and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
8.1 Definition, Site and Incidence
Adenoid cystic carcinoma (ADCC) in its classic form has a very characteristic sieve-like pattern of small malignant, often basaloid cells. The tumour was described by Robin and Laboulbene and by Billroth in the mid-nineteenth century and first termed cylindroma (Zylindrome) [25, 153]. The term adenoid cystic carcinoma was coined by Spies in 1930 [171] but gained wider acceptance first after the classic work on salivary gland tumours by Foote and Frazell in the 1950’s [64]. Several synonyms are still in use but more seldom so, such as cribriform adenocarcinoma (which nowadays also carries another connotation; please see Chap. 15), cylindromatous adenocarcinoma, adenocystic carcinoma and adenoid cystic adenocarcinoma. Adenoid cystic carcinoma consists of ductal (luminal) and basal/myoepithelial (abluminal) cells that usually are arranged in a typical cribriform (glandular) pattern but also in tubular and solid growth patterns. It has a slow but relentless progression with long-term (15–20 years) fatal outcome inasmuch as 60–90 % of cases and is hence always best regarded as a high-grade malignancy [65, 77, 91, 94, 97, 144]. To classify all adenoid cystic carcinoma to be of high-grade malignancy is a practical approach, possibly regarded as pragmatic by some, but makes the concept of high-grade transformation, or dedifferentiation, possibly of less importance in cases of ADCC, contrary to, e.g. acinic cell carcinoma.
As stressed in previous chapters, an accurate estimate of the incidence of salivary gland tumours is almost impossible to achieve. It is also emphasised again that there is also considerable variability amongst different geographical regions. A reasonable, but in no way complete, compilation of published series in the literature indicates a mean crude incidence of 1.0–1.3/100,000/year. Adenoid cystic carcinomas comprise approximately 10 % of all epithelial salivary neoplasms, 7–18 % of parotid malignancies and 20 % of all malignant salivary gland tumours. In a recent large population-based US study of 6,391 major salivary gland carcinomas, adenoid cystic carcinoma occurred equally in the submandibular and parotid glands, whilst other types of salivary carcinoma had considerably lower incidence rates in the submandibular gland [29]. Recent data from the SEER programme (US National Cancer Institute’s Surveillance, Epidemiology, and End Results) indicate indeed a decline of ADCCs in the USA [55]. Sublingual salivary tumours are rare but unfortunately very frequently malignant. Adenoid cystic carcinoma is the most common sublingual carcinoma and mucoepidermoid carcinoma the second most common [156, 201, 202]. Adenoid cystic carcinomas comprise 33–58 % of minor salivary gland malignancies with the highest frequency in the palate followed by the tongue, buccal mucosa and lip. Only mucoepidermoid carcinoma is a more common malignant intraoral salivary gland tumour. In the AFIP registry, ADCC is only the fourth most common malignant tumour after mucoepidermoid carcinoma, adenocarcinoma NOS and acinic cell carcinoma [56]. In a Danish population, ADCC was the most common carcinoma (25.2 %) and mucoepidermoid carcinoma second, only representing 16.9 % of carcinomas [26]. Adenoid cystic carcinoma is the most common malignant tumour in the submandibular gland as well as being the most common malignant minor salivary gland neoplasm of the entire upper respiratory tract [10, 58, 115]. Large recent studies from China show similar incidences of ADCC, and other salivary carcinomas, with the exception of a much higher incidence of lymphoepithelial carcinoma and a lower incidence of polymorphous low-grade carcinoma [185, 193]. Pleomorphic adenoma is the most common lacrimal salivary tumour, followed by adenoid cystic carcinoma which hence is the most common malignant lacrimal salivary tumour [57, 139, 157, 203].
Intraosseous adenoid cystic carcinomas are rare but well documented and so far primarily described in the mandible and only the odd case in the maxilla. The origin is still unknown, but naturally an ectopic embryogenic inclusion is the most likely explanation in most cases but a pluripotential nature of derivatives of oral ectoderm could be another possibility. This latter theory is illustrated by a reported mandibular case of ADCC with odontogenic features [107]. Al-Sukhun and associates estimated in 2006 that only 16 cases of intraosseous ADCC had been reported to that date [5]. Since then, a few more have been added to the literature making the total number of cases to only about 20 [33, 47, 70, 75].
Adenoid cystic carcinomas also arise in many other sites apart from the typical sites, i.e. the major salivary glands and oral minor salivary glands including the base of the tongue [1, 2, 80]. Salivary gland neoplasm of the breast is nowadays a well-known separate entity of breast tumours, and before the recent SEER report (see below), still some 150 cases of breast ADCC were reported. Breast adenoid cystic carcinoma interestingly carries a considerably better prognosis than do head and neck ADCCs [23, 28, 98, 109, 112, 166–168]. Other sites with reported ADCCs are as mentioned above the lacrimal gland [9, 102, 135] but also the sinonasal tract [3, 102, 137, 141] and larynx [48, 158, 205]. Indeed, a recent large Danish study showed adenoid cystic carcinoma to be the most common laryngeal salivary malignancy representing 46 % of salivary carcinomas on this site [131]. Adenoid cystic carcinomas also arise in the female genital tract [53, 188, 196, 198], the external auditory canal [34, 128], skin [22, 69, 99, 130, 150], prostate [83, 113] and lung [23, 95, 111]. Oesophageal adenoid cystic carcinomas appear to be more common in countries like China and Japan and arise most commonly in the middle to lower third of the oesophagus. It may also be associated with oesophageal basaloid squamous cell carcinoma [46, 76, 93, 96, 100, 125, 187]. A rare example of a bilateral ADCC has been reported, appearing synchronously in the left palatine tonsil and the right peritonsillar tissue [12]. Likewise exceedingly rare, two simultaneous tumours of two different major salivary glands have been described, e.g. a case of left parotid pleomorphic adenoma and contralateral sublingual gland adenoid cystic carcinoma [136]. ADCC has also been part of multiple metachronous salivary carcinomas [195] (see also Chap. 7). Further, adenoid cystic carcinoma is one of the most common components of so-called salivary hybrid tumours (see Chap. 15). Adenoid cystic carcinoma also arises as the malignant component in carcinoma ex pleomorphic adenoma; however, it is surprisingly rare [84] (see Chap. 10). It is of interest to note that in one of the 2012 reports from the American SEER (US National Cancer Institute’s Surveillance, Epidemiology, and End Results), 5,349 patients with ADCC were identified. Of these, 1,850 cases were found in the major salivary glands, 2,077 in the minor salivary glands, 696 cases in the breast, 291 in the skin, 203 in the lung and bronchus, 132 in the female genital system and 100 cases in the eye and orbit [101].
Adenoid cystic carcinoma typically presents as a slowly growing mass, and in approximately one-third of parotid cases, pain and facial nerve paralysis will develop. Macroscopically they are solid and well circumscribed but not encapsulated, firm and often light tan (Fig. 8.1a). In contrast to many of the other high-grade salivary malignancies, patients with parotid adenoid cystic carcinoma usually have been aware of the mass for months or even years before seeking medical advice. Adenoid cystic carcinoma of minor salivary glands is likewise a slow-growing, insidious disease. The high tendency for intra- and perineural invasion and spread via nerves is well known, but its true prognostic significance is in fact not known [15]. A large recent Chinese study of 616 cases of head and neck ADCCs showed regional lymph node metastasis in 10 % of cases. The tongue, and the base of the tongue in particular, and the floor of the mouth were the most common sites of lymph node metastasis (together approximately 50 % of cases). They also found that most cases occurred via a classic ‘tunnel-style’ metastasis and the level Ib and level II were the most frequently involved [116]. Fixation to the skin in cases of parotid adenoid cystic carcinoma occurs late. Adenoid cystic carcinomas typically have frequent local recurrences and late distant metastases. Palatal ADCC may spread along the palatine branches of trigeminal nerve and can on rare occasions give its first presentation as a tumour in the cavernous sinus or pterygopalatine fossa [73]. There are numerous other very rare and odd presentations of salivary adenoid cystic carcinoma. For example, pleural effusion was the presentation in a patient with a palatal ADCC [122] and pericardial effusion in patient with cutaneous ADCC in the scalp [22]. Nasopharyngeal adenoid cystic carcinoma may present as Horner’s syndrome [169] or even as Garcin’s syndrome (‘half base syndrome’; unilateral paralysis of most if not all cranial nerves) [85].
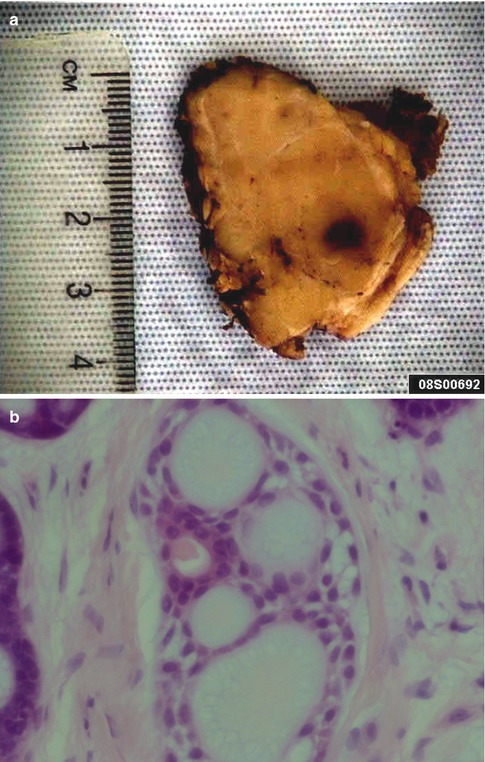
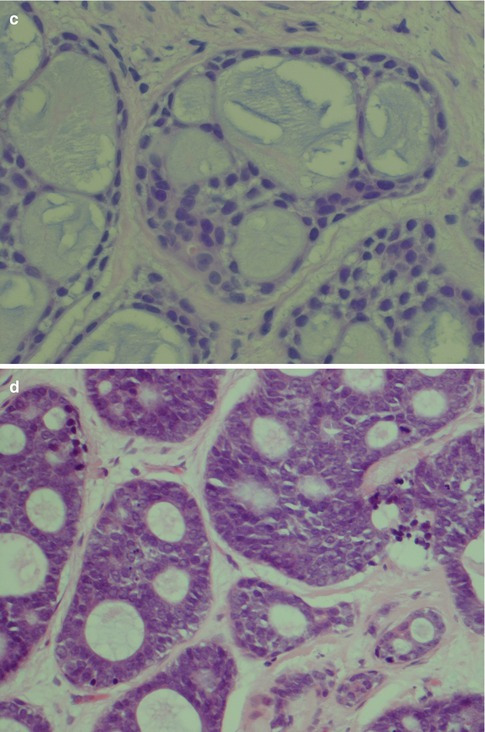


Fig. 8.1
(a) Parotid ADCC presenting as a light-tan and firm mass. (b) ADCC with four pseudocysts and one true cyst. Flat myoepithelial cells with clear cytoplasm line the pseudocysts whilst luminal eosinophilic cells line the glandular lumen. (c) Cylindromatous microcystic mucopolysaccharide-filled haematoxyphilic spaces. (d) The pseudocysts appear as ‘punched out’ holes in the cell islands
8.2 Histopathology
Adenoid cystic carcinoma is composed of two cell types typically arranged in three growth patterns and combinations thereof. The cells grow in a stroma that usually is prominent and that consists of mucopolysaccharides, collagen-like fibres and basal lamina components such as laminin, fibronectin, type IV collagen and glycosaminoglycans. The two cell types are modified basal/myoepithelial cells, the abluminal cells and the ductal, luminal cells. The myoepithelial cells are flat, spindle-shaped with scanty, indistinct cytoplasm. They tend to have hyperchromatic, angular nuclei and typically line the pseudocysts in the cribriform areas of an adenoid cystic carcinoma. The ductal or luminal cell is cuboidal or columnar with broader, eosinophilic cytoplasm and typically line the true cysts. Both these two cell types are always found in adenoid cystic carcinoma although in varying proportions, the basaloid cell type the more common. Small true duct and cysts are hence invariably present but may not be immediately apparent in the solid types of ADCC, neither in small incisional biopsies (Fig. 8.1b–d). There are three defined growth patterns:
1.
The typical and most common is the cribriform (glandular) type, a pattern created by numerous pseudocysts and some true cysts scattered in cell islands. The pseudocysts dominate in number and have an appearance of having been ‘punched out’ of the cell islands and contain pools of haematoxyphilic glycosaminoglycans. Furthermore to the glycans, the pseudocysts contain basal lamina components. This material is hyaluronidase sensitive and alcian blue positive but only weakly PAS positive after diastase treatment. The true cysts, on the other hand, contain secretory material that is neutral rather than acid and is diastase resistant, PAS positive and mucicarmine positive. The true cysts are lined with cuboidal or, occasionally, columnar cells (Figs. 8.2 and 8.3).
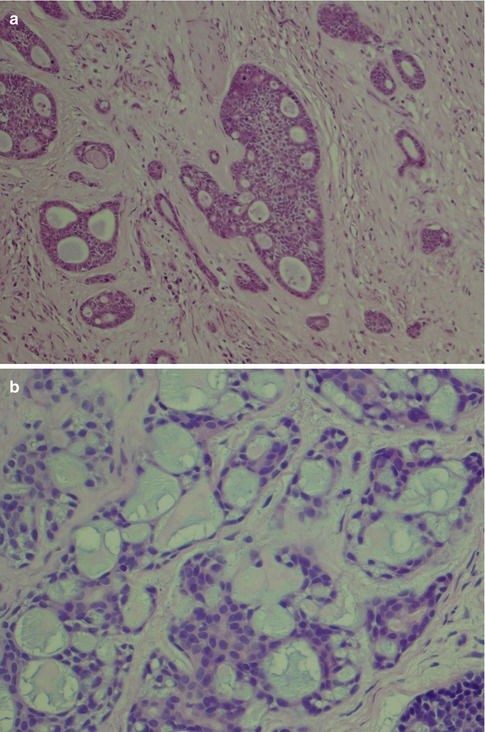
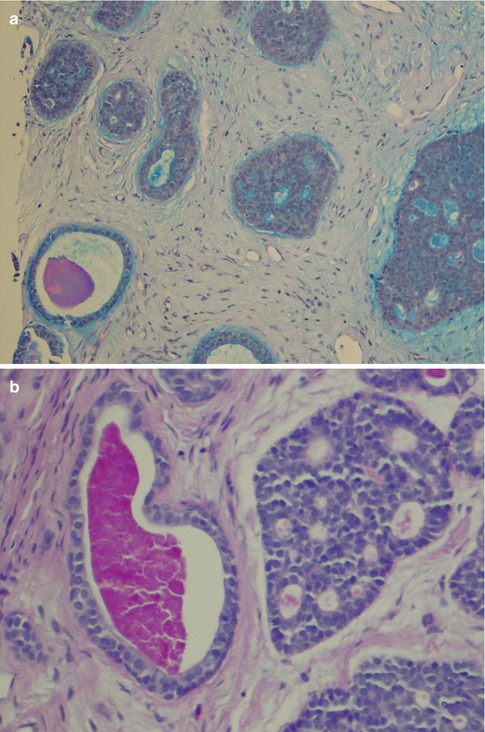
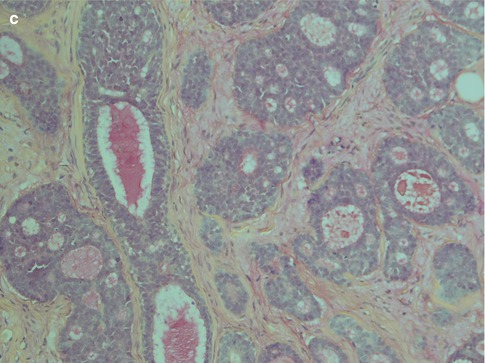

Fig. 8.2
(a) Cribriform ADCC. (b) Area with pseudocysts and flattened cells


Fig. 8.3
(a) AbPAS stain showing the acid material of the pseudocysts staining blue and the more neutral mucin of a true cyst magenta. (b) True cysts with diastase-resistant, PAS-positive material whilst pseudocysts are negative or only weakly positive (PASD). (c) Mucicarmine
2.
The second arrangement is tubular with well-formed ducts and tubules and frequently surrounded by a hyaline stroma. These ducts and tubules are lined by inner epithelial duct-like cells and one or several layers of outer flat, spindle-shaped myoepithelial cells. The ductal structures often contain an eosinophilic material. The tubular variant is almost invariable mixed with the other growth patterns, particularly the cribriform, and may form a pseudotrabecular pattern. Occasionally, not only the myoepithelial cells but also the epithelial ductal cells tend to be hydropic and of a clear cell appearance. The tubules can occasionally be arranged longitudinally; the cells are sometimes arranged in a palisading pattern (Fig. 8.4).
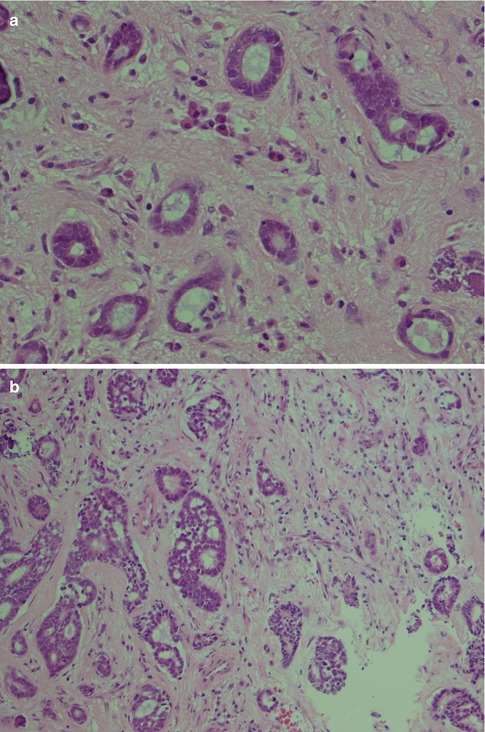
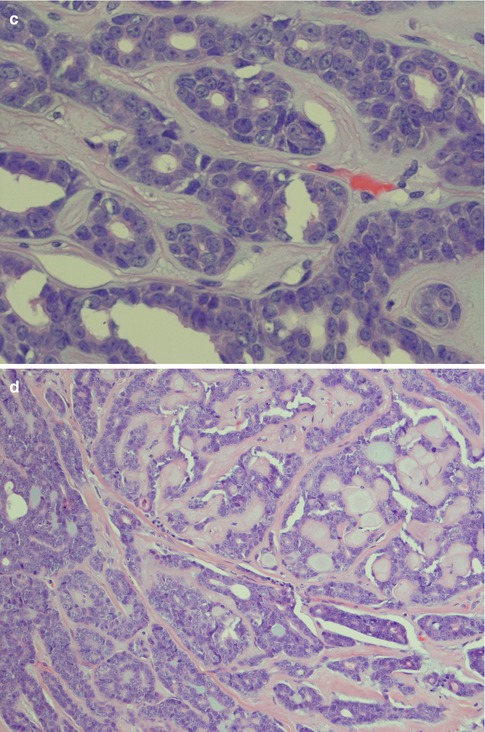
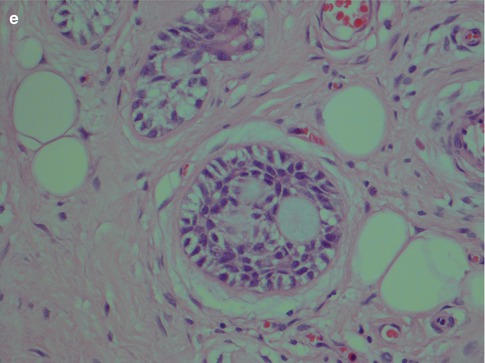



Fig. 8.4
(a) Tubular ADCC with well-formed tubules and duct-like structures. (b) Predominantly tubular type but also cribriform and trabecular patterns. (c) Tubular ADCC with cellular atypia. (d) Tubular ADCC with prominent hyalinised stroma. (e) Hydropic change and clear cell appearance
3.
In the solid type, myoepithelial and epithelial cells form solid masses, and there are only few glandular structures and pseudocysts. Between the relatively well-demarcated islands of these rather small cells, there are strands of stroma. The cell masses are sometimes fenestrated. On other occasions, the tumour forms a diffuse solid mass with very little intervening stroma (Fig. 8.5). Occasionally the solid type may take the appearance of a dedifferentiated ADCC (see below).
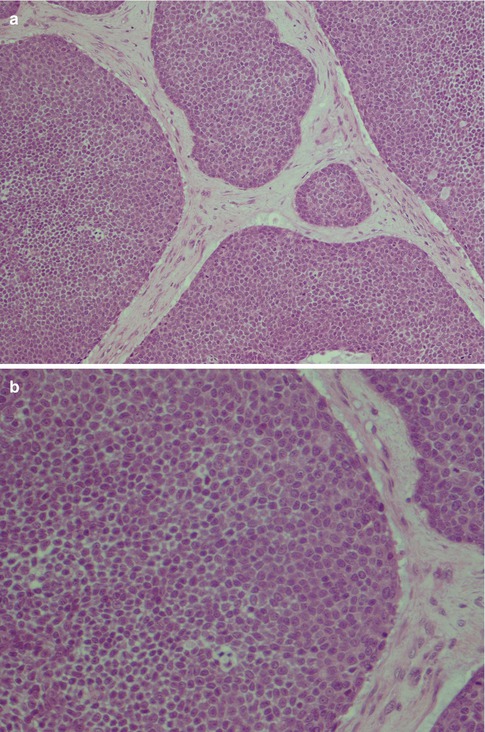
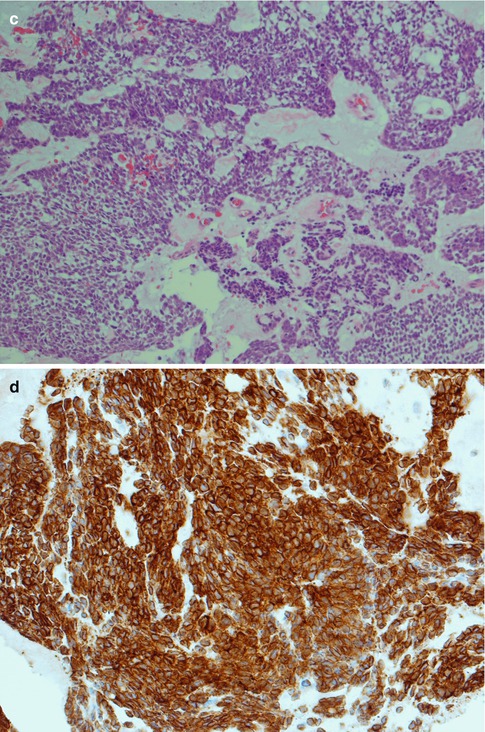


Fig. 8.5
(a) Solid type of ADCC with tumour cells in nests, partly fenestrated. (b) Higher magnification showing small closely packed, atypical cells, very few mitotic figures. (c) Diffuse solid ADCC not organised in nests and lobules. (d) Many if not most adenoid cystic carcinomas (like acinic cell carcinoma) show strong positivity for bcl-2
Although these three growth patterns are well defined, most tumours show mixtures of these patterns. In all three types of ADCC, mitoses are scarce but the cellular atypia is usually obvious, contrary to cases of polymorphous low-grade adenocarcinoma and adenomas. There may be areas of necrosis, particularly in solid areas and sometimes of considerable size (Fig. 8.6). Particularly in cases of solid adenoid cystic carcinoma, numerous sections are often needed to reveal the true nature of the tumour. This sampling procedure with numerous sections should be carried out in most cases of salivary neoplasms. Small excisional biopsies from mucosal sites can pose difficult diagnostic dilemmas when only the solid growth pattern of an ADCC is present. Numerous levels have to be cut and frequently immunohistochemistry performed to rule out other salivary gland tumours such as polymorphic low-grade adenocarcinoma, or pleomorphic adenoma, but also solid variants of mucosal squamous or adenosquamous carcinomas.
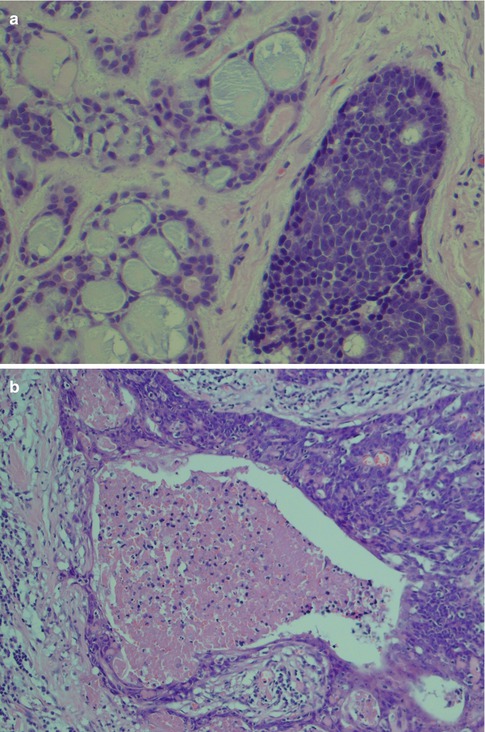

Fig. 8.6
(a) Cribriform pattern and to the right a more solid pattern with a few pseudocysts. (b) Adenoid cystic carcinoma with necrosis
Perineural and also intraneural invasion is a common and well-recognised feature of adenoid cystic carcinoma and can extend along nerves for a considerable distance from the main tumour. Its tendency for centring of not only nerves but also vessels is less well known. Attempts to use the immunohistochemical expression of neural cell adhesion molecule (NCAM) as a biological marker for perineural invasion in ADCCs have been performed but with not very encouraging results. One study showed the sensitivity and specificity for positive staining to be only 73 and 75 %, respectively [165]. Adenoid cystic carcinomas also frequently and extensively invade bone (Fig. 8.7). The tendency for peri- and intraneural invasion is shared by other salivary malignancies but in particular by polymorphous low-grade adenocarcinoma. Adenoid cystic carcinomas are slowly growing neoplasms in spite of being high-grade malignancy. Patients with parotid tumours usually present with several months’ or even a year’s history of gradual swelling. Occasionally though parotid adenoid cystic carcinoma may be as small as those detected in minor salivary glands. ADCCs are invariable invasive with infiltrative margins and only rarely are pushing invading borders seen (Fig. 8.8). Adenoid cyst carcinoma may show papillary and pseudopapillary growth patterns and can then rather easily be confused with polymorphous low-grade adenocarcinoma. Cellular atypia and a high Ki-67 labelling index will help in the differential diagnosis (Fig. 8.9). Adenoid cystic carcinomas essentially have no macrocysts but occasionally cystic spaces are observed, some of which may be artefactual. Solid variants of adenoid cystic carcinomas may also show pseudorosettes around vessels; however, the cells are negative for neuroendocrine markers like CD56, chromogranin and synaptophysin. Calcifications are not common but do occur, both as deposits in carcinoma tubules and in the surrounding fibrous tissue (Fig. 8.10). A three-tiered histological grading scheme of ADCC was proposed by Szanto and associates in 1984 and may still be applied in some institutions [16, 178]. Tumours with tubular and cribriform areas but no solid components constitute grade I, whilst grade II tumours have mixed patterns but less than 30 % of solid areas. Grade III tumours have predominantly a solid pattern. This grading system was shown to have a bearing on the prognosis, but shortcomings may be sampling of the tumour and, as always, the experience of the individual pathologist. We share the opinion that all ADCCs should be considered to be of high-grade malignancy and histological grading is of less importance. In order to make such a three-tiered histological grading accurately, the pathologist would need a considerable experience of adenoid cystic carcinoma, an experience not all of us have. Also, the treatment regime of ADCC is primarily decided upon the clinical staging and settings in the individual case rather than histological grading.
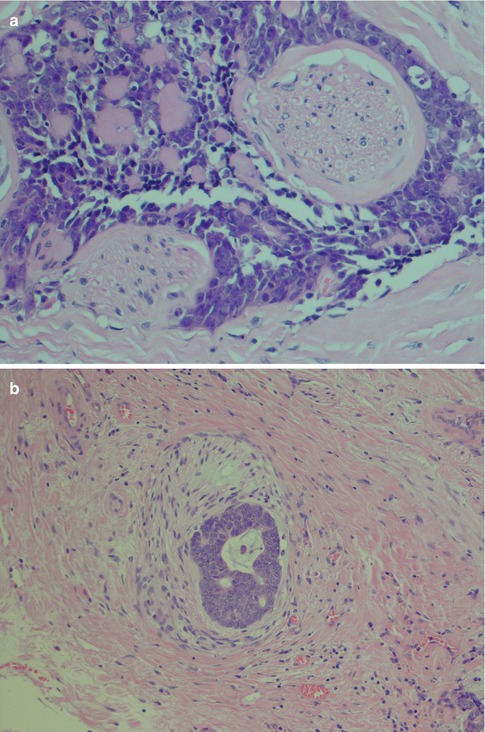
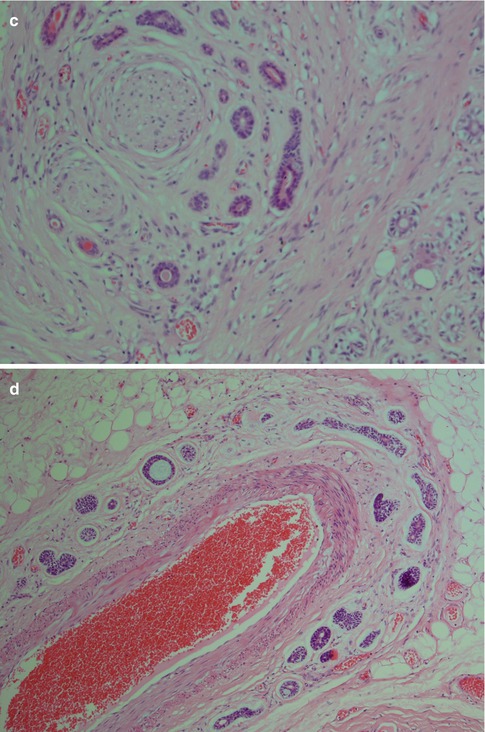
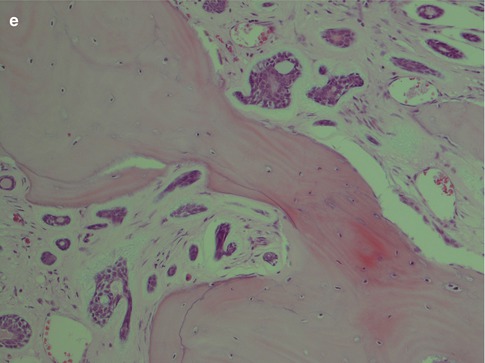
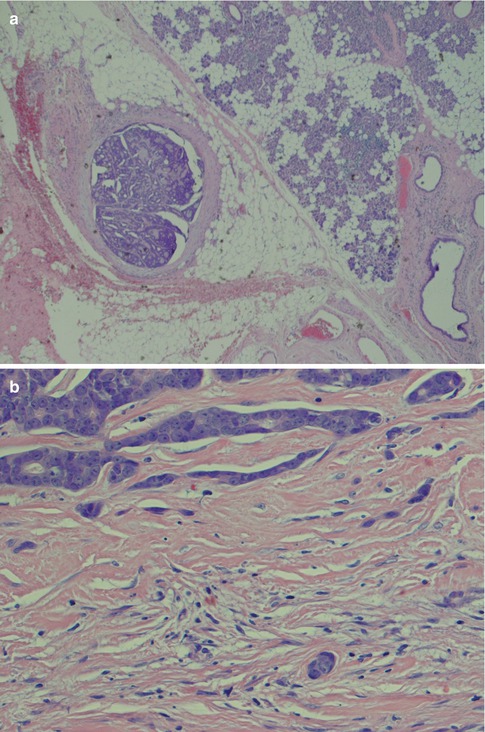
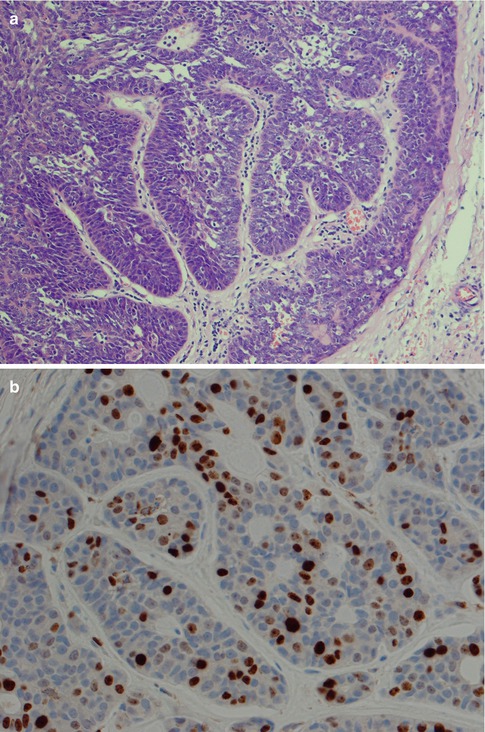
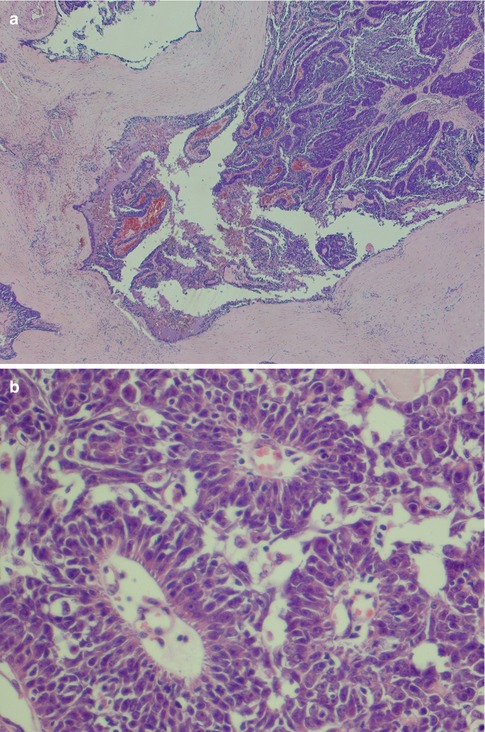
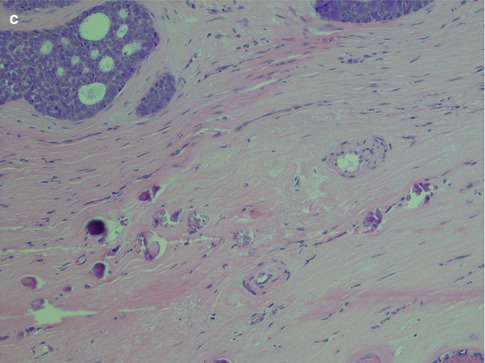



Fig. 8.7
(a) Typical perineural invasion. (b) Intraneural invasion. (c) ADCC centred around a nerve. (d) ADCC centred in a similar fashion around a vessel. (e) Extensive bone invasion

Fig. 8.8
(a) A very small superficial parotid ADCC. (b) ADCCs have infiltrative rather than pushing invasive borders

Fig. 8.9
(a) Pseudopapillary growth pattern that may mimic polymorphous low-grade adenocarcinoma. (b) A relatively high Ki-67 index supports ADCC rather than polymorphous low-grade adenocarcinoma


Fig. 8.10
(a) Cystic spaces in an ADCC. (b) Three pseudorosettes around vessels. (c) Calcification in ADCC
High–grade transformation (dedifferentiation) occurs on rare occasions in adenoid cystic carcinoma (ADCC-HGT) as it does in other salivary gland tumour entities (see Chap. 16). The first case was described by Cheuk and associates in 1999, and since then a number of case reports and also smaller series have been published, and by 2007 only approximately 25 cases of ADCC-HGT had been described in the English literature [36, 40, 126, 160, 162]. The high-grade component appears histologically as a distinct cell population of anaplastic cells with altered immunophenotype. High-grade transformation in ADCC is histologically characterised by nuclear enlargement and irregularity, higher mitotic counts, comedonecrosis, desmoplastic stroma and loss of the typical biphasic ductal-myoepithelial differentiation. The latter is visualised by loss of an abluminal layer of myoepithelial cells by immunohistochemistry (e.g. p63). Ki-67 and p53 labelling indices are also elevated whilst C-kit and cyclin-D1 are not. The most striking clinical feature is likely the high propensity for lymph node metastasis [162].
In 2006, a ‘new’ histological variant of adenoid cystic carcinoma was reported, but it appears as no, or very few, further cases have been published. These were two cases of a sclerosing variant of ADCC, one located in the parotid gland and the other case in the submandibular gland. The tumours were composed predominantly of varying-sized large sclerotic and hypocellular nodules. These nodules contained myoepithelial cells and pseudovascular spaces, the latter the authors concluded probably to be the result of artefactual retraction. There were also numerous small globules or spherules surrounded by myoepithelial cells similar to those of collagenous or mucinous spherulosis. There were no eosinophils present as is the case in sclerosing mucoepidermoid carcinoma. The biological behaviour of this morphological subtype is yet not known [4]. Stromal melanocytosis, distinct from sclerosis, is occasionally present in adenoid cystic carcinoma, which also may be the case in mucoepidermoid carcinoma [50, 180].
8.3 Molecular Pathology and Immunohistochemistry
ADCC is one of the five known translocation-associated salivary neoplasm (see Chap. 16). Cytogenetic findings include translocations of chromosome 9p13-23 (with 6q) which occurs in approximately 30 % of ADCCs [68, 90, 133]. In a chromosomal comparative genomic hybridisation and tissue microarray study of 27 ADCCs, copy number gains were found as well as copy number losses. The most common finding was copy number gain of 22q13 which was suggested to be involved in the initial pathogenesis of ADCC by proto-oncogene activation [67]. More recent studies have shown that in general, in adenoid cystic carcinomas, chromosomal losses are more frequent than gains. A cytogenetic profile specific for ADCC is however so far not known, but studies have shown that loss of 6q or 8p23 is associated with the total number of DNA copy number aberrations in ADCC and significantly greater in ADCCs with loss of 6q compared to other ADCCs. The frequent DNA copy number aberrations are loss of 6q23-27 and 8p23 and gains of 6p, 6q23, 8p23 and 22q13 [134]. One major study showed that the most frequent genetic change in ADCCs is the loss of 1p32-p36 (in more than 40 % of cases), followed by 6q23-q27 and 12q12-q14 [149]. Recent studies from 2013 by Zhang and associates demonstrated that not only for ADCCs but also for mucoepidermoid and salivary duct carcinoma, the most commonly shared copy number abnormalities (CNAs) are losses at chromosomes 6q23-26 and the 9p21 region. The same investigation revealed that in ADCC, subtype-specific CNAs included a loss at 12q11-12 and focal copy number losses included 1p36.33-p36.22. Tumour-specific amplicons were seen at 11q23.3. Interestingly, the study also demonstrated that fusion-positive ADCCs have relatively lower CNAs than their fusion-negative counterparts [204]. Currently the potential role of variable microRNAs (miRNA) in the tumorigenesis of ADCC is starting to evolve. For example, overexpression of miR-17 and miR-20a appears to be associated with poor outcome in cases of ADCC [120]. Other studies indicate that miR-181a can inhibit adenoid cystic carcinoma cell migration, metastasis and proliferation in vitro (via the MAPK-Snai2 pathway) and may hence be a new therapeutic target [78].
The PLAG1 gene, activated in a subset of pleomorphic adenoma, is apparently not playing any role in the tumorigenesis of ADCC, and no PLAG1 fusion has been detected in ADCC [44]. Similarly, SMAD4 (DPC4), a tumour suppressor gene located on 18q21 and frequently deleted in pancreatic (and colorectal) carcinoma, does not participate in the tumorigenesis of ADCC [43]. No mutations in codon 816 of the C-kit gene has been identified [170], and DNA sequencing showed no genetic alteration of C-kit juxtamembrane domain (exon 11) and tyrosine kinase domain (exon 17), although ADCCs rather consistently overexpress the receptor tyrosine kinase (RTK) C-kit. More recent studies confirm that C-kit gene mutations are rare but do occasionally occur (e.g. nucleotide (nt) switches nt1990G → A in exon 13 and nt2368A → G in exon 17) [124, 182, 192]. Therefore, the overexpression of C-kit protein may be involved in the pathogenesis of ADCC, but mutation of the gene does not appear to be the mechanism of C-kit activation [88]. Mitochondrial mutations, on the other hand, are frequent in ADCC [121]. Slug (other symbols, e.g. SNAI2, SNAIL2) is a member of the SNAIL superfamily of zinc finger transcription factors and functions as an antiapoptotic factor. Slug has been demonstrated to contribute to the function of the stem cell factor C-kit signalling pathway [143], and a recent study of 121 cases of adenoid cystic carcinoma showed positive expression (immunohistochemical) of C-kit and Slug in 90 and 72 %, respectively. These findings indicate that C-kit/Slug pathway may participate in the invasion and metastasis of ADCCs [181]. EMMPRIN (extracellular matrix metalloproteinase inducer) is a transmembrane glycoprotein that has shown to contribute to in vitro invasion of adenoid cystic carcinoma cells. Similarly, silencing of EMMPRIN demonstrated inhibition of both proliferation and perineural invasion of ADCC cells in vitro and in vivo [199, 200].
One currently possibly defining feature of ADCC is the recurrent translocation t(6;9)(q22 ~ 23; p23 ~ 24), with the fusion transcript that involves the MYB and NFIB genes [17, 119]. The downstream target genes of MYB are C-kit, Cox-2 and Bcl-2. The MYB-NFIB fusion was detected in 28 % of primary and 35 % metastatic ADCCs but in none of other salivary carcinomas analysed suggesting that the MYB-NFIB fusion characterises a subset of ADCCs contributing to MYB overexpression. MYB may thus be a new relatively specific target for tumour intervention in patients with ADCC [118]. These findings have also been confirmed by others, and, e.g. West and associated found that a balanced translocation between MYB and NFIB was present in 49 % of adenoid cystic carcinoma but not identified in non-adenoid cystic carcinomas [194]. Bell and associates also found 55 % of ADCCs to have increased MYB protein expression and mainly confined to the myoepithelial cells [19]. The MYB-NFIB gene fusion is not always associated with chimeric transcript formation [119]. Further, the MYB-NFIB gene fusion has been demonstrated in dermal cylindromas [63].
As emphasised in other chapters, immunohistochemistry plays a limited, even though important, role in diagnosing salivary gland tumours and should be used to assist the final diagnosis. Very few, if any, tumour-specific markers are available at present [127]. In ADCCs, both the ductal and the modified myoepithelial cells are strongly positive for pancytokeratins, low and high molecular weight cytokeratins CK8/18 and 34βE12, respectively, CK7, CK5/6 and Ber-EP4. None of these cytokeratin markers will hence discriminate between ductal and modified myoepithelial tumour cells in a solid ADCC nor in the more solid islands of a cribriform adenoid cystic carcinoma. Identification of the ductal structures is important in the differential versus myoepithelial carcinoma or different variants of mucosal squamous carcinomas. Virtually all salivary gland tumours are CK7+/CK20- which does not help in distinguishing different entities but facilitates to differentiate between primary salivary gland neoplasia from metastatic tumours and squamous cell carcinoma [114]. Most adenoid cystic carcinomas are strongly positive for both C-kit (78–92 %) and bcl-2 (90 %) [11, 32, 81, 170]. EMA shows a luminal positivity similar to that seen in normal salivary gland tissue (Fig. 8.11). There is only scattered and weak diffuse positivity for CK17, and CEA is negative or shows weak luminal positivity. The staining with CK14 is variable. CK14 is often negative in solid parts or shows a tendency for peripheral staining only but is strongly positive in nonsolid areas of cribriform adenoid cystic carcinoma with numerous pseudocysts. The tendency for CK14 positivity in the periphery of tumour nests has also been noticed with mucoepidermoid carcinomas (see Chap. 7). As with mucoepidermoid carcinoma, this staining pattern is far from always the case in ADCC. In tubular variants of adenoid cystic carcinoma, the CK14 staining is stronger in the inner luminal cells (Fig. 8.12). Adenoid cystic carcinomas invariably express a myoepithelial immunophenotype which however may vary in intensity and extent. Myoepithelial and basal/stem cell markers (smooth muscle actin, smooth muscle myosin heavy chain, p63, calponin and metallothionein) almost invariably highlight the myoepithelial cell components of adenoid cystic carcinoma (and of epithelial-myoepithelial carcinoma). p63 is a p53 homologue that is a selective marker of basal/stem cells and myoepithelial cells. The outer myoepithelial cells in tubular areas are well demonstrated by p63 and often SMA, whilst in solid parts of adenoid cystic carcinoma both p63 and SMA usually are negative or have only weakly scattered positivity (Fig. 8.13). The majority of cases of ADCC are positive for p63, and hence primarily in the non-luminal myoepithelial(-like) cells surrounding the luminal cells [52]. Of the above myoepithelial/basal cell markers, smooth muscle actin expression has been shown to have the highest accuracy (91.5 %) for ADCC [146]. Contrary to most other myoepithelial markers, S-100 is usually clearly positive in solid types of ADCC. The expression of GFAP may be controversial, and some authors reported adenoid cystic carcinoma to be negative [164], whilst others reported ADCC positive for GFAP in most of cases [104]. On balance, a positive GFAP immunostaining can be of some help in distinguishing difficult cases of adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma as this latter tumour more frequently is negative for GFAP (see Chap. 12) [6, 42, 74, 132, 151]. The expression of vimentin varies but is usually more pronounced in cribriform and tubular types than in solid types of adenoid cystic carcinoma (Fig. 8.14). HER2 is usually not expressed but has been described in some high-grade carcinomas [160]. CD43, a marker of T cells and histiocytes, has been reported in one series to be expressed in 100 % of cases of adenoid cystic carcinoma but considerably less in polymorphous low-grade adenocarcinoma and monomorphic adenomas. The series was however small consisting of only 12 cases of adenoid cystic carcinoma and 14 cases of PLGA [197]. Another recent study showed a selective expression of CD43 in 8 out 17 salivary adenoid cystic carcinomas indicating that CD43 may be selectively expressed in a subset of ADCC [163].
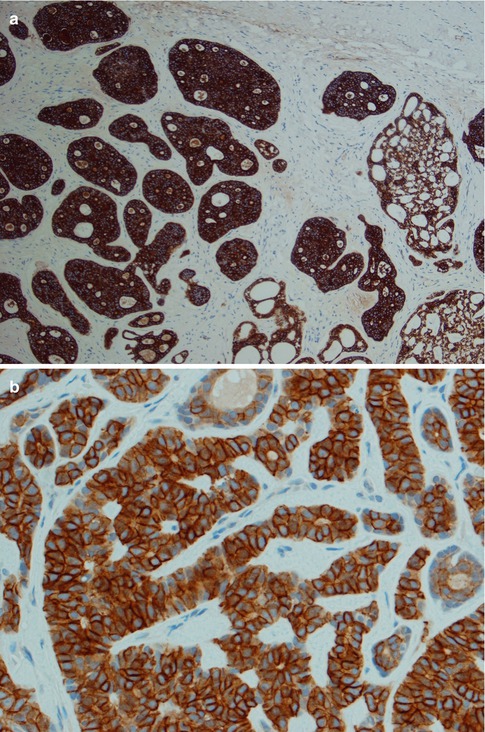
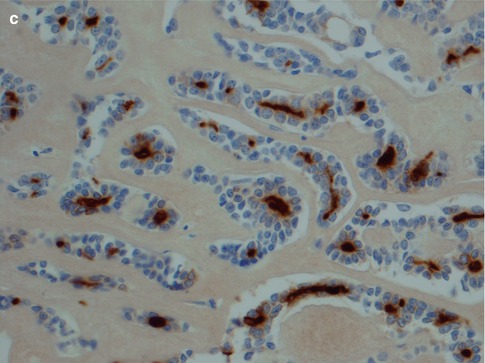
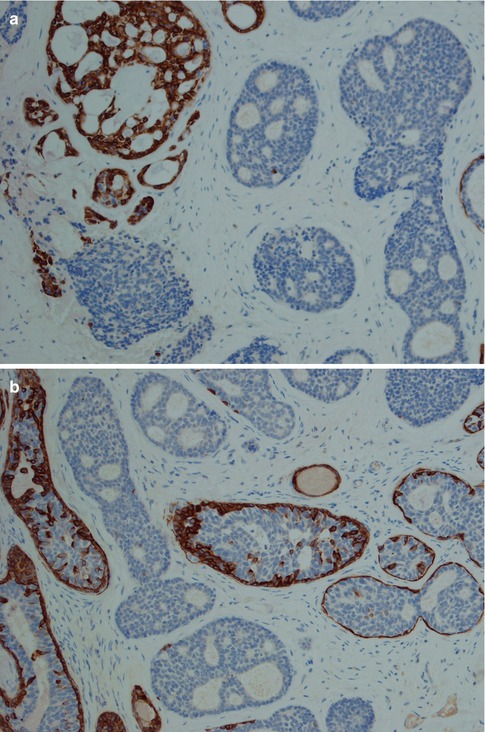
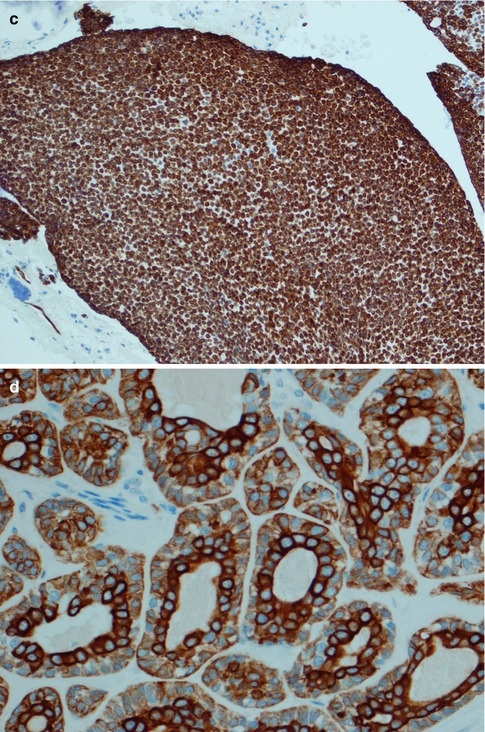
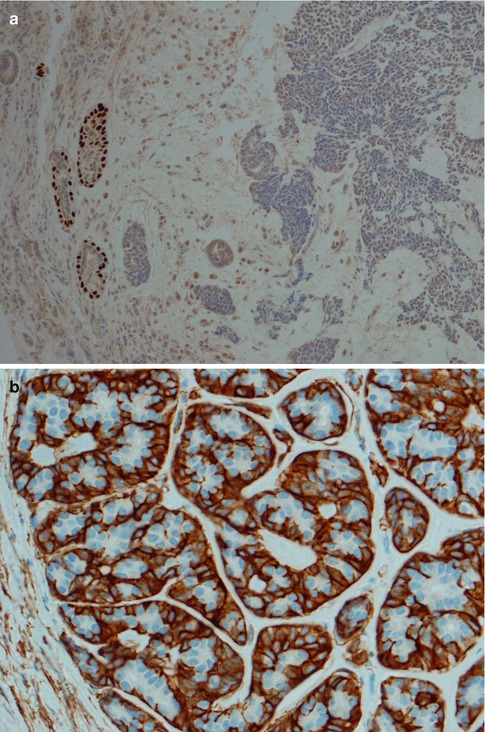
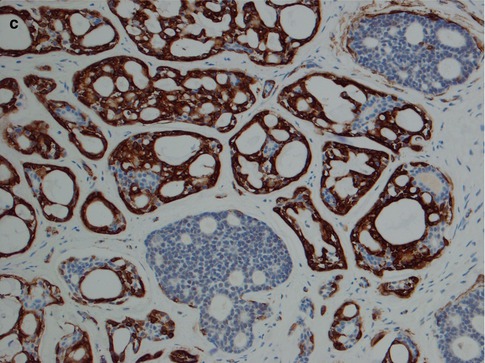
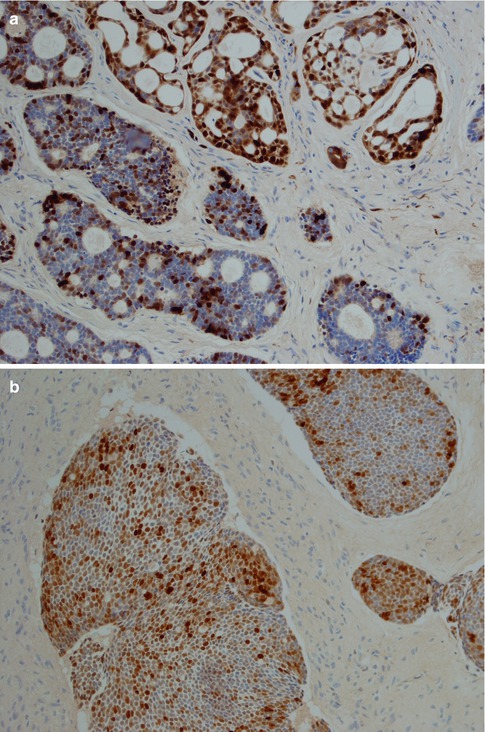
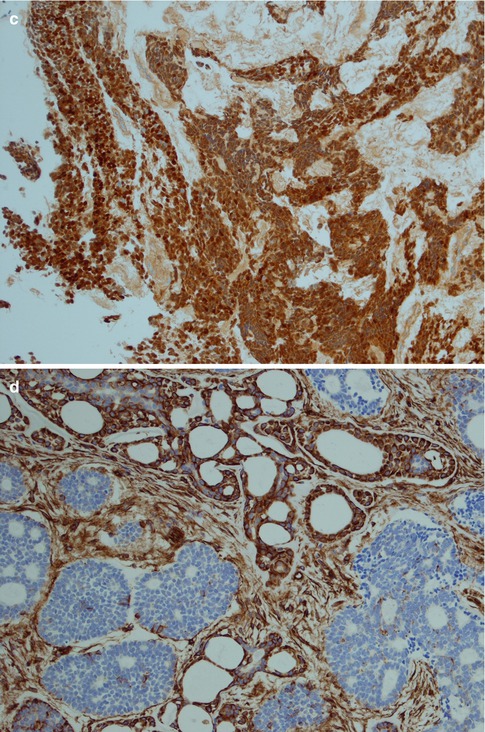
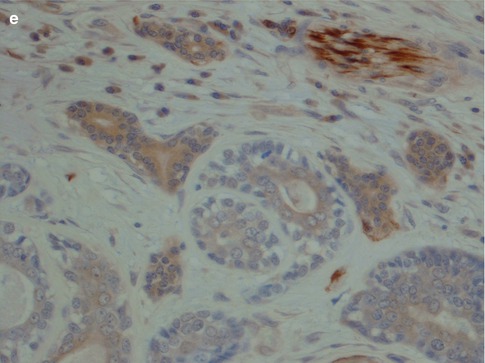


Fig. 8.11
(a) Strong uniform staining with CK7 (similar staining is seen with most cytokeratins except CK14). (b) Strong uniform staining with C-kit. (c) Luminal positivity for EMA in a tubular ADCC


Fig. 8.12
Different CK14 staining patterns in ADCC. (a) Areas with numerous pseudocysts stain positive for CK14, whilst the more solid cell islands are negative in cribriform ADCC. (b) The more solid islands are negative or show a peripheral staining for CK14. Note that the solid islands in this case do contain small pseudocysts. (c) CK14 positivity in a solid type without any pseudocysts. (d) Tubular area with stronger CK14 immunoreactivity in the inner luminal cells


Fig. 8.13
(a) p63-positive outer cells in tubular structures but negative or weakly scattered in solid areas. (b) Peripheral staining with SMA in tubular areas. (c) Strong SMA positivity in cribriform pseudocystic areas but negative in solid areas



Fig. 8.14




(a) Strong staining for S-100 in cribriform ADCC, somewhat less staining in more solid parts of the tumour. (b) Moderate positivity for S-100 in solid nests of a solid-type ADCC. (c) Even stronger S-100 positivity in a diffuse solid type. (d) Vimentin positivity in cribriform pseudocystic area (top) but negative in more solid areas. (e) Negative or only weak and patchy GFAP staining compared to crisp staining of nerve (top right)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


