Adenosis and Microglandular Adenosis
EDI BROGI
ADENOSIS
Adenosis is a lobulocentric proliferation predominantly derived from the terminal duct lobular unit (TDLU). Larger duct structures are sometimes incorporated into the lesion, but they are less involved than lobules. Epithelial and myoepithelial cells participate in adenosis. Ewing1 referred to adenosis as “fibrosing adenomatosis.” Foote and Stewart2 subsequently described the lesion as “sclerosing adenomatosis” and “sclerosing adenosis.” The earliest clinicopathologic studies of sclerosing adenosis were published in 19493 and 1950.4
Clinical Presentation
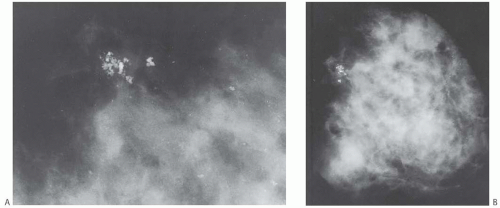
Adenosis occurs most often as part of the spectrum of proliferative alterations commonly referred to as fibrocystic changes. The entire complex may produce a palpable mass, with adenosis accounting for part or most of the lesion. Adenosis limited to isolated lobules that are not part of fibrocystic changes is a microscopic lesion that comes to clinical attention when it harbors mammographically detectable calcifications (Fig. 7.1). Microcalcifications frequently occur in the sclerosing type of adenosis.
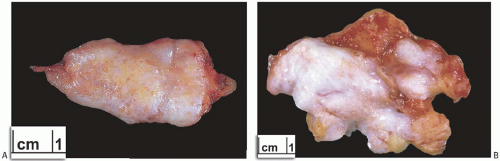
Adenosis forms a distinct clinically palpable or radiographically detectable mass when the affected lobules are closely adjacent or merge together to create an adenosis tumor5 (Figs. 7.2 and 7.3). Patients with adenosis tumor are almost always premenopausal, averaging about 30 years of age at diagnosis. Adenosis tumor is usually smaller than 2 cm. It is rarely attached to the skin, and the lesion is usually a firm, clinically discrete, or ill-defined mass easily mistaken for a fibroadenoma.3,6,7 A few patients report pain or tenderness.5,8,9
Radiology
Non-palpable adenosis tumors that contain calcifications may be detected by mammography (Fig. 7.1). Sonography usually reveals a solid, well-defined tumor.5 Gill et al.10 studied the radiologic findings in lesions that contained sclerosing adenosis in needle core biopsy samples. Thirtythree lesions in which sclerosing adenosis represented the major finding were deemed to have concordant radiologic and pathologic findings. Seventeen of the 33 lesions were detected radiologically as masses. Ten of 17 (59%) foci were circumscribed on ultrasound and eight by mammography, five had indistinct margins, and two were partially circumscribed. Fifteen of the 33 lesions were detected mammographically as clustered calcifications not associated with a mass. The calcifications were amorphous or indistinct in nine (60%) cases, pleomorphic in four (27%), and punctate in two (13%). In the same study, sclerosing adenosis was a minor component in 44/88 (50%) lesions sampled by core biopsy. Mammographically, ductal carcinoma in situ (DCIS) in sclerosing adenosis was associated with pleomorphic calcifications and atypical ductal hyperplasia (ADH) with amorphous calcifications. Taskin et al.8 evaluated the radiologic findings in 76 breast lesions that yielded a diagnosis of sclerosing adenosis, as either a major or a minor component. The lesions were detected mammographically as masses (25/69, 36%), microcalcifications (25/69, 30%), asymmetrical opacities (15/69, 22%), and architectural distortion (8/69, 12%). The remaining seven lesions were detected by ultrasound. Two lesions appeared as spiculated mammographic and sonographic masses. Sclerosing adenosis was the main finding in 17/37 lesions that underwent needle core biopsy. It constituted a minor component of the lesion in 20/37 (54%) cases in which fibroadenoma (eight cases), fibrocystic changes (seven cases), invasive carcinoma (two cases) and intraductal papilloma, ADH, and DCIS (one case each) were thought to account for the radiologic abnormality. Oztekin et al.9 described a 37-year-old woman with sclerosing adenosis that presented as bilateral palpable and tender masses with indistinct margins. Multiple oval masses with angulated margins, oriented parallel to the skin, were seen by ultrasound. They had complex posterior acoustic shadowing and mild vascularity on color Doppler ultrasound. Magnetic resonance imaging showed multiple ovoid masses with indistinct borders, intermediate enhancement on T1-weighted and T2-weighted images, and a homogeneous signal.
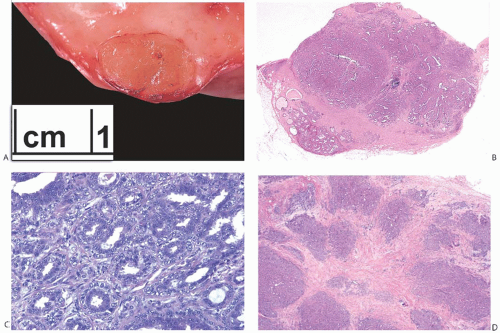
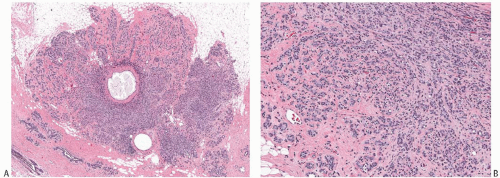 FIG. 7.3. Adenosis tumor. A: A whole-mount histologic section showing a well-circumscribed tumor with microcysts. B: Confluent sclerosing adenosis. |
Gross Pathology
Excised adenosis tumors vary in gross appearance depending upon their microscopic composition (Figs. 7.2 and 7.3). Lesions consisting of florid adenosis with a prominent glandular component are typically well-circumscribed nodules composed of gray or pale tan, firm homogeneous tissue. With increasing sclerosis, adenosis tumors are likely to be grossly less well defined at the borders, multinodular, and more fibrous in appearance. Lesions with abundant calcifications may seem gritty when cut. Gross cyst formation is infrequent. Necrosis and/or infarction are occasionally encountered in an adenosis tumor, usually during pregnancy or lactation.
Nontumorous adenosis in isolated lobules is difficult to detect grossly and it usually mingles with other proliferative lesions. Rarely, minute discrete foci of sclerosing adenosis may be palpated in unfixed breast tissue as fine granules by brushing a finger gently over the cut surface. With tangential light, these foci are sometimes visible as pale tan dots or a granular surface in white breast stroma (Fig. 7.4).
Microscopic Pathology
The structural histologic features described here may be encountered in individual microscopic foci of adenosis and in adenosis tumors. Adenosis tends to have a more prominent glandular pattern in premenopausal women, whereas sclerosis and diminished gland formation are more conspicuous after menopause. In some individual patients, there is very little variability in the spectrum of adenosis, whereas others exhibit diverse patterns.
Florid Adenosis
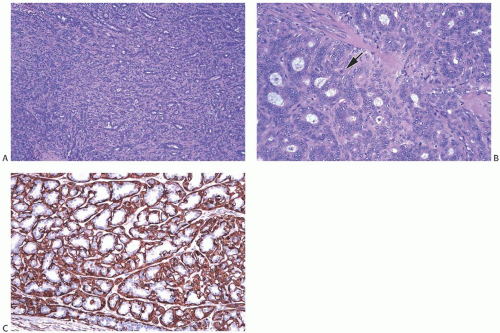
Florid adenosis is the most cellular type of adenosis. It is characterized by hyperplasia of epithelial and myoepithelial cells. Proliferation of ductules and lobular glands severely distorts and usually effaces the architecture of the underlying lobules. The hyperplastic structures appear to
be elongated, becoming tortuous and entwined, resulting in many more ductular cross sections than are present in an anatomically normal lobule (Fig. 7.5). In the plane of section, the complex proliferative structure has a swirling pattern, punctuated by glands cut transversely. Some glands may maintain round, open lumens, but the majority of the ductular structures are cut tangentially or longitudinally. They display elongated and often collapsed lumens, usually with smooth, non-angular contours. The caliber of the lumens is relatively constant regardless of the plane of section. Cystic dilation of ductules or glands is not prominent in florid adenosis.
be elongated, becoming tortuous and entwined, resulting in many more ductular cross sections than are present in an anatomically normal lobule (Fig. 7.5). In the plane of section, the complex proliferative structure has a swirling pattern, punctuated by glands cut transversely. Some glands may maintain round, open lumens, but the majority of the ductular structures are cut tangentially or longitudinally. They display elongated and often collapsed lumens, usually with smooth, non-angular contours. The caliber of the lumens is relatively constant regardless of the plane of section. Cystic dilation of ductules or glands is not prominent in florid adenosis.
Epithelial cells lining the tubules and glands are flattened, cuboidal, or slightly columnar and are arranged in one or two orderly layers surrounded by myoepithelial cells. Increase in cell size and nuclear pleomorphism can be found in florid adenosis, especially during pregnancy or lactation. Hyperplastic change in the epithelial component of florid adenosis is mirrored by myoepithelial hyperplasia (Figs. 7.2, 7.5, 7.6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree