Acute Decompensated Heart Failure
KEY CONCEPTS
![]() Unlike chronic heart failure therapies whose primary role is to improve survival, treatment goals for acute decompensated heart failure (ADHF) are directed toward relief of congestive symptoms, restoration of systemic oxygen transport and tissue perfusion through improved myocardial contractility, and minimization of further cardiac damage and other adverse effects.
Unlike chronic heart failure therapies whose primary role is to improve survival, treatment goals for acute decompensated heart failure (ADHF) are directed toward relief of congestive symptoms, restoration of systemic oxygen transport and tissue perfusion through improved myocardial contractility, and minimization of further cardiac damage and other adverse effects.
![]() Maximizing oral chronic heart failure therapy may assist with optimizing cardiac output and relieving congestion.
Maximizing oral chronic heart failure therapy may assist with optimizing cardiac output and relieving congestion.
![]() Patients presenting to the hospital with ADHF can be categorized into four subsets based upon fluid status (euvolemic or “dry” vs. fluid overloaded or “wet”) and cardiac function (adequate cardiac output or “warm” vs. hypoperfusion or “cold”). Therefore, patients are either warm and dry, warm and wet, cold and dry, or cold and wet.
Patients presenting to the hospital with ADHF can be categorized into four subsets based upon fluid status (euvolemic or “dry” vs. fluid overloaded or “wet”) and cardiac function (adequate cardiac output or “warm” vs. hypoperfusion or “cold”). Therefore, patients are either warm and dry, warm and wet, cold and dry, or cold and wet.
![]() While invasive hemodynamic monitoring using a PA catheter does not alter outcomes in a broad population of ADHF patients, it is indicated in those who are refractory to initial therapy, whose volume status is unclear, or who have clinically significant hypotension (i.e., systolic blood pressure less than 80 mm Hg) or worsening renal function despite therapy.
While invasive hemodynamic monitoring using a PA catheter does not alter outcomes in a broad population of ADHF patients, it is indicated in those who are refractory to initial therapy, whose volume status is unclear, or who have clinically significant hypotension (i.e., systolic blood pressure less than 80 mm Hg) or worsening renal function despite therapy.
![]() Key hemodynamic parameters to monitor with a PA catheter include pulmonary capillary wedge pressure (PCWP; reflecting fluid status or “preload”), cardiac output or cardiac index (CI; reflecting the innate contractility of the heart), and systemic vascular resistance (reflecting vascular tone or “afterload”). While a normal PCWP (6 to 12 mm Hg) is desirable in healthy patients, higher filling pressures (15 to 18 mm Hg) are often necessary in patients with heart failure.
Key hemodynamic parameters to monitor with a PA catheter include pulmonary capillary wedge pressure (PCWP; reflecting fluid status or “preload”), cardiac output or cardiac index (CI; reflecting the innate contractility of the heart), and systemic vascular resistance (reflecting vascular tone or “afterload”). While a normal PCWP (6 to 12 mm Hg) is desirable in healthy patients, higher filling pressures (15 to 18 mm Hg) are often necessary in patients with heart failure.
![]() Three major therapeutic categories exist for the management of ADHF including diuretics, inotropes, and vasodilators. No therapy studied to date has conclusively been shown to decrease mortality and several may potentially worsen outcomes.
Three major therapeutic categories exist for the management of ADHF including diuretics, inotropes, and vasodilators. No therapy studied to date has conclusively been shown to decrease mortality and several may potentially worsen outcomes.
![]() IV loop diuretics are considered first-line therapy for the management of ADHF associated with fluid overload nonresponsive to orally administered diuretics. While a variety of therapeutic options may be considered for refractory fluid overload, a recent clinical trial demonstrated no difference in outcomes between bolus and continuous administration of IV diuretics; however, administering high-dose IV diuretic (2.5-times the previous oral regimen) is associated with greater fluid removal rate. If patients continue to be refractory to, or experience worsening renal function with diuretic therapy, vasodilatory and inotropic therapy may be indicated. Placement of a pulmonary artery (PA) catheter may be helpful in guiding therapy in such patients.
IV loop diuretics are considered first-line therapy for the management of ADHF associated with fluid overload nonresponsive to orally administered diuretics. While a variety of therapeutic options may be considered for refractory fluid overload, a recent clinical trial demonstrated no difference in outcomes between bolus and continuous administration of IV diuretics; however, administering high-dose IV diuretic (2.5-times the previous oral regimen) is associated with greater fluid removal rate. If patients continue to be refractory to, or experience worsening renal function with diuretic therapy, vasodilatory and inotropic therapy may be indicated. Placement of a pulmonary artery (PA) catheter may be helpful in guiding therapy in such patients.
![]() IV inotropes are recommended for symptom relief or end-organ dysfunction in patients with left ventricular dysfunction and low cardiac output. Such therapy may be especially useful in patients with low systolic blood pressure (less than 90 mm Hg) or symptomatic hypotension in the setting of adequate filling pressures. Inotropic therapy may also be considered in patients who do not tolerate or respond to IV vasodilators or in patients with worsening renal function, but should be avoided in patients with reduced left heart filling pressures. Patients receiving these agents should be monitored continuously for arrhythmias.
IV inotropes are recommended for symptom relief or end-organ dysfunction in patients with left ventricular dysfunction and low cardiac output. Such therapy may be especially useful in patients with low systolic blood pressure (less than 90 mm Hg) or symptomatic hypotension in the setting of adequate filling pressures. Inotropic therapy may also be considered in patients who do not tolerate or respond to IV vasodilators or in patients with worsening renal function, but should be avoided in patients with reduced left heart filling pressures. Patients receiving these agents should be monitored continuously for arrhythmias.
![]() Given the potential risks associated with inotropic therapy, vasodilators should be considered prior to their use.
Given the potential risks associated with inotropic therapy, vasodilators should be considered prior to their use.
![]() IV vasodilators may be added to diuretics for rapid symptom resolution, especially in patients with acute pulmonary edema or severe hypertension. Such therapy may also be considered in patients who fail to respond to aggressive treatment with diuretics. Vasodilators should be avoided in patients with symptomatic hypotension and frequent blood pressure monitoring is necessary to ensure their safe use. In addition, these agents should not be used in patients with reduced left heart filling pressures. If patients fail to respond to IV diuretics or vasodilators or experience worsening renal function, IV inotropic therapy should be considered.
IV vasodilators may be added to diuretics for rapid symptom resolution, especially in patients with acute pulmonary edema or severe hypertension. Such therapy may also be considered in patients who fail to respond to aggressive treatment with diuretics. Vasodilators should be avoided in patients with symptomatic hypotension and frequent blood pressure monitoring is necessary to ensure their safe use. In addition, these agents should not be used in patients with reduced left heart filling pressures. If patients fail to respond to IV diuretics or vasodilators or experience worsening renal function, IV inotropic therapy should be considered.
![]() Vasopressin antagonists provide a new therapeutic option for managing hyponatremia in patients with euvolemic or hypervolemic hyponatremia. Tolvaptan is the only vasopressin antagonist indicated for hyponatremia associated with heart failure. Despite being an oral agent, tolvaptan should only be initiated in the hospital setting to allow for monitoring of volume status and serum sodium concentrations, as rapid correction of serum sodium may result in adverse neurological sequalae.
Vasopressin antagonists provide a new therapeutic option for managing hyponatremia in patients with euvolemic or hypervolemic hyponatremia. Tolvaptan is the only vasopressin antagonist indicated for hyponatremia associated with heart failure. Despite being an oral agent, tolvaptan should only be initiated in the hospital setting to allow for monitoring of volume status and serum sodium concentrations, as rapid correction of serum sodium may result in adverse neurological sequalae.
![]() Given extended wait times for patients on the cardiac transplantation list, implantation of a ventricular assist device may be considered for those in whom extended time to identify a suitable donor is anticipated (i.e., “bridge” to transplant) or in whom transplantation is not an option (i.e., “destination” therapy).
Given extended wait times for patients on the cardiac transplantation list, implantation of a ventricular assist device may be considered for those in whom extended time to identify a suitable donor is anticipated (i.e., “bridge” to transplant) or in whom transplantation is not an option (i.e., “destination” therapy).
INTRODUCTION
As discussed in the Systolic and Diastolic Heart Failure chapter (Chap. 4), the number of patients with heart failure (HF) is substantial and continues to increase. Despite survival from HF having improved over time, the 5-year mortality rate remains 50%. In addition, the growing number of patients with this disorder and the progressive nature of the disease have led to substantial increases in hospitalizations for HF. In addition, an estimated 5.1 million U.S. adults over 20 years of age have HF; it is estimated that by 2030, an additional 3 million people will have HF, representing a 25% increase in prevalence from 2013 estimates. At 40 years of age, the lifetime risk for developing HF is one in five in both men and women. Recent data indicate that over 1 million patients are hospitalized for HF annually, resulting in significant morbidity, mortality, and consumption of large quantities of healthcare resources.1,2 Hospital admission for HF is associated with an increased risk of subsequent hospitalization and decreased survival.3 The economic impact of heart failure is considerable with costs driven primarily by inpatient care.2
A number of terms have been used to characterize patients with worsening heart failure requiring hospitalization. Patients with persistent symptoms or refractory heart failure requiring specialized interventions despite optimal oral therapies are classified as Stage D in the American College of Cardiology/American Heart Association (ACC/AHA) classification scheme.4–6 These patients typically fall into the category of New York Heart Association (NYHA) class III or IV. Specialized interventions may include the addition of select medications beyond standard therapy or consideration for various surgical options. The terms acute decompensated heart failure (ADHF) or exacerbation of heart failure refer to those patients with new or worsening signs or symptoms (often as a result of volume overload and/or hypoperfusion) requiring additional medical care such as emergency department visits and hospitalizations. The term acute heart failure may be misleading as it more often refers to the patient with a sudden onset of signs or symptoms in the setting of previously normal cardiac function. This chapter will focus on the management of patients with ADHF. Clinical syndromes within decompensated heart failure include pulmonary or systemic volume overload, low cardiac output, and acute pulmonary edema. Clinicians should recognize that patients may present with impaired or preserved left ventricular systolic function and a variety of etiologies may be responsible for the primary disease process. The clinical course of heart failure manifests as periods of relative stability with an increasing frequency in episodes of decompensation as the underlying disease progresses.7
Despite the considerable morbidity and mortality associated with ADHF, the first randomized placebo-controlled trials in this patient population were published in 2002.8,9 In addition, it was not until 2005 that guidelines specifically addressing ADHF were promulgated. The Heart Failure Society of America (HFSA) and the ACC/AHA guidelines address the management of ADHF; however, the HFSA guidelines are more detailed and will be the focus of the remainder of this chapter.4,6,10
PATHOPHYSIOLOGY AND CLINICAL PRESENTATION
Patients requiring intensive therapy for ADHF may have a variety of underlying etiologies and clinical presentations.10 Patients with worsening chronic heart failure comprise approximately 70% of heart failure hospitalizations. These patients can become refractory to oral therapies and decompensate following even a relatively mild insult (e.g., dietary indiscretion, nonsteroidal antiinflammatory drug use), medication noncompliance, or a concurrent noncardiac illness (e.g., infection). New or worsening cardiac processes, such as myocardial infarction, atrial fibrillation, hypertensive urgency/emergency, myocarditis, or acute valvular insufficiency may also result in decompensation in an otherwise stable patient. Secondly, de novo heart failure may occur when left ventricular dysfunction results from a large myocardial infarction or sudden elevation in blood pressure; such cases represent approximately 25% of admissions. A third group of patients with severe left ventricular systolic dysfunction associated with progressive worsening of cardiac output and refractoriness to therapy represents about 5% of heart failure admissions.11 Additional insight into the clinical characteristics of patients presenting unexpectedly with ADHF indicates that a high percentage have hypertension and preserved left ventricular systolic function.12
Several studies have provided a better understanding of the prognostic factors associated with ADHF. Data from the Acute Decompensated Heart Failure National Registry (ADHERE), an archive of hospitalized patients with a primary diagnosis of ADHF, found blood urea nitrogen (BUN) greater than or equal to 43 mg/dL (15.4 mmol/L) to be the best individual predictor of in-hospital mortality, followed by systolic blood pressure less than 115 mm Hg and serum creatinine greater than or equal to 2.75 mg/dL (243 μmol/L). Using these three parameters, patients may be identified as low, intermediate, high, and very high risk with in-hospital mortalities of 2%, 6%, 13%, and 20%, respectively.13 Hyponatremia, elevations in troponin I, ischemic etiology, and poor functional capacity are also negative prognostic factors.11 Importantly, patients who survive a hospitalization for ADHF remain at high risk for rehospitalization or death. Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Registry, another archive of ADHF patients, indicated overall mortality and rehospitalization rates of 8.6% and 29.6%, respectively, at 60 to 90 days postdischarge.14 In patients who survive a hospitalization for ADHF, low blood pressure and poor renal function are also negative prognostic markers for subsequent readmission or death.14 However, use of standard heart failure therapies at discharge as well as coronary angiography or implantable cardioverter-defibrillator placement during hospitalization are associated with improved prognosis,14 suggesting that optimal management of these patients during their hospitalization can yield beneficial effects on subsequent prognosis.
GENERAL APPROACH TO TREATMENT
![]() The overall goals of therapy in ADHF are to provide symptomatic relief while optimizing volume status and low cardiac output so that a patient can be discharged in a stable compensated state on oral drug therapy. Although diuretic, vasodilator, and positive inotropic therapy can be very effective at achieving these goals, their efficacy must be balanced against the potential for serious toxicity. Thus, another important goal is to minimize the risks associated with these therapies including renal dysfunction, myocardial injury, electrolyte depletion, hypotension, and arrhythmias.
The overall goals of therapy in ADHF are to provide symptomatic relief while optimizing volume status and low cardiac output so that a patient can be discharged in a stable compensated state on oral drug therapy. Although diuretic, vasodilator, and positive inotropic therapy can be very effective at achieving these goals, their efficacy must be balanced against the potential for serious toxicity. Thus, another important goal is to minimize the risks associated with these therapies including renal dysfunction, myocardial injury, electrolyte depletion, hypotension, and arrhythmias.
In addition, all patients should be evaluated for potential etiologies contributing to decompensation as well as other precipitating factors, including atrial fibrillation and other arrhythmias, worsening hypertension, myocardial ischemia or infarction, anemia, hypothyroidism or hyperthyroidism, or other causes. Medications (including noncardiac medications) that may worsen cardiac function should also be considered as precipitating or contributing factors. Patients who may benefit from coronary revascularization should also be identified. Prior to discharge, optimization of chronic oral therapy and patient education are critical to preventing future hospitalizations. When available and appropriate, patients should be referred to a heart failure disease management program.10
A careful history and physical examination are key components in the diagnosis of ADHF. The history should focus on the potential etiologies of heart failure, the presence of any precipitating factors, onset, duration, and severity of symptoms, and a careful medication history. Current guidelines recommend making the diagnosis of ADHF based primarily on signs and symptoms.10 The more common presentation of ADHF is severe fluid overload, and orthopnea is the symptom that best correlates with elevated pulmonary pressures.15 Important elements of the physical examination include assessment of vital signs and weight, cardiac auscultation for heart sounds and murmurs, pulmonary auscultation for rales, and evaluation for the presence of peripheral edema. Jugular venous pressure is the most reliable indicator of volume status and should be carefully evaluated on admission and closely followed during hospitalization as an indicator of the efficacy of diuretic therapy.15 An S3 gallop, suggestive of increased volume in the left ventricle, has high diagnostic specificity for heart failure decompensation.16 Other physical findings such as pulmonary crackles and lower extremity edema have low specificity and sensitivity for the diagnosis of ADHF. The development of a bedside assay for plasma B-type natriuretic peptide (BNP) has focused considerable attention on the use of natriuretic peptide levels as an aid in the diagnosis of suspected heart failure.17 Plasma BNP and N-terminal pro-BNP concentrations are positively correlated with the degree of left ventricular dysfunction and heart failure, and these assays are now frequently used in acute care settings to assist in the differential diagnosis of dyspnea (heart failure vs. asthma, chronic obstructive pulmonary disease, or infection). A low BNP concentration, often defined as less than 100 pg/mL (ng/L; 29 pmol/L), has a 96% predictive value for excluding heart failure as the underlying etiology of a patient presenting with dyspnea. In addition, an elevated BNP concentration prior to discharge is associated with an increased risk of poor long-term outcomes. However, some limitations exist with the use of BNP. For example, any disease process that increases right heart pressures will elevate BNP, including pulmonary emboli, chronic obstructive lung disease, and primary pulmonary hypertension. In addition, BNP concentrations may be mildly increased with advanced age, female gender, and renal dysfunction, and lower in the setting of obesity.15 Although ongoing research will better characterize the role of BNP in the diagnosis and treatment of heart failure, current guidelines recommend obtaining a BNP concentration in conjunction with assessing signs and symptoms when the diagnosis of ADHF is uncertain.10
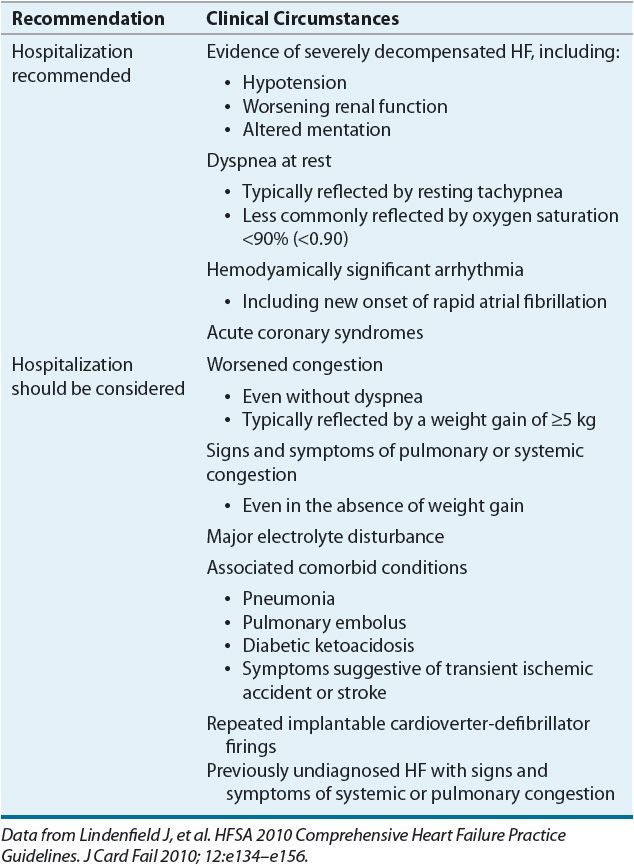
Hospitalization for ADHF is recommended or should be considered depending on patient presentation (Table 5-1). Most patients do not require admission to an intensive care unit and may be admitted to a monitored unit or general medical floor. If a patient experiences hemodynamic instability necessitating frequent monitoring of vital signs, invasive hemodynamic monitoring, or rapid titration of IV medications (with concurrent monitoring), admission to an intensive care unit may be required to assure safe and effective outcomes.
TABLE 5-1 Recommendations for Hospitalizing Patients Presenting with ADHF
![]() The first step in the management of ADHF is to ascertain that optimal treatment with oral medications has been achieved.10 If fluid retention is evident on physical examination, aggressive diuresis should be pursued. Although increasing the dose of oral diuretic therapy may be effective in some cases, the use of IV diuretics is often necessary. Every effort should be made to optimize standard heart failure therapy including an ACE inhibitor and β-blocker. β-blocker therapy should generally be continued during a hospitalization unless recent dose initiation or uptitration was responsible for decompensation. In such cases, β-blocker therapy may be temporarily held or dose-reduced. Otherwise, discontinuation of β-blockers is discouraged as it has been associated with worse outcomes in patients in ADHF.18,19 Appropriateness of initiating β-blockers prior to discharge will be discussed later in this chapter.
The first step in the management of ADHF is to ascertain that optimal treatment with oral medications has been achieved.10 If fluid retention is evident on physical examination, aggressive diuresis should be pursued. Although increasing the dose of oral diuretic therapy may be effective in some cases, the use of IV diuretics is often necessary. Every effort should be made to optimize standard heart failure therapy including an ACE inhibitor and β-blocker. β-blocker therapy should generally be continued during a hospitalization unless recent dose initiation or uptitration was responsible for decompensation. In such cases, β-blocker therapy may be temporarily held or dose-reduced. Otherwise, discontinuation of β-blockers is discouraged as it has been associated with worse outcomes in patients in ADHF.18,19 Appropriateness of initiating β-blockers prior to discharge will be discussed later in this chapter.
Discontinuation of ACE inhibitor or β-blocker therapy may be necessary in the setting of cardiogenic shock or symptomatic hypotension. Certain therapies may also need to be temporarily held in the setting of renal dysfunction, especially in the setting of oliguria or hyperkalemia (e.g., ACE inhibitor, angiotensin receptor blocker, aldosterone antagonist) or elevated serum digoxin concentrations. Therapies that can cause worsening renal function (e.g., ACE inhibitor) should only be initiatied or uptitrated cautiously during aggressive fluid removal with IV diuretic therapy. In addition, serum potassium concentrations should be monitored closely as IV diuretic therapy is transitioned to oral diuretic therapy, especially if an aldosterone antagonist was initiated during the hospital stay; this ensures such therapy can be tolerated on the oral diuretic dose prescribed at discharge. Most patients may continue to receive digoxin at low doses targeting a trough serum concentration of 0.5 to 1 ng/mL (0.6 to 1.3 nmol/L).10 Discontinuation of digoxin is generally discouraged as an association between withdrawal of therapy and worsening HF has been well-documented.20,21 Digoxin should only be discontinued if serum concentrations cannot be maintained in a desirable range.
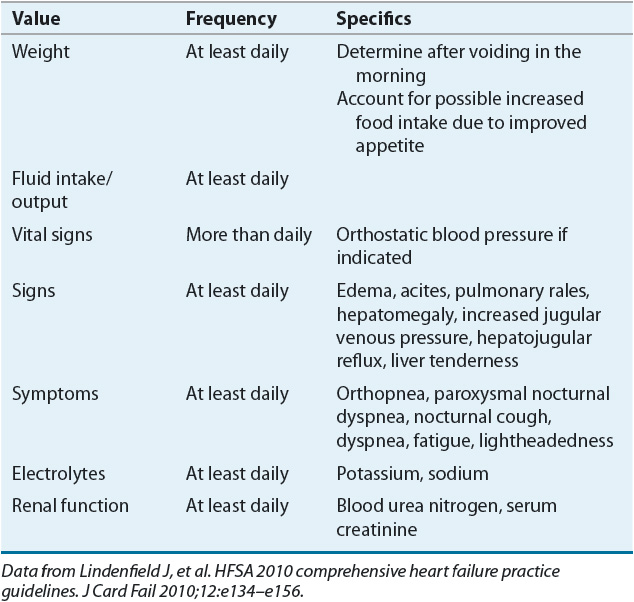
Two general approaches exist for maximizing therapy in the ADHF patient. One is to use simple clinical parameters (e.g., signs and symptoms, blood pressure, renal function) and the other is to combine these parameters with invasive hemodynamic monitoring. In all ADHF patients, close monitoring is essential for ensuring an optimal response to therapy while avoiding adverse effects (summarized in Table 5-2). Daily monitoring to assess the efficacy of drug therapy should include weight, strict fluid intake and output, and heart failure signs and symptoms. Foley catheter placement is not recommended unless close monitoring of urine output is not otherwise possible. As safety endpoints, monitoring for electrolyte depletion, symptomatic hypotension, and renal dysfunction should be assessed frequently. While many of the above parameters may be monitored daily, some will need to be monitored more frequently as dictated by patient clinical status. Vital signs should be assessed multiple times throughout the day at a frequency that is appropriate for the patient’s degree of stability. Orthostatic blood pressure should be assessed at least once daily.10
TABLE 5-2 Monitoring Recommendations for Patients Hospitalized with ADHF
PRINCIPLES OF THERAPY BASED ON CLINICAL PRESENTATION
![]() Appropriate medical management of ADHF is guided by determining whether the patient has signs and symptoms of fluid overload (“wet” heart failure) or low cardiac output (“cold” heart failure).15,22 As previously discussed, most patients present with fluid overload (or the “wet” profile). Symptoms consistent with pulmonary congestion include orthopnea and dyspnea with minimal exertion and those of systemic congestion include GI discomfort, ascites, and peripheral edema. Patients with no or minimal fluid overload (or the “dry” category of ADHF) may have symptoms that are more difficult to distinguish. Such patients may present with a syndrome of low cardiac output (“cold” heart failure) which is characterized principally by extreme fatigue as well as poor appetite, nausea, and early satiety, although GI symptoms may be a sign of congestion rather than low cardiac output to the GI tract. Patients with “cold” heart failure frequently exhibit worsening renal function and a decline in serum sodium concentrations, which, as previously discussed, are both associated with poor prognosis.
Appropriate medical management of ADHF is guided by determining whether the patient has signs and symptoms of fluid overload (“wet” heart failure) or low cardiac output (“cold” heart failure).15,22 As previously discussed, most patients present with fluid overload (or the “wet” profile). Symptoms consistent with pulmonary congestion include orthopnea and dyspnea with minimal exertion and those of systemic congestion include GI discomfort, ascites, and peripheral edema. Patients with no or minimal fluid overload (or the “dry” category of ADHF) may have symptoms that are more difficult to distinguish. Such patients may present with a syndrome of low cardiac output (“cold” heart failure) which is characterized principally by extreme fatigue as well as poor appetite, nausea, and early satiety, although GI symptoms may be a sign of congestion rather than low cardiac output to the GI tract. Patients with “cold” heart failure frequently exhibit worsening renal function and a decline in serum sodium concentrations, which, as previously discussed, are both associated with poor prognosis.
Many patients will present with signs and symptoms of both wet and cold types of ADHF. In these patients, low-output symptoms may not be obvious until congestion is optimally treated.
PRINCIPLES OF THERAPY BASED ON HEMODYNAMIC SUBSETS
Patients with ADHF may have critically reduced cardiac output, usually with low arterial blood pressure and systemic hypoperfusion resulting in organ system dysfunction (i.e., cardiogenic shock). They may also have pulmonary edema with hypoxemia, respiratory acidosis, and markedly increased work of breathing. With cardiopulmonary support, response to interventions should be assessed promptly to allow for timely adjustments in treatment. Since cardiopulmonary support must be instituted and adjusted rapidly, immediate assessment of each intervention limits risks and allows for more prompt adjustments in therapy. Continuous monitoring of ECG, continuous pulse oximetry, urine flow, and automated blood pressure recordings are standards of care for critically ill patients with cardiopulmonary decompensation. Peripheral or femoral arterial catheters may be utilized for continuous and accurate assessment of arterial pressure.
Hemodynamic Monitoring
![]() The role of invasive hemodynamic monitoring in patients with ADHF remains controversial. In a clinical trial assessing the routine use of this strategy, PA catheter placement had no impact on survival after hospital discharge, although patients with a clear indication for its use were excluded.23 Based on these results, the routine use of invasive monitoring is not currently recommended. However, invasive monitoring often provides essential information for adjusting drug therapy in patients with a confusing or complicated clinical picture and during dose titration of rapidly acting medications. Therefore, invasive monitoring should be considered in patients who are refractory to initial therapy, those in whom volume status is unclear, or those who have clinically significant hypotension (e.g., systolic blood pressure less than 80 mm Hg) or worsening renal function despite therapy. In addition, documentation of an adequate hemodynamic response to inotropic therapy is often necessary prior to committing patients to chronic outpatient inotropic therapy.10 Finally, assessment of hemodynamic parameters is required to document adequate reversal of pulmonary hypertension prior to cardiac transplantation.15
The role of invasive hemodynamic monitoring in patients with ADHF remains controversial. In a clinical trial assessing the routine use of this strategy, PA catheter placement had no impact on survival after hospital discharge, although patients with a clear indication for its use were excluded.23 Based on these results, the routine use of invasive monitoring is not currently recommended. However, invasive monitoring often provides essential information for adjusting drug therapy in patients with a confusing or complicated clinical picture and during dose titration of rapidly acting medications. Therefore, invasive monitoring should be considered in patients who are refractory to initial therapy, those in whom volume status is unclear, or those who have clinically significant hypotension (e.g., systolic blood pressure less than 80 mm Hg) or worsening renal function despite therapy. In addition, documentation of an adequate hemodynamic response to inotropic therapy is often necessary prior to committing patients to chronic outpatient inotropic therapy.10 Finally, assessment of hemodynamic parameters is required to document adequate reversal of pulmonary hypertension prior to cardiac transplantation.15
![]() Invasive hemodynamic monitoring is usually performed with a flow-directed PA catheter (also known as Swan-Ganz catheter) placed percutaneously through a central vein and advanced through the right side of the heart and into the PA. Inflation of a balloon proximal to the end port allows the catheter to “wedge,” yielding the PA occlusion pressure, which estimates the pulmonary venous (left atrial) pressure and, in the absence of intracardiac shunt, mitral valve disease or pulmonary disease, the left ventricular end-diastolic pressure. While the term pulmonary artery occlusion pressure has previously been used to describe the filling pressure of the heart, the term pulmonary capillary wedge pressure (PCWP) is used more commonly in clinical practice and will be used henceforth. The PCWP is a useful marker of volume status; an elevated PCWP indicates fluid overload while a reduced PCWP indicates dehydration or inadequate filling pressures. Cardiac output may also be measured and represents the volume of blood being pumped by the heart (in particular by the left ventricle) in a minute. The cardiac index (CI) normalizes the cardiac output for body surface area, thus allowing measurements of heart performance to be made without being influenced by body size. Systemic vascular resistance is calculated using cardiac output and thus is inversely related to cardiac output. Mixed venous oxygen saturation represents the end result of both oxygen delivery and consumption at the tissue level.
Invasive hemodynamic monitoring is usually performed with a flow-directed PA catheter (also known as Swan-Ganz catheter) placed percutaneously through a central vein and advanced through the right side of the heart and into the PA. Inflation of a balloon proximal to the end port allows the catheter to “wedge,” yielding the PA occlusion pressure, which estimates the pulmonary venous (left atrial) pressure and, in the absence of intracardiac shunt, mitral valve disease or pulmonary disease, the left ventricular end-diastolic pressure. While the term pulmonary artery occlusion pressure has previously been used to describe the filling pressure of the heart, the term pulmonary capillary wedge pressure (PCWP) is used more commonly in clinical practice and will be used henceforth. The PCWP is a useful marker of volume status; an elevated PCWP indicates fluid overload while a reduced PCWP indicates dehydration or inadequate filling pressures. Cardiac output may also be measured and represents the volume of blood being pumped by the heart (in particular by the left ventricle) in a minute. The cardiac index (CI) normalizes the cardiac output for body surface area, thus allowing measurements of heart performance to be made without being influenced by body size. Systemic vascular resistance is calculated using cardiac output and thus is inversely related to cardiac output. Mixed venous oxygen saturation represents the end result of both oxygen delivery and consumption at the tissue level.
Systemic vascular resistance (also referred to as total peripheral resistance) reflects the “afterload” or resistance applied to the left ventricle, which represents the force impeding ejection of blood from the left ventricle. Vasoconstriction (i.e., decrease in blood vessel diameter) increases vascular resistance, whereas vasodilation decreases it. An elevated systemic vascular resistance is common in untreated heart failure and is generally responsive to oral or IV arterial vasodilators. Conversely, a reduction in resistance is consistent with vasodilatory shock (e.g., sepsis) and is routinely managed with IV vasopressor therapy. In lieu of inotropic therapy, arterial vasodilators are the therapy of choice to reduce elevated systemic vascular resistance in ADHF.
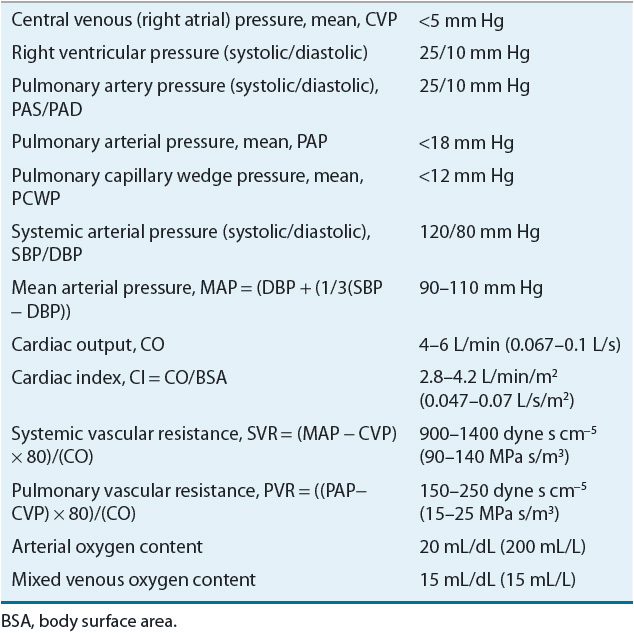
Resistance present in the vasculature of the lungs is known as the pulmonary vascular resistance, and represents the impedance of blood flow from the right ventricle to the pulmonary circulation. Pulmonary hypertension and pulmonary edema are two common causes of elevated pulmonary vascular resistance. Patients with pulmonary hypertension must have proven reversibility in elevated pulmonary pressures prior to being listed for heart transplantation. If these pressures are irreversible, isolated right ventricular failure is likely to occur immediately following heart transplantation. Just as systemic resistance is calculated using mean arterial pressure, pulmonary vascular resistance is calculated using mean PA pressure, which incorporates the PA systolic and diastolic pressures. The PA diastolic pressure may be useful if the PA catheter fails to wedge (making it impossible to obtain PCWP). If PCWP and PA diastolic pressure correlate prior to the failure to wedge, then PA diastolic pressure can be followed as a surrogate marker of fluid status. Normal values for hemodynamic parameters are listed in Table 5-3.
TABLE 5-3 Hemodynamic Monitoring: Normal Values
In addition to understanding the initial clinical presentation, invasive hemodynamic monitoring assists with the classification of patients into specific subsets and subsequent selection of appropriate medical therapy. These hemodynamic subsets were first proposed for patients with left ventricular dysfunction following an acute myocardial infarction but also are applicable to patients with acute or severe heart failure from other causes (Fig. 5-1).24 This classification scheme has four subsets and is based on a CI above or below 2.2 L/min/m2 (0.037 L/s/m2) and a PCWP above or below 18 mm Hg. A treatment algorithm, based on hemodynamic subsets, is provided in Figure 5-2. In addition to utilizing the above profiles or categories to stratify patients with ADHF, these four hemodynamic profiles are also predictive of clinical outcomes. Patients in the wet-warm and wet-cold profiles have a twofold and 2.5-fold greater risk of death at 1 year, respectively, compared to dry-warm patients.15 Patients may experience compromised CI in the setting of significant fluid overload, which may improve as diuresis occurs. The underlying mechanism for how increasing fluid overload further worsens cardiac function is not clearly understood and is depicted in Figure 5-3.
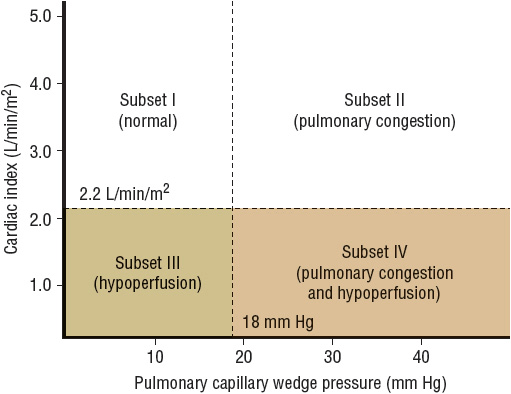
FIGURE 5-1 Hemodynamic subsets of heart failure based on cardiac index and pulmonary artery occlusion pressure. Cardiac index is expressed in conventional units of mL/min/m2, and can be converted to SI units of mL/s/m2 by multiplying by 0.0167. (Forrester JS, Diamond G, Chatterjee K, et al. Medical Therapy of Acute Myocardial Infarction by Application of Hemodynamic Subsets. N Engl J Med 1976;295:1356–1362. Copyright © 1976 Massachusetts Medical Society. All rights reserved.)
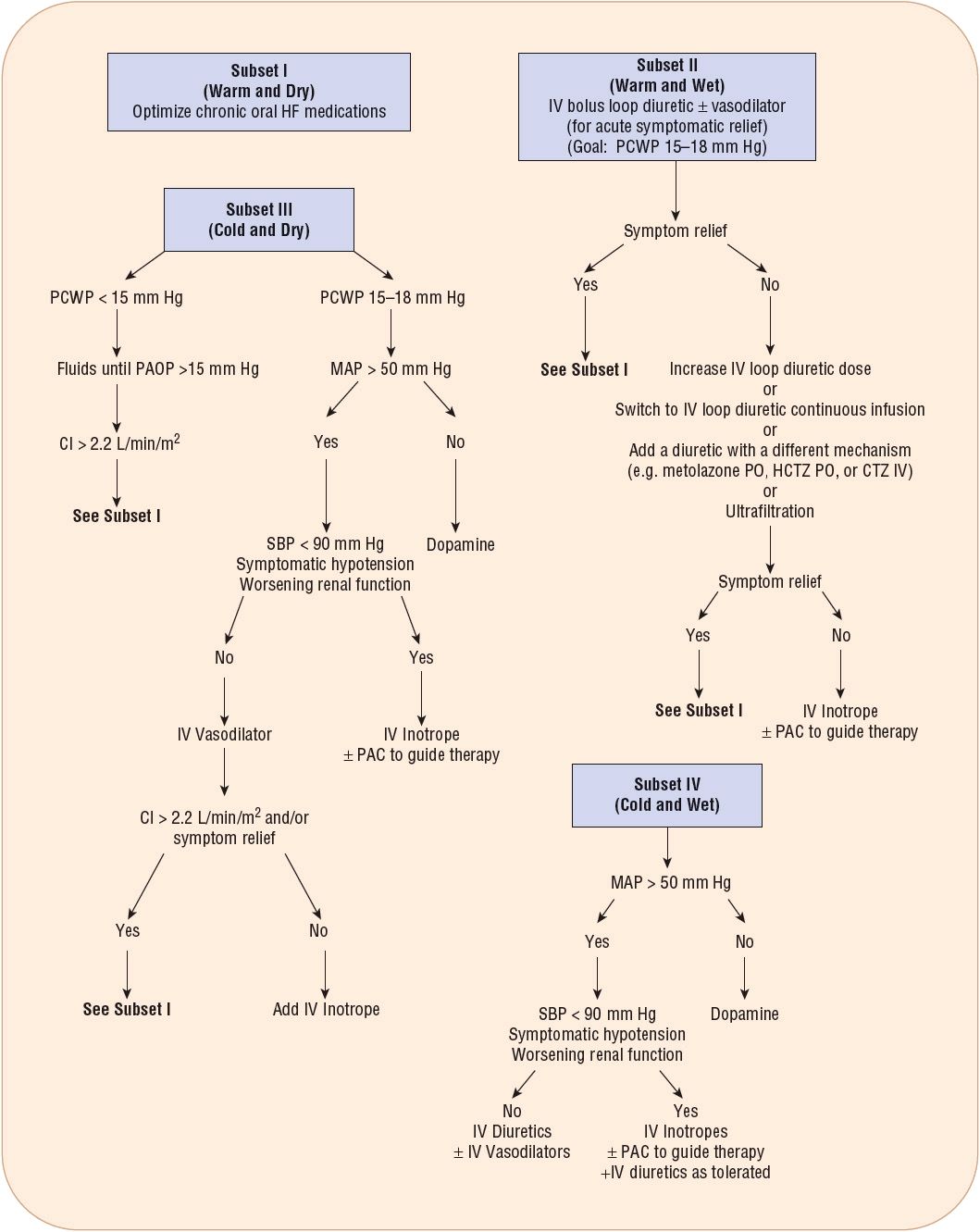
FIGURE 5-2 General treatment algorithm for ADHF based on clinical presentation. IV vasodilators that may be used include nitroglycerin, nesiritide, or nitroprusside. Metolazone or spironolactone may be added if the patient fails to respond to loop diuretics and a second diuretic is required. IV inotropes that may be used include dobutamine or milrinone. (CI, cardiac index; CTZ, chlorothiazide; HCTZ, hydrochlorothiazide; HF, heart failure; MAP, mean arterial pressure; PAC, pulmonary artery catheter; PCWP, pulmonary capillary wedge pressure; PO, by mouth; SBP, systolic blood pressure.) Adapted from HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Fail 2010;16:e1–e2.
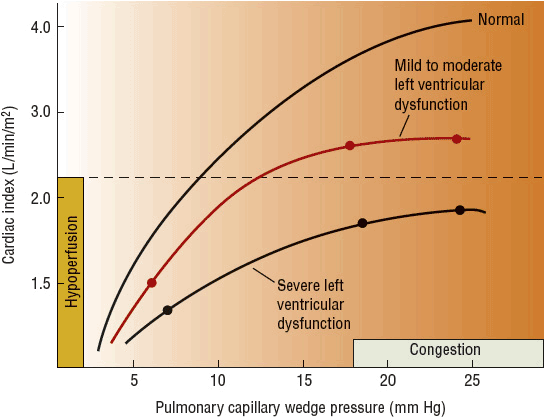
FIGURE 5-3 Relationship between cardiac output (shown as cardiac index which is CO/BSA) and preload (shown as pulmonary capillary wedge pressure). Cardiac index is expressed in conventional units of mL/min/m2, and can be converted to SI units of mL/s/m2 by multiplying by 0.0167.
Subset I
Patients in hemodynamic subset I have a CI and PCWP within generally acceptable ranges and have the lowest mortality of any subset. These patients do not need immediate specific interventions other than maximizing oral therapy and monitoring. Patients with significant left ventricular dysfunction may still present in subset I because normal compensatory mechanisms and/or appropriate drug therapy may at least partially correct an otherwise abnormal hemodynamic profile.
Subset II
As shown in Figure 5-1, patients in subset II have an adequate CI but a PCWP greater than 18 mm Hg. These patients are likely to have congestion (i.e., “wet” heart failure) secondary to increased hydrostatic pressure in the pulmonary and systemic circulation but no evidence of peripheral hypoperfusion. The primary goal of therapy in these patients is to reduce congestion by lowering PCWP without reductions in cardiac output, increases in heart rate, or further neurohormonal activation. Therefore, it is critically important that PCWP not be decreased excessively. Although the normal range of PCWP is 5 to 12 mm Hg for individuals without cardiac dysfunction, higher pressures (i.e., 15 to 18 mm Hg) are often necessary in patients with heart failure in order to optimize CI. Generally, the PCWP can be lowered to 15 to 18 mm Hg with relatively little decrease in cardiac output because the Frank-Starling curve is flatter at higher PCWP values in patients with heart failure (depicted in Figure 5-3). Cardiac output also declines when the PCWP desired in a heart failure patient (i.e., PCWP 15 to 18 mm Hg) is exceeded. This phenomenon may explain why patients may experience enhanced diuresis and improved renal function when the PCWP range of 15 to 18 mm Hg is achieved in a heart failure patient with fluid overload. IV administration of agents that reduce preload (i.e., loop diuretics, nitroglycerin, or nesiritide) are the most appropriate acute therapy to achieve the therapeutic goal for patients in subset II. Despite a very rapid onset with diuretic therapy, the time required for significant improvement in oxygenation with IV loop diuretics may take several hours in select patients. Thus, IV venodilators such as nitroglycerin and nesiritide may be utilized for rapid venodilation, which can acutely aid in improving hypoxia (Fig. 5-2).9
Current guidelines recommend loop diuretics as first-line therapy for patients with fluid overload and that such agents typically be administered IV.10 The rate of diuresis should achieve a desirable volume status without causing a rapid reduction in intravascular volume, which may result in symptomatic hypotension or renal dysfunction. Electrolyte depletion should be monitored closely especially when high doses or combination diuretic therapy is utilized. In addition to sodium restriction (less than 2 g daily), supplemental oxygen should be administered as needed for hypoxemia. In patients with moderate hyponatremia (less than 130 mEq/L [130 mmol/L]), fluid restriction (less than 2 L daily) should be considered, and in patients with worsening or severe hyponatremia (less than 125 mEq/L [125 mmol/L]), stricter fluid restriction may be necessary.10 The arginine vasopressin (AVP) antagonists are a new class of agents indicated for the management of euvolemic or hypervolemic hyponatremia in a variety of disease states including heart failure.25 Currently available vasopressin antagonists are discussed in greater detail later in this chapter.
IV vasodilators may be added to diuretics for rapid symptom resolution, especially in patients with acute pulmonary edema or severe hypertension. Such therapy may also be considered in patients who fail to respond to aggressive treatment with diuretics. Vasodilators should be avoided in patients with symptomatic hypotension, and frequent blood pressure monitoring is necessary to ensure their safe use. In addition, these agents should not be used in patients with reduced left heart filling pressures. If symptomatic hypotension occurs with vasodilator therapy, the dose should be reduced or the agent discontinued. If patients fail to respond to the above therapies or experience worsening renal function, IV inotropic therapy should be considered.10
Subset III
Patients in hemodynamic subset III have a CI of less than 2.2 L/min/m2 but without an abnormal elevation in PCWP (Fig. 5-1). These patients usually present without evidence of congestion, but low cardiac output results in signs and symptoms of peripheral hypoperfusion (i.e., decreased urine output, weakness, peripheral vasoconstriction, weak pulses). The mortality rate of subset III patients is reportedly higher than that of patients without hypoperfusion.24 Although the treatment goal is to alleviate signs and symptoms of hypoperfusion by increasing CI and perfusion to essential organs, therapy may differ based on initial presentation. If the PCWP is significantly below 15 mm Hg, IV fluids should be administered to provide a more optimal left ventricular filling pressure (i.e., 15 to 18 mm Hg), consequently improving CI (Fig. 5-2). Alternatively, diuretic therapy should be held and fluid restriction liberalized. When only mild left ventricular dysfunction is present, IV fluid administration may be all that is necessary to achieve a CI above 2.2 L/min/m2 (0.037 L/s/m2). However, many patients will have significant left ventricular dysfunction and depressed Frank-Starling relationship despite adequate filling pressures. In such patients, IV administration of positive inotropic agents (e.g., dobutamine, milrinone) and/or arterial vasodilators (e.g., nitroprusside or nesiritide) may be necessary to achieve an adequate CI (Fig. 5-2). Some positive inotropic medications also have arterial vasodilating activity (see specific drug classes that follow).
Current guidelines recommend IV inotropes for symptom relief or end-organ dysfunction in patients with left ventricular dysfunction and low cardiac output syndrome.10 Such therapy may be especially useful in patients with low systolic blood pressure (less than 90 mm Hg) or symptomatic hypotension in the setting of adequate filling pressures. As previously discussed (see Subset II), inotropic therapy may be considered in patients who do not tolerate or respond to IV vasodilators or in patients with worsening renal function. As with vasodilators, inotrope administration requires frequent blood pressure monitoring as well as continuous monitoring for arrhythmias. If arrhythmias occur, dose reduction or discontinuation of inotropic therapy should be performed. As with vasodilators, these agents should be avoided in patients with low left heart filling pressures. Given the potential risks associated with inotropic therapy, vasodilators should be considered prior to using inotropes.10
In general, inotropic therapy should not be used routinely in the ADHF population. Instead, they should be reserved for the purpose of increasing cardiac output in the specific patients described above. These agents may also be used to “bridge” patients to heart transplantation or a left ventricular assist device, or as palliative therapy to improve functional status and quality of life in patients who are not candidates for these definitive therapies.10
Subset IV
Patients with a CI of less than 2.2 L/min/m2 (0.037 L/s/m2) and a PCWP higher than 18 mm Hg are in hemodynamic subset IV (Fig. 5-1). This subset is characterized by the worst prognosis of the four and represents the most common hemodynamic profile for patients with end-stage heart failure. Given the severity of systolic failure, such patients cannot maintain an adequate CI despite elevated left ventricular filling pressure and increased myocardial fiber stretch. Patients in subset IV will present with signs and symptoms of both congestion and hypoperfusion. Treatment goals for these patients include the alleviation of signs and symptoms by increasing CI above 2.2 L/min/m2 (0.037 L/s/m2) and reducing PCWP to 15 to 18 mm Hg while maintaining an adequate mean arterial pressure. As a consequence, therapy will involve a combination of agents used in Subsets II and III in order to achieve these goals (i.e., combination of diuretic plus positive inotrope). These targets may be difficult to achieve and often necessitate careful monitoring and individualization of drug therapy. Nitroprusside may be a particularly useful agent in this setting because of its mixed arterial-venous vasodilating effects. However, in the presence of significant hypotension and low mean arterial pressures, inotropic agents with vasopressor activity (e.g., dopamine) may be required initially to achieve an adequate end-organ perfusion pressure and can then be combined, if necessary, with diuretics and/or therapies to obtain the desired hemodynamic effects and clinical response (Fig. 5-2).
PHARMACOLOGIC THERAPY OF ACUTE DECOMPENSATED HEART FAILURE
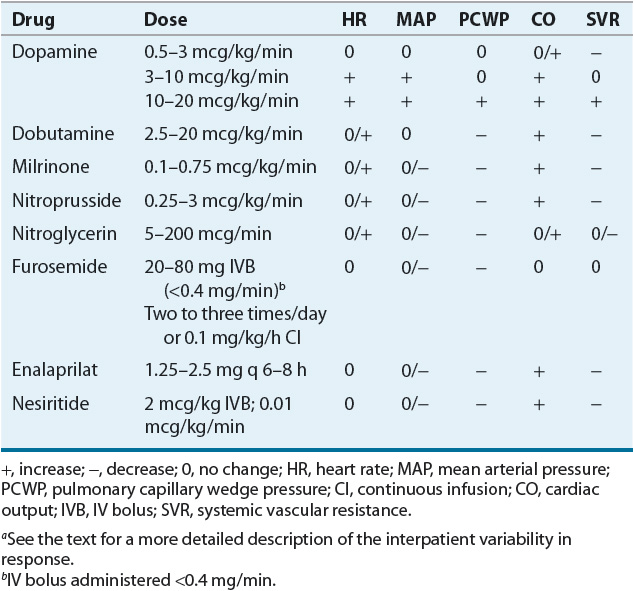
![]() Unfortunately, drug therapies utilized in the management of ADHF have not improved substantially in the last decade due primarily to a dearth of clinical trial data in this population. The agents used to treat patients with ADHF rarely, if ever, produce a single cardiovascular action. Even when intended for a specific purpose (e.g., positive inotropic effects), other cardiovascular effects (tachycardia, vasodilation, or vasoconstriction) may either add to the therapeutic effect of the drug, or cause adverse effects that negate or even outweigh its intended therapeutic benefit. How an individual patient will respond to an intervention is often difficult to anticipate. For this reason, hemodynamic monitoring is often useful, and many drugs are considered first-line therapy due in part to their short half-lives and ease of titration. The description of expected drug actions outlined below should be viewed as a general guide to the clinician and patients should be continually reassessed for desired therapeutic outcomes. Table 5-4 contains a summary of the expected hemodynamic effects of the various drugs discussed below.
Unfortunately, drug therapies utilized in the management of ADHF have not improved substantially in the last decade due primarily to a dearth of clinical trial data in this population. The agents used to treat patients with ADHF rarely, if ever, produce a single cardiovascular action. Even when intended for a specific purpose (e.g., positive inotropic effects), other cardiovascular effects (tachycardia, vasodilation, or vasoconstriction) may either add to the therapeutic effect of the drug, or cause adverse effects that negate or even outweigh its intended therapeutic benefit. How an individual patient will respond to an intervention is often difficult to anticipate. For this reason, hemodynamic monitoring is often useful, and many drugs are considered first-line therapy due in part to their short half-lives and ease of titration. The description of expected drug actions outlined below should be viewed as a general guide to the clinician and patients should be continually reassessed for desired therapeutic outcomes. Table 5-4 contains a summary of the expected hemodynamic effects of the various drugs discussed below.
TABLE 5-4 Usual Hemodynamic Effects of IV Agents Commonly Used for Treatment of Advanced or Decompensated Heart Failurea