Acute Myeloid Leukemia with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), CBFB-MYH11
Kaaren K. Reichard, MD
Key Facts
Terminology
AML with abnormal eosinophils
AML with CBFB-MYH11 fusion
Microscopic Pathology
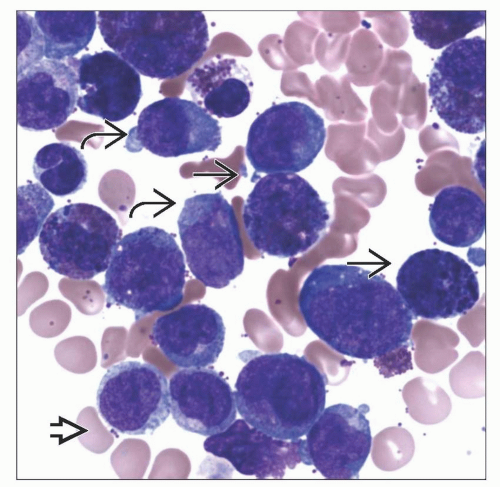
Increased blasts and blast equivalents: Myeloblasts, monoblasts, and promonocytes
Abnormal eosinophils with mixed eosinophil and basophil-type granules
May not see abnormal eosinophil component in peripheral blood
Hypercellular marrow
Ancillary Tests
Flow cytometric analysis
Myeloblasts: CD34, CD33, and CD45 (weak) positive
Monocytic cells: CD33 (bright), CD45, and CD36/CD64 positive
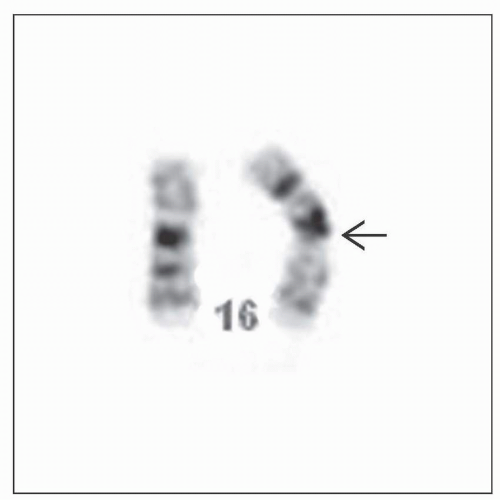
Cytogenetic &/or FISH analysis: Essential
Abnormality may be subtle &/or overlooked
Molecular testing for CBFB-MYH11
For diagnosis and residual disease monitoring
Top Differential Diagnoses
AMLs ± recurrent genetic abnormalities [e.g., t(8;21), t(9;11)] or other
Therapy-related inv(16) (need clinical history)
Reporting Considerations
Diagnose as AML with inv(16)(p13.1q22) or t(16;16) (p13.1q22); CBFB-MYH11
Low blast count AML; required threshold of ≥20% blasts for AML not required
TERMINOLOGY
Abbreviations
Acute myeloid leukemia (AML)
AML with inv(16)(p13.1q22) (WHO)
Synonyms
AML with abnormal eosinophils
AML M4eo in French-American-British classification
Often referred to as core binding factor acute myeloid leukemia (along with AML with t[8;21])
Definitions
Acute myeloid leukemia harboring distinctive cytogenetic/molecular finding
inv(16)(p.13.1q22)
Translocation (16;16)(p13.1;q22)
CBFB-MYH11 genetic fusion
Core binding factor beta (CBFB)
Myosin heavy chain gene (MYH11)
AML with recurring genetic abnormality (WHO)
Low blast count AML
Diagnosis is established despite occasional cases with blast count < usual requisite 20%
Presence of genetic abnormality warrants diagnosis of AML
ETIOLOGY/PATHOGENESIS
Environmental Exposure
None presently identified for de novo AML with inversion 16
Exposure to cytotoxic agents &/or radiotherapy may result in therapy-related AML with inversion 16
Molecular Pathogenesis
In normal cells: Core binding factor (CBF) beta protein interacts with CBF alpha subunit (a.k.a. RUNX1) to form CBF transcription factor complex
In cells with inv(16) or variant: Genetic rearrangement results in a CBFB-MYH11 molecular fusion
Persistent transcriptional activation and leukemogenesis
Abnormal CBFB-MYH11 chimeric protein acts by preventing normal proteolysis of CBF complex
“Multi-hit” model of AML
Both class 1 and class 2 mutations are needed for leukemogenesis
CBFB-MYH11 fusion is considered class 2 mutation
Development of overt leukemia requires class 1 mutation (e.g., KIT, FLT3, or RAS mutation)
CLINICAL ISSUES
Epidemiology
Incidence
Approximately 6-12% of pediatric AML
Approximately 8% of adult AML
Age
Median: 40-45 years
Gender
Equal male:female ratio
Presentation
Abnormal CBC
Anemia
Neutropenia
Thrombocytopenia
Variable white blood cell count (WBC)
Variable percent blast count
Variable percent monocytic cells
Absolute monocytosis common
Extramedullary involvement common
More common than AML with t(8;21)
Skin, especially scalp; central nervous system
Prognosis
Favorable risk
Complete remission very common
Rates reach ˜ 90% after standard induction therapy
Prognostic genetic factors
1 or more secondary chromosome abnormalities
Trisomy 22 may be associated with lower risk of relapse
KIT mutations
May be found in 22-27% of cases
Prognostic impact not well defined
Some studies show higher risk of relapse
Minimal residual disease (MRD) monitoring
Quantitative RT-PCR studies
Identify molecular remission while on therapy
Molecular remission predictive of durable complete remission
Complete absence of CBFB-MYH11 fusion may not be necessary for long-term remission
Anticipate impending disease relapse prior to morphologic or hematologic relapse
Intervene appropriately
MACROSCOPIC FEATURES
General Features
Extramedullary involvement
Gingival hyperplasia
Subcutaneous nodules
Splenomegaly in 30% of patients
Hepatomegaly
Reported but rare
MICROSCOPIC PATHOLOGY
Predominant Cell/Compartment Type
Blast
Eosinophil precursor
Monocyte
Myeloblast
Key Microscopic Features
Peripheral blood
Anemia
Thrombocytopenia
Neutropenia
Generally leukocytosis dominated by blasts and immature-appearing monocytic cells
WBC may exceed 100 × 109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree