070-1080; Harris, J. D., & Kotob, F. (2006). Management of opioid-related side effects. In O. A. de Leon-Casasola (Ed.), Cancer pain. Pharmacological, interventional and palliative care approaches, Philadelphia, Saunders; Ho, K. Y., Gan, T. J., & Habib, A. S. (2006). Gabapentin and postoperative pain—A systematic review of randomized controlled trials. Pain, 126(1-3), 91-101; Ilfeld, B. M., Morey, T. E., & Enneking, F. K. (2003). Continuous infraclavicular perineural infusion with clonidine and ropivacaine compared with ropivacaine alone: A randomized, double-blinded, controlled study. Anesth Analg, 97(3), 706-712; Kim, S. W., Shin, I. S., Kim, J. M., et al. (2008). Effectiveness of mirtazapine for nausea and insomnia in cancer patients with depression. Psychiatry Clin Neurosci, 62(1), 75-83; Lacy, C. F., Armstrong, L. L., Goldman, M. P., et al. (Eds.). (2008). Drug information handbook, ed 17, Hudson, OH, Lexi-Comp Inc; Maizels, M., & McCarberg, B. (2005). Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am Fam Physician, 71(3), 483-490; May, A., Leone, M., Afra, J., et al. (2006). EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol, 13(10), 1066-1077; Moss, J., & Glick, D. (2005). The autonomic nervous system. In R. D. Miller (Ed.), Miller’s anesthesia, ed 6, Philadelphia, Elsevier; Moulin, D. E., Clark, A. J., Gilron, I., et al. (2007). Pharmacological management of chronic neuropathic pain—Consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag, 12(1), 13-21; Odom-Forren, J., & Watson, D. (2005). Practical guide to moderate sedation/analgesia. St. Louis, Mosby; Reves, J., Glass, P. S. A., Lubarsky, D. A., et al. (2005). Intravenous nonopioid anesthetics. In R. D. Miller (Ed.), Miller’s anesthesia, ed 6, Philadelphia, Churchill Livingstone; Semenchuk, M. R., & Sherman, S. (2000). Effectiveness of tizanidine in neuropathic pain: An open-label study. J Pain, 11(4), 285-292; Sharma, S., Rajagopal, M. R., Palat, G., et al. (2009). A phase II pilot study to evaluate use of intravenous lidocaine for opioid-refractory pain in cancer patients. J Pain Symptom Manage, 37(1), 85-93; Silberstein, S. D., Neto, W., Schmitt, J., et al. (2004). Topiramate in migraine prevention: Results of a large, controlled trial. Arch Neurol, 61(4), 490-495; Stacey, B. R., Dworkin, R. H., Murphy, K., et al. (2008). Pregabalin in the treatment of refractory neuropathic pain: Results of a 15-month open-label trial. Pain Med, 9(8), 1202-1208; Tremont-Lukats, I. W., Challapalli, V., McNicol, E. D., et al. (2005). Systemic administration of local anesthetics to relieve neuropathic pain: A systematic review and meta-analysis. Anesth Analg, 101(6), 1738-1749;Tremont-Lukats I.W., Hutson, P. R., & Backonja, M. (2006). A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain, 22(3), 266-271; White, P. F., Tufanogullari, B., Taylor, J., et al. (2009). The effect of pregabalin on postoperative anxiety and sedation levels: A dose-ranging study. Anesth Analg, 108(4), 1140-1145; Zed, P. J., Abu-Laban, R. B., Chan, W. W. Y., et al. (2007). Efficacy, safety and patient satisfaction of propofol for procedural sedation and analgesia in the emergency department: A prospective study. Can J Emerg Med, 9(6), 421-427. Pasero C, McCaffery M. May be duplicated for use in clinical practice. Methylphenidate (Sarhill, Walsh, Nelson, 2001) and the newer agent modafinil (Prommer, 2006) are most commonly used to counteract sedation and fatigue in cancer populations. Clinical reviews discuss their usefulness in reducing opioid-induced sedation and improving fatigue and overall well-being, but more studies are needed to measure their efficacy in the treatment plans of individuals with pain (Bruera, Neumann, 1998; Fishbain, Cutler, Lewis, et al., 2004a; Reissig, Rybarczyk, 2005; Shaiova, 2005). This was confirmed in a Cochrane Collaboration Review that concluded that methylphenidate has been shown to improve concentration and is effective for fatigue in cancer patients based on studies with small sample sizes, and further research was encouraged (Minton, Stone, Richardson, et al., 2008). A review of the literature between 1996 and 2002 concluded that methylphenidate effectively attenuated opioid-induced somnolence, augmented opioid analgesia, treated depression, and improved cognitive function in cancer patients and recommended its use in palliative care (Rozans, Dreisbach, Lertora, et al., 2002). Methylphenidate has also been used in the acute care setting. An interesting publication reported that methylphenidate facilitated the weaning from mechanical ventilation in 5 of 7 patients who exhibited psychomotor retardation associated with markedly depressed mood (Rothenhausler, Ehrentraut, von Degenfeld, et al., 2000). The drug has also been researched for treatment of postanesthesia shivering, though it is rarely used for this purpose as more effective drugs are available (Kranke, Eberhart, Roewer, et al., 2004). The use of modafinil is gaining more popularity in practice, most likely because the drug has been shown to be very effective in treating fatigue and increasing alertness (Prommer, 2006). One randomized controlled trial found that modafinil decreased fatigue and increased “alertness” and “energy” in 71% of those given the drug, compared with 18% of those given a placebo, in the immediate postoperative period following general anesthesia (Larijani, Goldberg, Hojat, et al., 2004). The drug was well-tolerated, and those in the modafinil group experienced less pain and nausea. The drug is especially effective for cognitive impairment that can accompany both analgesic therapy and chemotherapy in cancer patients (Biegler, Chaoul, Cohen, 2009). A comprehensive evidence-based review of modafinil provides several indications and guidelines for its use (Kumar, 2008). Further research is needed to establish its analgesic potential. Dextroamphetamine is used less today than in the past as the newer and better-tolerated psychostimulants have become available. A placebo-controlled study comparing dextroamphetamine, caffeine, and modafinil in healthy volunteers who were subjected to 44 hours of continuous wakefulness found that duration of vigilance and wakefulness were shortest with caffeine and longest with dextroamphetamine (Killgore, Rupp, Grugle, et al., 2008). Adverse effects with modafinil were similar to placebo, caffeine was associated with the most adverse effects, and restorative sleep was adversely affected by dextroamphetamine. A randomized controlled trial administered dextroamphetamine 10 mg or placebo to fatigued older adults with advanced cancer receiving palliative care (Auret, Schug, Bremner, et al., 2009). Although improvements in fatigue were noted on the second day, there were no significant differences in fatigue or quality of life at the end of the 8-day study period. Caffeine has been used for many years to treat opioid-induced sedation or somnolence related to other adjuvant analgesics. It also can reduce general fatigue associated with drug therapies or disease states. More importantly, research has shown that caffeine has analgesic properties, for example, in the treatment of headaches. Clinical trials indicate that caffeine in doses of more than 65 mg may enhance the analgesia of other agents (Zhang, 2001). Caffeine is widely available in both medications and food items, particularly coffee and tea. See Chapter 9 for a more detailed discussion of the use of caffeine as an adjuvant analgesic, and refer to Table 9-1 (p. 241) for dietary sources of caffeine and Table 9-2 (pp. 242-245) for nonopioid analgesics that contain caffeine. Donepezil, an oral acetylcholinesterase inhibitor approved for the treatment of Alzheimer’s disease, is the drug in this class that is most frequently tried as a strategy to reduce opioid-induced sedation. In one case series, 6 patients with cancer pain who were experiencing marked sedation from their pain therapy (opioid doses higher than 200 mg of morphine equivalents per day) had notable improvements after taking donepezil (Slatkin, Rhiner, Bolton, 2001). Patients indicated fewer episodes of spontaneous sleeping during the day, fewer myoclonic twitches, and better function and social interaction. Sleep at night also improved. Clearly, more research is needed to measure the benefits of this agent for opioid- and adjuvant-induced sedation. The use of calcitonin for bone pain has been previously addressed in Chapter 29. Additionally, this agent has also been used for the treatment of other types of pain syndromes. Unfortunately, there are insufficient data to support widespread use beyond bone pain. It has little to no effectiveness for phantom limb pain (Albazaz, Wong, Homer-Vanniasinkam, 2008; Halbert, Crotty, Cameron, 2003; Smith, Dubin, Parikh, 2009). However, intranasal calcitonin was shown to relieve pain with movement and rest and improve ability to work in patients with complex regional pain syndrome (Gobelet, Waldburger, Meier, et al., 1992; Perez, Kwakkel, Zuurmond, 2001). It has also been used to effectively treat intractable painful diabetic neuropathy (Quatraro, Minei, De Rosa, et al., 1992; Zieleniewski, 1990). The literature suggests that it may be useful for acute neuropathic pain (Gray, 2008) and pain from trigeminal neuralgia (Qin, Cai, Yang, 2008). The exact mechanisms for possible analgesic effects for neuropathic pain remain unknown. The optimal dose and dosing frequency for calcitonin are not established (Perez, Kwakkel, Zuurmond, 2001). Dosing recommendations discussed previously in relation to bone pain may be used for neuropathic pain (see Chapter 39). In an evidence-based review, it was concluded that some neuroleptics, including at least one of the newer, so-called atypical neuroleptics, may have analgesic activity, but there are far too few randomized controlled and other clinical trials to draw definitive conclusions (Fishbain, Cutler, Lewis, et al., 2004b). There is no clear evidence that the phenothiazines (e.g., chlorpromazine [Thorazine], fluphenazine [Prolixin]) specifically either relieve pain or potentiate opioid analgesia (American Pain Society [APS], 2003). Olanzapine (Zyprexa) is a newer, so-called atypical neuroleptic and has a somewhat better safety profile than the traditional neuroleptics. This drug may have some benefit when added to opioid therapy in those with severe cancer pain associated with cognitive impairment or anxiety (Khojainova, Santiago-Palma, Kornick, et al., 2002). In a case series, olanzapine also was reported to be effective in rapidly aborting episodic and persistent cluster headache (Rozen, 2001) and relieving refractory fibromyalgia symptoms, including pain (Kiser, Cohen, Freedenfeld, et al., 2001). Evidence that traditional neuroleptics other than methotrimeprazine are analgesic is equally sparse. Pimozide (Orap) was effective in patients with trigeminal neuralgia, and this has suggested a role for neuroleptics in patients with refractory neuropathic pain (Lussier, Portenoy, 2004). Given its adverse effect liability, pimozide specifically is not considered. Other neuroleptics, such as haloperidol (Haldol) and fluphenazine, would be preferred in this rare circumstance. The mechanism of neuroleptic analgesia is unknown, but may involve the effect of dopaminergic blockade on endogenous pain-modulating systems (Lussier, Portenoy, 2004) (see Section I). Dopamine receptors, specifically the D2 subtype, are represented among the numerous pathways that subserve pain modulation (Yaksh, 2006). Studies in animals have suggested that selective dopamine antagonists can potentiate morphine analgesia, and controlled clinical trials have demonstrated that metoclopramide, a relatively selective blocker of the D2 receptor, is mildly analgesic in humans (Kandler, Lisander, 1993; Rosenblatt, Cioffi, Sinatra, et al., 1991). This evidence, however, does not confirm that a dopaminergic mechanism underlies the analgesic effects of neuroleptic drugs, because all these drugs interact with other receptors that could potentially mediate analgesic effects. Metoclopramide has a dopaminergic mechanism, and there is no strong evidence that this drug is analgesic (Gupta, 2006). The neuroleptics are started at low doses and increased to whatever dose the literature suggests is usually analgesic, and then discontinued if no analgesia ensues (Lussier, Portenoy, 2004). For example, low initial doses of fluphenazine can be escalated to 1 to 2 mg 3 times daily, and the dose of haloperidol can be slowly increased to 2 to 5 mg 2 to 3 times a day. Common adverse effects of neuroleptic drugs include sedation, orthostatic dizziness, and anticholinergic effects (Lussier, Portenoy, 2004). Some patients experience mental clouding or confusion. Phenothiazines, such as chlorpromazine and fluphenazine, are more likely to produce these effects than other subclasses, such as the butyrophenones (e.g., haloperidol). The sedation produced by the neuroleptics can be additive to other CNS depressants. The possibility of extrapyramidal adverse effects is perhaps the greatest concern in the clinical use of neuroleptic drugs (Lussier, Portenoy, 2004). The incidence of these disorders varies with the drug, duration of therapy, and dose. Fluphenazine and haloperidol are more likely to produce these effects than olanzapine (Tardy, Baldessarini, Tazari, 2002). The most serious extrapyramidal reaction is the neuroleptic malignant syndrome that is characterized by rigidity, autonomic instability, and encephalopathy. Successful management requires prompt diagnosis, discontinuation of the neuroleptic, and intensive supportive measures. The use of dantrolene (Dantrium) and bromocriptine (Parlodel) has been suggested in severe cases. Other extrapyramidal effects can be distressing or seriously impair function. These include acute dystonic reactions (muscle contraction and jerking movements), akathisia (restlessness), parkinsonism, and tardive dyskinesia. The management of these complications usually involves discontinuation of the neuroleptic, with or without the administration of an anticholinergic drug, such as benztropine (Lussier, Portenoy, 2004). The benzodiazepines, clonazepam (Klonopin) (Fishbain, Cutler, Rosomoff, et al., 2000; Wiffen, Collins, McQuay, et al., 2005) and alprazolam (Xanax) (Fernandez, Adams, Holmes, 1987) have been used in the management of both musculoskeletal pains and neuropathic pains. Although clonazepam often is considered specifically for neuropathic pain, a critical review finds little support (Dellemijn, Fields, 1994; Reddy, Patt, 1994) (see Chapter 23 for more research and discussion of clonazepam). Negative findings also have been reported in an early controlled trial of lorazepam (Ativan) for postherpetic neuralgia (Max, Schafer, Culnane, et al., 1988) and in a small controlled repeated-dose study of oral chlordiazepoxide (Librium) for persistent pain (Yosselson-Superstine, Lipman, Sanders, 1985). Thus, the evidence for benzodiazepine analgesia is limited and conflicting, leading the APS (2003) to conclude that benzodiazepines are not effective analgesics except for muscle spasm (see Chapter 25). As discussed previously, the evidence for use of benzodiazepines as muscle relaxants also is minimal (see Chapter 25). Diazepam (Valium) and other benzodiazepines are widely used for this purpose, but benefits are limited, especially when given orally. Adenosine (Adenocard, Adenoscan) is an antiarrhythmic agent commercially available for IV administration. The basis for its analgesic potential lies within the fact that adenosine is an important modulator of neurotransmission associated with many physiologic functions, including the regulation of arousal and sleep, anxiety, cognition, and memory, and may contribute to the etiology of pathologic pain (Ribeiro, Sebastiao, de Mendonca, 2002; Sawynok, Liu, 2003). Early research of adenosine suggested that the drug had analgesic effects for postoperative pain (Segerdahl, Ekblom, Sandelin, et al., 1995) and neuropathic pain (Sollevi, Belfrage, Lundeberg, et al., 1995). A later comprehensive review of adenosine also supported a role in relieving both postoperative and persistent neuropathic pain states (Hayashida, Fukuda, Fukunaga, 2005). Although adenosine has been identified as having potential as an intrathecal analgesic, further research is needed to more clearly define its place in pain management by this route of administration (Schug, Saunders, Kurowski, et al., 2006). Many antihistamines are available, including diphenhydramine (Benadryl), hydroxyzine (Vistaril parenterally), phenyltoloxamine (Phenylgesic, a nonprescription cold remedy), pyrilamine (an ingredient in several cold remedies [e.g., Codimal PH syrup, ND-Gesic tablets]), and orphenadrine (Norflex). Orphenadrine is an analogue of diphenhydramine and is combined with aspirin and caffeine for analgesia but is classified as a skeletal muscle relaxant (see Chapter 25). There is little evidence that this drug, or other antihistamines, relax muscles or effectively relieve pain. A randomized study of women undergoing hysterectomy found a lower incidence of severe nausea with perioperative diphenhydramine but no differences in pain or opioid consumption compared with placebo (Lin, Yeh, Yeh, et al., 2005). At best, diphenhydramine has been observed anecdotally to augment the treatment of pain that has failed to respond to opioids and other adjuvant analgesics (Santiago-Palma, Fischberg Kornick, et al., 2001). Suggested therapy can start at 25 mg of oral or parenteral diphenhydramine every 6 to 8 hours, and be titrated to effect. However, the coadministration of diphenhydramine with opioids is problematic in the postoperative setting because this can increase the risk of excessive sedation and respiratory depression (Anwari, Iqbal, 2003); increased monitoring of sedation levels is required with this practice (see Chapter 19 for more information). The purported analgesic properties of hydroxyzine, which has been traditionally combined with an opioid for an opioid dose-sparing effect, have never been confirmed (Gordon, 1995). Studies reporting analgesic activity for this agent suffer from major methodologic flaws, including the lack of placebo-controlled trials and scientific rigor in analyzing results. These have compromised the ability to draw definitive conclusions about its benefits (Glazier, 1990). With doses that may contribute to pain relief, hydroxyzine would have the potential for inducing significant sedation, which adds to the adverse effects of opioids, but would not be reversed with naloxone (Gordon, 1995). Further, when given parenterally, it can cause significant local pain and tissue damage. Antihistamines must also be used with caution in older patients as they have increased sensitivity to the anticholinergic and sedative effects of these drugs (see Table 13-4 on p. 336). Clinical experience suggests that treatment with antihistamines should be reserved for treating anxiety and opioid-induced nausea and pruritus in some patients (see Chapter 19). Botulinum toxin type A (Botox) is a fermented product derived from Clostriduium botulinum, an anaerobic spore-forming bacterium (Setler, 2002). It is approved for administration by injection for several conditions, including cervical dystonia, hemifacial spasm, and blepharospasm (Lew, 2002). Injection of botulinum produces partial chemical denervation and localized reduction in muscle activity. The toxin is used to treat spasticity (O’Brien, 2002), and clinical reports, open-label trials, and a few randomized controlled studies have shown that it may be effective for some types of persistent pain when more conservative approaches fail to provide relief (Arezzo, 2002; Gajraj, 2005; Smith, Audette, Royal, 2002). When effective, the toxin provides long-term (weeks) relief with a relatively low incidence of adverse effects (Smith, Audette, Royal, 2002). Adverse effects are usually transient but can last for months and include localized muscle weakness and pain at the injection site, skin rash, pruritus, and flulike symptoms. Prompted by reports that the botulinum toxin may spread from the area of injection to other parts of the body, the United States Food and Drug Administration (U.S. FDA) announced label changes in 2009 for all botulinum toxin products, which included a boxed warning. Reported symptoms were similar to those of botulism, including unexpected loss of strength or muscle weakness, hoarseness or trouble talking, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision, and drooping eyelids. Myofascial pain syndrome and fibromyalgia have been shown to be responsive to botulinum toxin injection into trigger points or tender points (Smith, Audette, Royal, 2002). A case report described successful use of botulinum injection for persistent perineal pain (Gajraj, 2005). It has been suggested as a possible treatment for chronic whiplash-associated disorder (Freund, Schwartz, 2002). A small randomized trial of postwhiplash neck pain found the best efficacy if the toxin is administered within 1 year of the injury (Braker, Yariv, Adler, et al., 2008). Whereas botulinum treatment has been used to relieve back pain in select patients (Difazio, Jabbari, 2002), a placebo-controlled, randomized study found that single-dose treatment without physical therapy is ineffective for persistent neck pain (Wheeler, Goolkasian, Gretz, 2001). Those who were injected experienced a high incidence of adverse effects in this study; further research on multidose therapy was encouraged. Botulinum treatment also has been considered a viable option for persistent headaches, with the advantage of a lack of systemic effects associated with conventional treatments (Loder, Biondi, 2002). However, a randomized, double-blind, placebo-controlled trial found it ineffective for the treatment of tension-type headaches (Schulte-Mattler, Krack, BoNTTH Study Group, 2004), and another well-controlled study showed that it had no efficacy for episodic migraine headaches (Saper, Mathew, Loder, et al., 2007). The underlying mechanism of the analgesic action of botulinum toxin type A is not entirely clear but is thought to be related to its ability to inhibit the release of the neurotransmitter acetylcholine from cholinergic nerve terminals (Setler, 2002). A study of human volunteers found that botulinum toxin type A preferentially targets C-fibers, blocks neurotransmitter release, and subsequently reduces pain and neurogenic inflammation (Gazerani, Pendersen, Staahl, et al., 2009). A decrease in active tender trigger points is suggested as an underlying mechanism for pain relief with soft-tissue pain syndromes (Smith, Audette, Royal, 2002). The reader is referred to a journal supplement devoted to botulinum toxin treatment for specific types of pain that might best respond, dosing guidelines, administration technique, expected responses, and adverse effects (Clinical Journal of Pain, 18[Suppl 6], 2002).
Adjuvants Less Often Used
Psychostimulants and Anticholinesterases
Calcitonin
Neuroleptics
Mechanism of Action
Dose Selection
Adverse Effects
Benzodiazepines
Adenosine
Antihistamines
Botulinum Toxin Type A
Adjuvants Less Often Used
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


