Fig. 13.1
The production of the disrupted adenovirus vaccine dAd5GNE. Recombinant, E1‾E3‾ replication-incompetent adenovirus serotype 5 is disrupted by detergent and heat, and the disassociated capsid proteins (fiber, hexon, and penton base) are chemically coupled to the cocaine analog GNE
13.6 dAd5GNE Design
The adenovirus-based vaccine platform was derived from the concept that the carrier protein provides the signaling to the immune system to overcome the lack of response to the small addictive molecules. Of the many serotypes of adenovirus, serotype 5 (Ad5) has the best-characterized immune profile as a gene therapy vector and an excellent safety record in the clinic (Crystal et al. 2002; Harvey et al. 2002). Rather than using a wild-type Ad5, the E1 genes of the vector genome were removed, creating a replication-incompetent starting material, minimizing potential safety concerns associated with residual vector DNA (Wold et al. 1999). Although the intact adenovirus evokes potent humoral immunity, there are several additional advantages of disrupting the E1‾E3‾ adenovirus. First, the disrupted capsid vaccine circumvents the minor risk associated with infectious adenovirus vectors, which already have a well-documented and clinically acceptable safety profile (Crystal et al. 2002; Harvey et al. 2002). Second, the disrupted proteins of the adenovirus circumvent preexisting anti-adenovirus immunity (De et al. 2013). Third, the disrupted capsid likely exposes additional sites for the conjugation of GNE haptens, increasing the effective dose of the antigen. Finally, the inclusion of the beta-galactosidase transgene in the vector’s expression cassette provides a sensitive assay used for in-process quality control and detection of residual infectious Ad5 after the disruption process.
Hapten design is very important for small-molecule-targeted vaccines. The first-generation cocaine hapten, GNC, was designed based on the need for it to be relatively easy to manufacture, safe to administer, and linkable to the carrier protein without disruption of the cocaine-like antigenic character (Hicks et al. 2011; Carrera et al. 1995). While GNC demonstrated efficacy, a new hapten, GNE, provided greater chemical stability and thus more sustained presentation to the host immune system. The carboxyl groups on the cocaine analogs GNE and GNC are activated with 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide and N-hydroxysulfosuccinimide for coupling to the primary amines of lysines on the adenovirus capsid, forming the covalently linked protein–hapten conjugates that serve as the key components of the vaccine. Optimization of several parameters was required to maximize the density of haptens. For example, the conjugation ratios of hapten to capsid protein differed for each hapten. The hapten GNC/carrier ratio of 100:1 was sufficient to saturate the binding to adenovirus capsid proteins, whereas for GNE that ratio was 300:1. A formulation had to be defined that provided stability to the dAd5GNE, was compatible with the adjuvant, and would not impact the safety profile, particularly at the site of injection. Lastly, the purification and formulation must remove conjugation residuals to avoid adverse effects on potency and safety. The formulation buffer for dAd5GNE enabled stable −80 °C storage for at least 1 year. Head-to-head comparison of several adjuvants with dAd5GNE demonstrated that Adjuplex® (Advanced BioAdjuvants LLC, Omaha NE) provided the greatest immunostimulatory boost, providing the optimal balance of potency and risk analysis for adjuvant components and potential safety concerns.
13.7 dAd5GNE-Evoked Immune Response
Studies with dAd5GNC in mice demonstrated classic vaccine immunity with early response anti-cocaine IgM antibody levels that dropped below the limit of detection by week 5, inversely related to rising anti-cocaine IgG titers (Hicks et al. 2011). An evaluation of IgG subtypes demonstrated that IgG1 levels were highest, followed by IgG2a and IgG2b. Additionally, the polyclonal response had similar avidities for cocaine and for the conjugated cocaine analog, suggesting excellent hapten/cocaine characteristics (Hicks et al. 2011). Although the most desired immunoglobulin isotype in mice is not clear, it is important to note that IgG1 is not the most potent of the immunoglobulin isotypes to activate complement, a process not needed for a response to addictive drugs (Schroeder and Cavacini 2010).
Subsequent experiments with dAd5GNE confirmed the capacity of the vaccine to stimulate robust anti-cocaine IgG immunity in mice, rats, and non-human primates (De et al. 2013; Hicks et al. 2014; Maoz et al. 2013; Wee et al. 2012). Evaluation of the serum from dAd5GNE-vaccinated non-human primates revealed antibodies with high specificity not only for cocaine but also for the active cocaine metabolite cocaethylene and lower, but measureable, specificity for the active cocaine metabolite norcocaine (Hicks et al. 2014). Further, reduced affinity for the non-active metabolites benzoylecgonine and ethyl methyl ester suggests that the anti-cocaine immunity to the hapten recognized the essence of the cocaine molecule that embodies the functional action of the drug.
Many studies evaluating vaccines for the treatment of substance use disorders have demonstrated that vaccines with the highest antibody titers tend to demonstrate some measure of efficacy (Cornuz et al. 2008; Haney et al. 2010; Hatsukami et al. 2011; Martell et al. 2009). Similarly in preclinical studies, dAd5GNE mediated CNS protection following cocaine administration in non-human primates with anti-cocaine antibody titers >4 × 105, whereas animals with anti-cocaine antibody titers <4 × 105 had only mildly reduced cocaine levels in the brain. Cocaine administration at the weight-adjusted typical human dose showed significant reduction in cocaine binding at the site of the dopamine transporter in high-titer non-human primates relative to non-immunized animals and animals with titers <4 × 105 (Maoz et al. 2013). Similarly, a study in mice demonstrated that following cocaine challenge, there was no significant reduction of cocaine levels in the brain when anti-cocaine antibody titers were <5 × 105. However, when anti-cocaine antibody titers were >5 × 105, cocaine levels in the brain were reduced significantly.
Vaccination regimen and dAd5GNE dose were evaluated with the goal of achieving the high, persistent anti-cocaine antibody titers required for efficacy. Long-term sustained titers are necessary for the vaccine to provide coverage in the context of unpredictable instances of addictive drug use. Dose escalation results from mice, rats, and non-human primates were neither consistent nor predictable by body weight or surface area, and therefore, the definitive extrapolation to humans will require clinical study. However, the evaluation of anti-cocaine antibody titers over time in two studies of vaccinated non-human primates demonstrated that the anti-cocaine antibody titer half-life was approximately 4 weeks. Consistent with this, monthly vaccinations of non-human primates yielded sustained, high anti-cocaine antibody titers, which remained stable for 6 months, the duration of the study, suggesting that the optimal dosing regimen for humans will be monthly.
13.8 dAd5GNE Efficacy in the Context of Preexisting Anti-adenovirus Immunity
The translation of a vaccine to widespread use requires that it is broadly effective in the target population. Vaccines against infectious agents have different criteria for success that include sufficient coverage to provide herd protection, limiting propagation of the target through a population. While this is not an important characteristic for a therapeutic vaccine for drug use, there is a shared need for efficacy in the context of a target population with preexisting immunity to the vaccine vector, as vaccines based on adenovirus have suffered from widespread seroprevalence in human populations (D’Ambrosio et al. 1982; Mast et al. 2010; Nwanegbo et al. 2004; Piedra et al. 1998). The possibility that preexisting anti-adenovirus immunity may limit the potency of dAd5GNE is therefore an important concern to address. However, in mice treated to evoke potent neutralizing anti-adenovirus immunity, subsequent vaccination with dAd5 did not significantly affect total or isotype-specific anti-cocaine antibody levels and did not affect the vaccine-mediated abrogation of cocaine access to the CNS (De et al. 2013).
13.9 dAd5GNE-Induced CNS Protection and Redistribution of Cocaine
Evaluation of the capacity of dAd5GNE to evoke high-titer anti-cocaine antibodies is necessary, but not sufficient for an efficacious and safe anti-cocaine vaccine. Assessment of the vaccine must include an evaluation of vaccine function in the context of cocaine use. As such, the distribution of a sequestered bolus of the drug throughout the body was evaluated, with assessment of local toxicity to organs that can be adversely affected by cocaine, such as the heart (Benowitz 1993; Brody et al. 1990; Derlet and Albertson 1989; Hollander et al. 1995; Kloss et al. 1984; Lange and Hillis 2001; Lipton et al. 2000; Muscholl 1961; Restrepo et al. 2007; Rump et al. 1995; Silva et al. 1991).
The biodistribution of 3H-cocaine was evaluated in naive and dAd5GNE-vaccinated rodents. In both mice and rats, dAd5GNE vaccination significantly increased the amount of 3H-cocaine maintained in the serum of vaccinated animals, most of which was IgG bound (De et al. 2013; Wee et al. 2012). Vaccination significantly reduced cocaine access to the brain in mice by 63 % (p < 0.003) and in rats by 61 % (p < 0.009) (De et al. 2013; Wee et al. 2012). Positron emission tomography (PET) was used to evaluate access of administered cocaine to the brains of naive and dAd5GNE-vaccinated non-human primates (Maoz et al. 2013). A high-affinity, dopamine transporter (DAT)-specific ligand 11C-PE2I was displaced by cocaine and the loss of the PET signal correlated with cocaine access. When naive animals were challenged with cocaine, there was a 62 % loss of 11C-PE2I-labeled DAT in the caudate and putamen, a level significantly greater than the 47 % threshold needed to evoke a subjective “high” in humans (Maoz et al. 2013; Volkow et al. 1997). In contrast, dAd5GNE-vaccinated animals showed less than 20 % cocaine-occupied DAT when anti-cocaine titers were >4 × 105.
Systemic distribution of administered cocaine and cocaine metabolites was assessed in vaccinated and naive non-human primates. As expected, dAd5GNE vaccination significantly reduced cocaine and cocaine metabolite levels in the brain, but despite increased levels in the serum, levels of cocaine and cocaine metabolites were reduced in peripheral organs (adrenal gland, spleen, lung, heart, kidney, and liver), suggesting that antibody-bound cocaine restricts access to, or deposition in, the viscera (Hicks et al. 2014). As such, dAd5GNE vaccination may prevent some of the systemic toxicities associated with cocaine overdose, including arrhythmia and cardiovascular collapse (Derlet and Albertson 1989; Lange and Hillis 2001; Lipton et al. 2000).
13.10 dAd5GNE-Induced Suppression of Cocaine-Induced Behavior
When a weight-adjusted typical human dose of cocaine (1 mg/kg) was delivered intravenously to naive mice, there was a marked increase in the locomotor activity. In dAd5GNC- and dAd5GNE-vaccinated mice, this increase was abrogated (Hicks et al. 2011) (see Fig. 13.2 for an example). The relative distribution of movement behaviors, including ambulation, stereotypy, vertical, and resting time, in vaccinated animals challenged with cocaine showed no significant difference relative to PBS-challenged controls. Behavior studies of dAd5GNE-vaccinated rats challenged intraperitoneally with 15 mg/kg cocaine demonstrated significant vaccine-mediated reduction of cocaine-induced locomotor activity (Wee et al. 2012). Cocaine-challenged naive rats traveled farther and spent more time exhibiting a vertical rearing behavior than vaccinated rats, which were similar to naive PBS-treated controls.
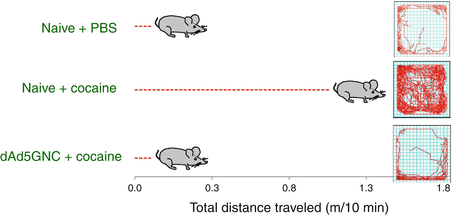

Fig. 13.2
dAd5GNE-mediated abrogation of cocaine-mediated hyper-locomotor activity. Naive or vaccinated mice were administered with cocaine, and the distance traveled over 10 min was measured in a rodent ambulatory behavior box. As a control, naive mice were treated with PBS (no cocaine). The cocaine-treated vaccinated mice were indistinguishable from the PBS controls and significantly different from the cocaine-treated naive mice. Shown at left is the total distance traveled for each group and at right the tracing of the mouse movement in the behavior box
13.11 dAd5GNE Protection in Binge Drug Use
An issue for the use of therapeutic vaccines to treat addictive drugs is the concern that the use of multiple administrations of the addictive drug or an increase in the dose will be used to overwhelm the antibody-mediated protection and leave vaccinated individuals vulnerable to binge drug use and its associated adverse effects (Orson et al. 2009; Kosten et al. 2002; Martell et al. 2009). In studies that address these concerns, dAd5GNE-vaccinated mice and NHPs retained high anti-cocaine titers 15 min following cocaine administration. Similarly, dAd5GNE-vaccinated non-human primates that received cocaine on a daily basis were compared with cocaine-naive NHPs, and the two groups exhibited no significant differences in anti-cocaine antibody titer half-life, suggesting that continuous cocaine use will not lead to antibody exhaustion and that vaccination likely remains protective soon after cocaine use. Notably, dAd5GNE protected the brain of vaccinated mice equally well whether cocaine was administered on a weekly or daily basis, and while naive mice exhibited marked hyperactivity following a daily and bihourly cocaine “binge,” vaccinated mice did not (Havlicek et al. 2014). Cocaine dose escalation produced increasing levels of hyperactivity in naive mice, but did not significantly affect the activity of vaccinated mice. Finally, vaccinated mice receiving high intravenous doses of cocaine had fewer cocaine-induced seizures, suggesting that vaccination not only prevents the behavior associated with high-dose cocaine use but also reduces the toxicity of high-dose use.
13.12 dAd5GNE-Induced Reductions in Cocaine Self-Administration
The ultimate goal of an anti-cocaine vaccine is to decrease motivation to self-administer cocaine; if vaccination removes the reward of drug use, it is expected that the reinforcing effects of addiction will fall off as well. While successful achievement of these end points will require evaluation in clinical trials, preclinical experiments with dAd5GNE suggest that vaccination can reduce cocaine self-administration in rodents and non-human primates. In rats trained to self-administer cocaine, vaccination did not reduce the frequency of 0.5 mg/kg intravenous cocaine self-administration, but when the effort to access cocaine was elevated, vaccinated animals self-administered less frequently than naive animals (Wee et al. 2012). Importantly, when access to cocaine was suspended, naive rats primed with cocaine resumed self-administration whereas the vaccinated rats did not, suggesting that dAd5GNE vaccination significantly reduced the reinforcing effects of cocaine use and may be effective at preventing recidivism.
Studies with dAd5GNE-vaccinated and non-vaccinated control non-human primates that were trained to self-administer cocaine and candy and become “addicted” were deprived of access to the cocaine with the candy alternative remaining (Evans et al. 2012). Subsequent reinstatement of cocaine was followed by a relatively fast return to self-administration in the control monkeys but not in those that were dAd5GNE vaccinated. Most of the vaccinated monkeys resisted the option of cocaine, even in the contexts of removal of the candy alternative, reduced work required for cocaine access, or administration of a cocaine prime. These three manipulations were required for reinstatement, and the time until reinstatement correlated with anti-cocaine antibody titer. Taken together, this evidence suggests that dAd5GNE vaccination may be effective for recovering cocaine addicts.
13.13 Safety of dAd5GNE
Prior to translation to the clinic, formal safety and toxicology studies are required for any drug product. During the preclinical efficacy studies, it was established that the vaccine-mediated redistribution of cocaine served to protect peripheral organs from the drug. Additionally, concerns that the vaccine may drive “binge” use were addressed by the protection of mice from seizures induced by very high doses of cocaine. These important findings complement our formal IND-enabling toxicology studies in mice and non-human primates.
A dose-ranging, placebo-controlled, murine study evaluated potential vaccine-mediated changes in serum chemistry, hematology, organ weight, gross pathology, histopathology, and general health over the course of six months. Aside from minor localized inflammation in mice, which was associated with repeated administrations of the adjuvant at the local site of injection, no adverse effects related to the intramuscular administration of dAd5GNE were observed. This local inflammation was due to the required large dose of adjuvant, which did not scale with body weight as did the active ingredient dAd5GNE. In contrast to the mice, a dose-ranging, placebo-controlled, toxicology study in non-human primates evaluating similar parameters over the course of six months showed no toxicity, including no local toxicity at the site of vaccine administration. Finally, these animals were challenged with cocaine on each of the 3 days leading up to sacrifice as a model to evaluate vaccine safety in the context of cocaine use.
13.14 Adenovirus-Based Anti-nicotine Vaccine HexonAM1
In light of efficacy demonstrated with the adenovirus-based anti-cocaine vaccine dAd5GNE, we have developed adenovirus-based vaccines against other highly addictive molecules. With the goal of improved efficacy with an easily characterized product, a single component of the adenovirus capsid, hexon, was chosen as the immunogen for its established high immunopotency. Thus, the nicotine vaccine HexonAM1 was developed as a potential immunotherapy for smoking cessation. Similar in design to the cocaine vaccine dAd5GNE, HexonAM1 uses conjugation of a non-immunogenic nicotine analog (AM1) to hexon to evoke an anti-nicotine immune response, sufficient to abrogate systemically administered nicotine. As with the anti-cocaine vaccine, the strategy is to block the transfer of nicotine across the blood–brain barrier to the CNS nicotinic acetylcholine receptors (nAChR). This immunotherapy, with many parallel concepts to the dAd5GNE cocaine vaccine, targets the nicotine molecule in order to block reward to drug cravings.
Similar to cocaine, nicotine is a small addictive molecule that evades immune surveillance and must be coupled to larger immunogenic proteins to evoke an immune response (Cerny and Cerny 2009; Moreno et al. 2010). Since conjugating the nicotine molecule to proteins requires the addition of reactive free carboxyl groups, nicotine analogs have been developed to mimic the chemical characteristics of nicotine while providing a handle for chemical conjugation (Moreno et al. 2010; Carrera et al. 2004; Isomura et al. 2001; Meijler et al. 2003). In studies comparing a series of these analogs (Nic, CNI, and AM1), we found that only AM1 produced a high-titer relevant response in mice, and thus, this hapten was used for all further studies.
Our first-generation adenovirus-based nicotine vaccine dAd5AM1 utilized the same immunogenic disrupted adenovirus (dAd5) platform as dAd5GNE (De et al. 2013). Studies with dAd5AM1 demonstrated that the vaccine produced high anti-nicotine titers in mice within 4 weeks (De et al. 2013). Further, anti-nicotine titers remained high throughout the course of the study and were not hindered by preexisting adenovirus immunity (De et al. 2013). However, the potency was not sufficient to protect rodents from high levels of nicotine, leading our laboratory to explore the individual adenoviral capsid proteins hexon, penton, and fiber as potential immunogenic carriers (Rosenberg et al. 2013). Prior studies have evaluated these adenoviral capsid components and have found that the hexon proteins elicit the highest anti-Ad antibody titers in mice and humans (Molinier-Frenkel et al. 2002). To apply this potency to the nicotine analog, purified hexon protein was conjugated to AM1 with the same reagents used for dAd5GNE production.
Studies in mice showed that HexonAM1 triggered persistently higher anti-nicotine titers than dAd5AM1 (~1.0 × 106). As an initial measure of efficacy, the capacity of HexonAM1-evoked anti-nicotine antibodies to rapidly bind nicotine and block access to the CNS was evaluated (Rosenberg et al. 2013). Vaccinated and non-vaccinated mice were challenged with radioactive 3H-nicotine and sacrificed 1 min later to evaluate the partition of nicotine in the blood (IgG bound and free) and CNS. Nicotine levels in the brain of HexonAM1-vaccinated mice were 53 % lower than naive control mice, generating a 3.4-fold reduction in the brain to blood nicotine ratio. In the serum 83 % of the nicotine was IgG bound, indicating that this was an immune-mediated effect.
The mice required preconditioning (sensitization) with nicotine before typical weight-adjusted human doses of nicotine produced changes in locomotor activity (Rosenberg et al. 2013). HexonAM1-vaccinated and control, non-vaccinated, sensitized mice were challenged daily with subcutaneously administered nicotine (0.5 mg/kg) and assessed for ambulatory activity. While control mice displayed typical nicotine-induced hypo-locomotion during each nicotine challenge, the behavior of HexonAM1-vaccinated mice was indistinguishable from control mice challenged with PBS. Repeat assessment over 5 weeks demonstrated that HexonAM1-mediated immunity abrogated nearly all nicotine-induced hypo-locomotor activity, i.e., the HexonAM1-mediated blockade of nicotine was sufficient to prevent nicotine-induced behavior in mice.
13.15 The Potential of Adenovirus-Based Vaccines
The adenovirus capsid protein-based vaccines to cocaine and nicotine hold promise as therapeutics for the treatment of these substance use disorders. Designed to leverage the potent immunity of the adenovirus capsid and redirect it to the conjugated small drug analogs, these vaccines blocked administered drugs from reaching their CNS receptors and abrogated drug-induced physiological responses. For the disrupted capsid platform applied to the cocaine target, formal safety and toxicology studies led to the conclusion of an excellent safety profile, and therefore, the vaccine platform is ready to be evaluated in clinical trials. Careful consideration of clinical trial design includes specifying the target population, the dosing regimen, safety and efficacy phenotypes, and a reasonable prospective definition of successful end points.
Targeting cocaine offers the risk/benefit advantage of having no competing, currently approved pharmacological therapy, and therefore, there is motivation to meet a clear-cut societal need. Clinical success of an adenovirus-based vaccine against cocaine will justify rapid application to other addictive substances such as nicotine, opiates, and methamphetamine.
References
Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112:1969–77.PubMed
Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;24:3232–40.PubMed
Aurisicchio L, Ciliberto G. Genetic cancer vaccines: current status and perspectives. Expert Opin Biol Ther. 2012;12:1043–58.PubMed
Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nat Rev Microbiol. 2014;12:765–71.PubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


