Acute Myeloid Leukemia with t(8;21)(q22;q22), RUNX1-RUNX1T1
Kaaren K. Reichard, MD
Key Facts
Terminology
AML with maturation
Diagnostic genetic abnormality: RUNX1-RUNX1T1 fusion
Microscopic Pathology
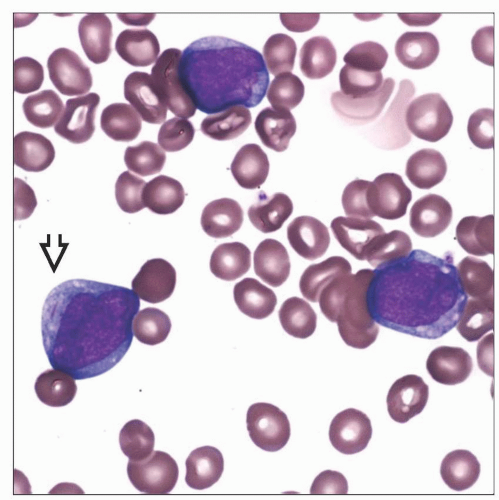
Increased myeloblasts
Evidence of neutrophilic maturation
Characteristic Auer rods
Single, thin, tapered ends
Abnormal neutrophilic precursors
Salmon-colored granules and dysplastic nuclear features
Ancillary Tests
Immunophenotyping
Increased myeloblasts; CD34(+), CD13(+), CD33(+)
May show expression of B-associated antigens; CD19, CD79a, Pax-5
May show expression of TdT or CD56
Cytogenetics/FISH/molecular
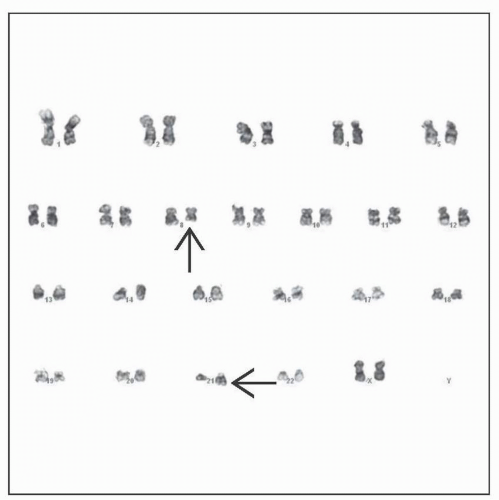
Demonstrate t(8;21) or RUNX1-RUNX1T1 fusion
Top Differential Diagnoses
AMLs ± recurrent genetic abnormalities (if blasts ≥ 20%)
Reactive neutrophilia (if low blast count)
Chronic myelogenous leukemia, neutrophilic variant
Reporting Considerations
Diagnose as AML with t(8;21)(q22;q22), RUNX1-RUNXIT1
“Low blast count” AML: Requisite ≥ 20% blasts not needed if genetic abnormality present
TERMINOLOGY
Abbreviations
Acute myeloid leukemia (AML)
AML with t(8;21)(q22;q22), RUNX1-RUNX1T1
Synonyms
AML with maturation
AML M2 in French-American-British classification
Core binding factor AML
AML with AML1-ETO fusion
Definitions
AML with specific genetic finding
t(8;21)(q22;q22) or variant or RUNX1-RUNX1T1 (a.k.a. AML1-ETO) gene fusion
Blast count may not meet typical requirement of ≥ 20%
So-called low blast count AML or oligoblastic AML
AML is defined by presence of genetic abnormality, regardless of blast count
ETIOLOGY/PATHOGENESIS
Environmental Exposure
None identified for de novo AML with t(8;21)
Therapy-related AML with t(8;21)
Prior exposure to cytotoxic agents &/or radiotherapy
Molecular Pathogenesis
Normal
Core binding factor (CBF) alpha subunit (a.k.a. RUNX1) interacts with CBF beta subunit
CBF alpha and beta form transcription factor complex
CBF alpha subunit binds to DNA promoter sequences involved in hematopoiesis
Abnormal
t(8;21) results in chimeric fusion protein RUNX1-RUNX1T1
Downregulates normal transcriptional activity
“Multi-hit” model of AML
Class 1 and class 2 mutations
RUNX1-RUNX1T1 fusion is considered a class 2 mutation
Insufficient alone for leukemia formation
Development of overt leukemia requires concurrent class 1 mutation (e.g., KIT, FLT3, or RAS mutation)
Discovery of t(8;21)
1st AML reciprocal translocation identified with common banding techniques (1975)
Association with Systemic Mastocytosis
AML with t(8;21) is most common AML associated with mastocytosis
WHO 2008 terminology
Systemic mastocytosis with associated hematological non-mast cell lineage disease (SM-AHNMD)
KIT D816V mutation in blasts and mast cells may indicate common progenitor
CLINICAL ISSUES
Epidemiology
Incidence
10-15% of pediatric AML cases
7% of adult AML cases
Age
Predominates in younger patients (20-40 years)
Gender
Equal male:female ratio
Ethnicity
More frequent in African-Americans than Caucasians compared to AML with inv(16)
Presentation
Abnormal CBC
Anemia
Thrombocytopenia
Single, bi-, or pancytopenia
Variable white blood cell count
Variable percent blast count
Neutropenia common
Myeloid neoplasm
Myeloid sarcomas common
Skin and gingival involvement
Treatment
Chemotherapy
Cytarabine-based regimen
Prognosis
Favorable risk
Complete remission common
Especially with intensive postremission treatment
Multiple cycles of high-dose cytarabine
50-60% cured with contemporary treatment
Prognostic genetic factors
˜ 70% of patients harbor an additional chromosomal abnormality
-Y; worse overall survival
-X; no obvious impact
del(9q); better overall survival in non-white individuals
+8; no impact
KIT mutations
Found in 12-47% of patients
Occur mostly in exon 17
May confer adverse prognosis
FLT3 mutations
Infrequent; 4-12%
Internal tandem duplication and point mutations in the tyrosine kinase domain
Prognostic significance unknown
Some reports suggest worse prognosis
Minimal residual disease monitoring
Quantitative RT-PCR studies
Goal: Identify molecular remission while on therapy
Molecular remission predictive of durable complete remission
Absence of RUNX1-RUNX1T1 fusion may not be necessary for long-term remission
> 1 log increase in transcript levels associated with increased relapse risk
MACROSCOPIC FEATURES
Extramedullary Involvement
Less common than in AML with inv16
Gingival hyperplasia
Splenomegaly rare
Cutaneous involvement
MICROSCOPIC PATHOLOGY
Key Microscopic Features
Peripheral blood
Anemia
Thrombocytopenia
Circulating blasts; possibly with Auer rods
Generally leukocytosis dominated by blasts
Evidence of neutrophilic maturation
Occasionally monocytic component
WBC count rarely exceeds 100 × 109/L
Bone marrow aspirate
Increased myeloid blasts
Variable blast count
Some cases less than the required 20% (low blast count AML)
Characteristic Auer rods
Thin with tapered, “cigar-shaped” ends
Usually single within a cell
Seen in cytoplasm of blasts and maturing/mature granulocytes
Abnormal neutrophilic precursors
Numerous pink/salmon-colored granules
Granules may abnormally aggregate in discrete region of cytoplasm
Dysplastic nuclei with megaloblastoid change and abnormal nuclear segmentation
In < 20% of cases, monocytic component
Erythroid and megakaryocytic lineages often decreased due to marrow replacement by AML
Increased and atypical, spindled mast cells if concurrent systemic mastocytosis
Bone marrow core biopsy
100% cellular
With an increasing proportion of neutrophilic maturation, sheets of blasts may not be conspicuous
Megakaryocytic lineage is decreased but morphologically unremarkable
May see associated mast cell disease
Focal, compact, dense aggregates of mast cells
Mast cell aggregates may be overshadowed by the AML (so-called occult mastocytosis)
Mastocytosis is often revealed after treatment of AML
Osteosclerotic bone
ANCILLARY TESTS
Cytology
Myeloperoxidase: Myeloblasts positive
Immunohistochemistry
Increased blasts: CD34, CD117 positive
May show Pax-5 positivity
Associated mast cell disease: Mast cells positive for tryptase &/or CD117, CD25 &/or CD2
Flow Cytometry
Myeloblasts: CD34, CD33, CD13, weak CD45
Aberrant antigen expression common
B-associated antigens: CD79a, CD19
TdT
CD56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree