29
ACTH, Adrenal Steroids, and Pharmacology of the Adrenal Cortex
This chapter will be most useful after having a basic understanding of the material in Chapter 42, ACTH, Adrenal Steroids, and the Pharmacology of the Adrenal Cortex in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition contains:
• A detailed discussion of the chemistry, actions, and regulation of the secretion of adrenocorticotropic hormone (ACTH)
• A description of the physiological functions of the adrenocorticosteroids
• A detailed description of the pharmacological effects of the adrenocorticosteroids
• A depiction of the chemical structures of the adrenocorticosteroids and their synthetic derivatives
• A discussion of inhibitors of the biosynthesis and action of the adrenocorticosteroids
LEARNING OBJECTIVES
 Understand the mechanisms of action and the physiological effects of the adrenocorticosteroids.
Understand the mechanisms of action and the physiological effects of the adrenocorticosteroids.
 Describe the differences in the anti-inflammatory and sodium-retaining potencies of the glucocorticoids.
Describe the differences in the anti-inflammatory and sodium-retaining potencies of the glucocorticoids.
 Describe the side effects of prolonged therapy with glucocorticoids.
Describe the side effects of prolonged therapy with glucocorticoids.
 Understand the effect of abrupt cessation of glucocorticoid therapy.
Understand the effect of abrupt cessation of glucocorticoid therapy.
DRUGS INCLUDED IN THIS CHAPTER
Aminoglutethimide (CYTADREN)
Corticorelin (ACTHREL)
Cosyntropin (COSTROSYN, SYNACTHEN)
Etomidate (AMIDATE)
Human corticotropin-releasing hormone (CRH)
Ketoconazole (NIZORAL; see also Chapter 43)
Metyrapone (METOPIRONE)
Mifepristone (MIFUPREX, RU-486; see also Chapter 28)
Mitotane (LYSODREN)
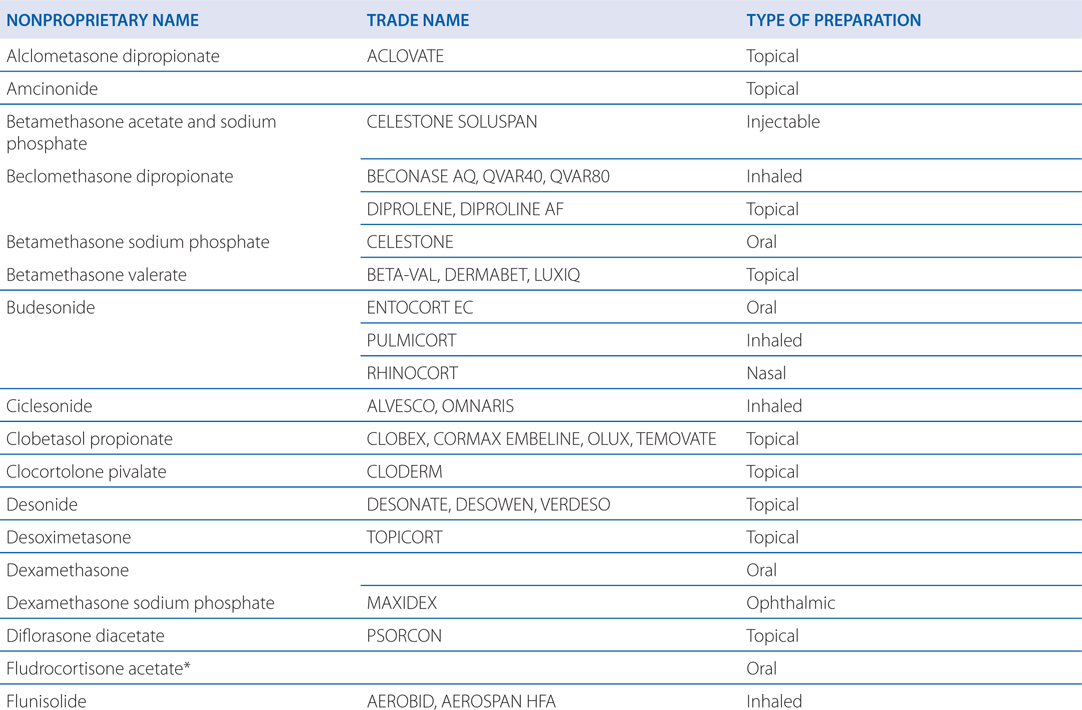
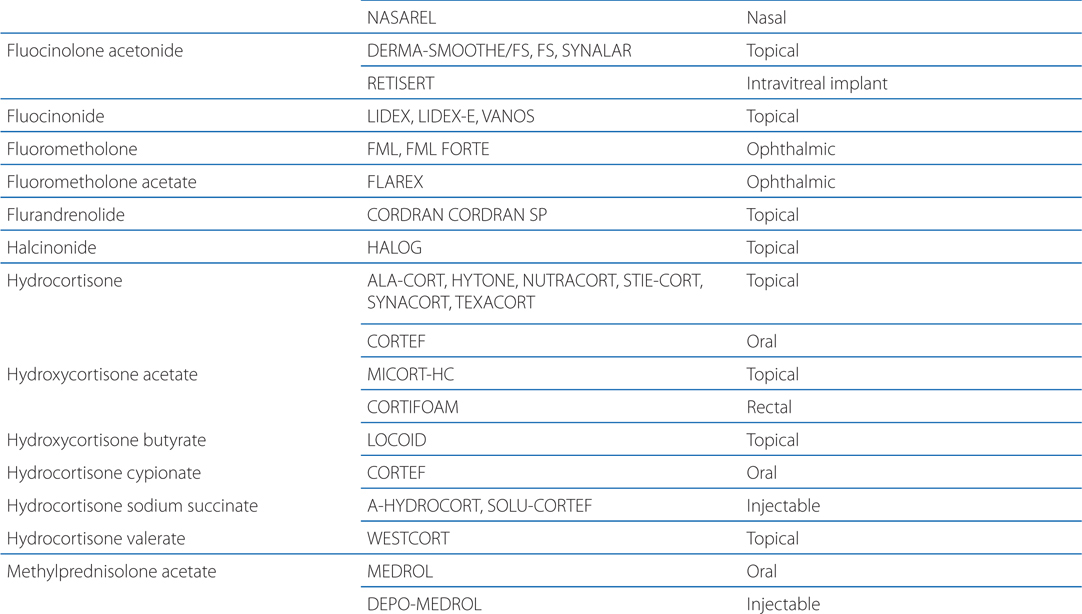
Table 29-1 lists available preparations of adrenocortical steroids and their synthetic analogs
TABLE 29-1 Available Preparations of Adrenocortical Steroids and Their Synthetic Analogs
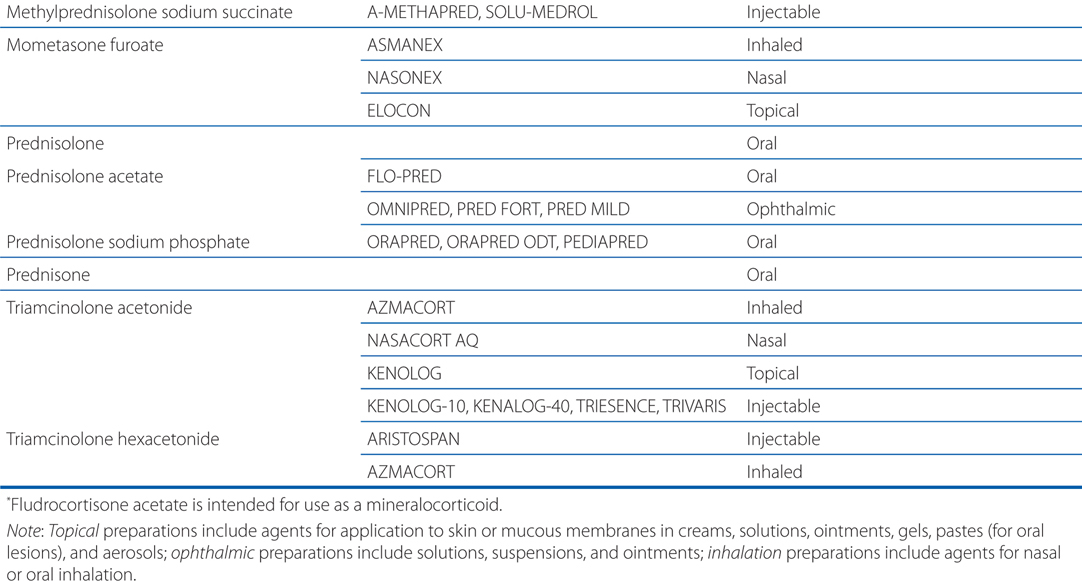
MECHANISMS OF ACTION OF ACTH, ADRENOCORTICOSTEROIDS, AND INHIBITORS OF THE SYNTHESIS OF ADRENOCORTICOSTEROIDS
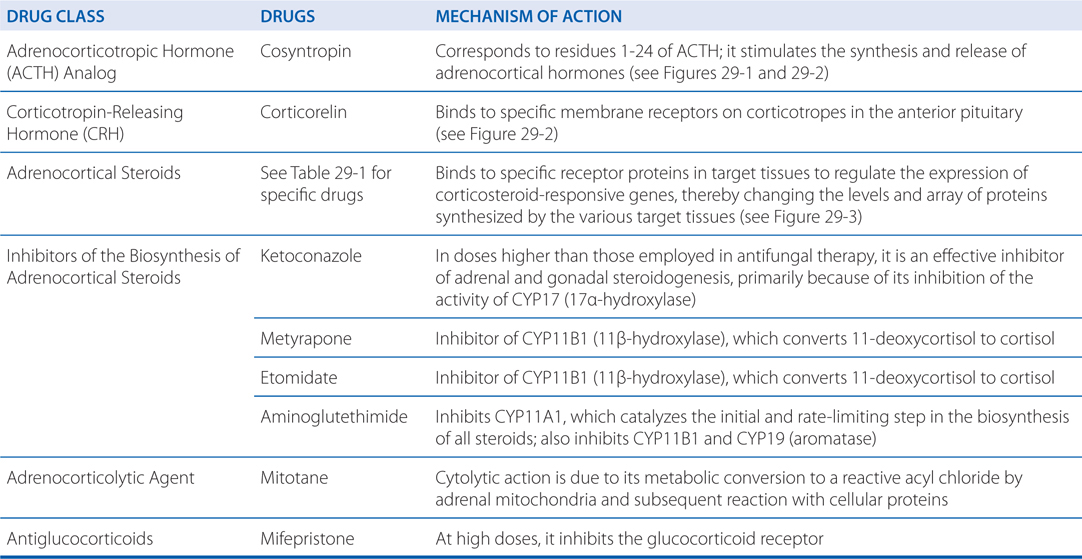
PHYSIOLOGIC FUNCTIONS AND PHARMACOLOGIC EFFECTS OF ADRENOCORTICAL STEROIDS
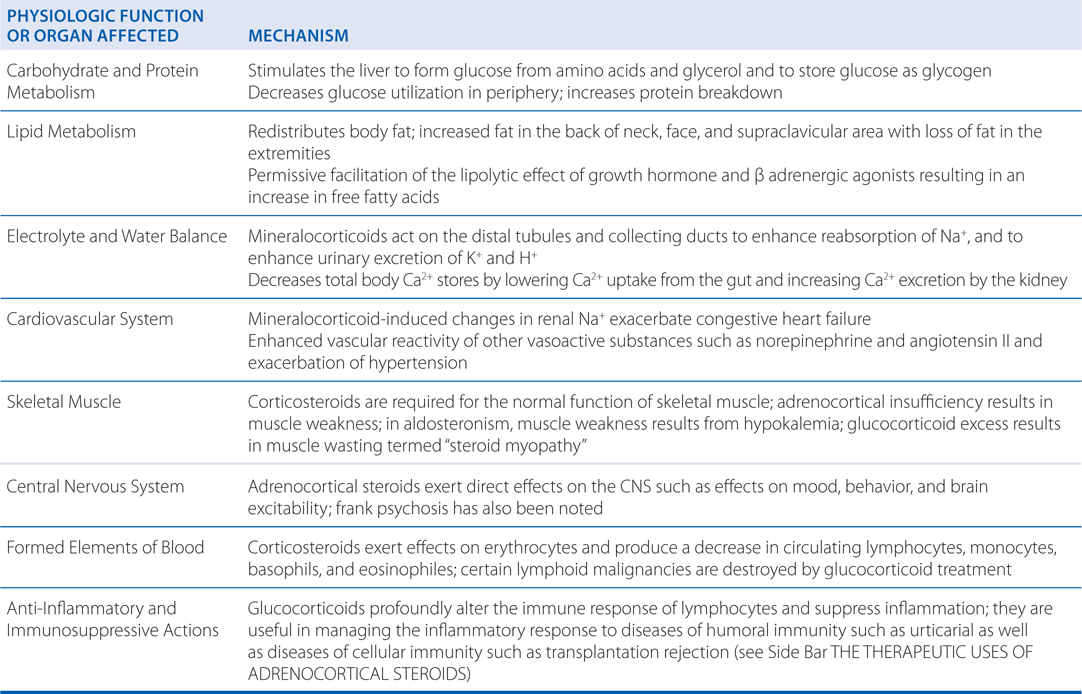
• Replacement therapy
▶ Acute adrenal Insufficiency
▶ Chronic adrenal insufficiency
▶ Congenital adrenal hyperplasia
• Nonendocrine diseases
▶ Rheumatic disorders
▶ Renal disease
▶ Allergic disease
▶ Bronchial asthma and other pulmonary conditions
▶ Infectious diseases
▶ Ocular diseases
▶ Skin diseases
▶ Gastrointestinal diseases
▶ Hepatic diseases
▶ Malignancies
▶ Cerebral edema
▶ Sarcoidosis
▶ Thrombocytopenia
▶ Autoimmune destruction of erythrocytes
▶ Organ transplantation
▶ Spinal cord injury
A 53-year-old woman with rheumatoid arthritis involving the joints of both hands and both hips has developed an exacerbation of her disease in the past two weeks. Fifteen years ago she responded well to a short course of prednisone. For the past 3 months, she has been receiving methotrexate. She is started back on prednisone now for relief of her symptoms.
a. Why was prednisone chosen?
Table 29-2 shows the relative potencies and equivalent doses of representative corticosteroids. Prednisone is a more potent anti-inflammatory agent than cortisol, but not as potent as dexamethasone. Prednisone has much less sodium-retaining potency than fludrocortisone, which has predominant mineralocorticoid activity. Prednisone is considered to be intermediate in its duration of action compared to cortisol and dexamethasone, which are short- and long-acting, respectively.
TABLE 29-2 Relative Potencies and Equivalent Doses of Representative Corticosteroids
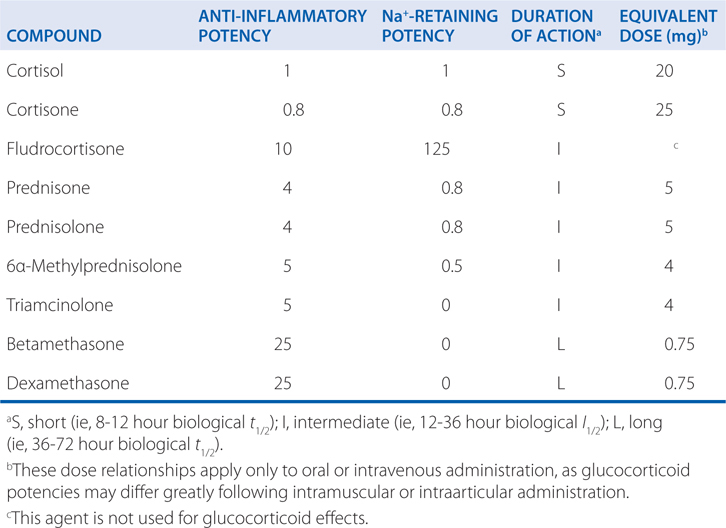
To facilitate drug tapering and/or conversion to alternate-day therapy, the intermediate-acting glucocorticoid such as prednisone is preferred to the longer-acting dexamethasone.
The use of glucocorticoids in a disease such as rheumatoid arthritis is largely empirical and the use of glucocorticoids should follow the principles in the Side Bar GENERAL PRINCIPLES FOR THE USE OF GLUCOCORTICOIDS.
b. What is the mechanism of action of adrenocortical steroids?
The glucocorticoids interact with receptors in the various target tissues that are members of the nuclear receptor family of transcription factors (see Figure 29-3). In the target tissues, proteins are expressed that alter cellular functions resulting in the physiologic functions and pharmacologic effects shown in the Table PHYSIOLOGICAL FUNCTIONS AND PHARMACOLOGICAL EFFECTS OF THE ADRENOCORTICAL STEROIDS.
GENERAL PRINCIPLES FOR THE USE OF GLUCOCORTICOIDS
• The decision to institute therapy requires careful consideration of the relative risks and benefits in each patient.
• For any disease and any patient, the appropriate dose to achieve a given therapeutic effect must be determined by trial and error and must be reevaluated periodically.
• A single dose of a glucocorticoid, even a large one, is virtually without harmful effects and a short course of up to 1 week, in the absence of specific contraindications, is unlikely to be harmful.
• As the duration of glucocorticoid therapy extends beyond 1 week, there are time and dose-related increases in the incidence of disabling and potential lethal effects.
• Except in the setting of replacement therapy the glucocorticoids are neither specific nor curative.
• Abrupt cessation of glucocorticoids after prolonged therapy is associated with the risk of adrenal insufficiency, which may be fatal.
A 43-year-man has been diagnosed with primary adrenal insufficiency (Addison’s disease). He requires replacement therapy with adrenocortical steroids.
a. What is the difference between primary and secondary adrenal insufficiency?
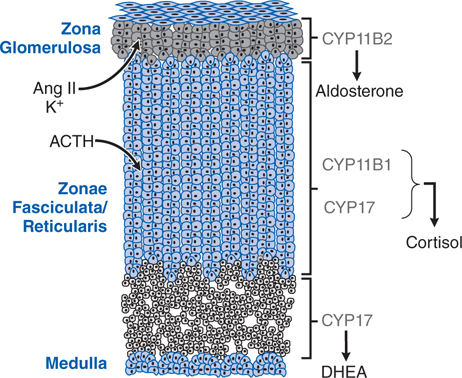
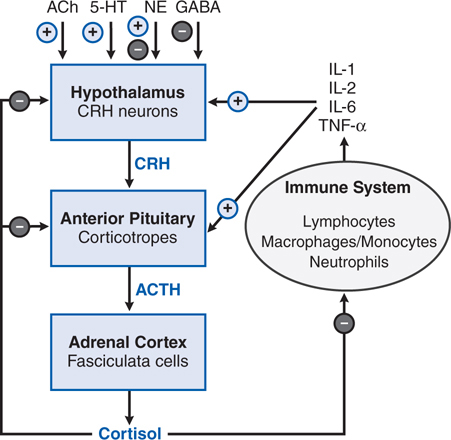
Adrenal insufficiency can result from structural or functional lesions of the adrenal cortex (primary adrenal insufficiency or Addison’s disease) or from functional or structural lesions of the anterior pituitary or hypothalamus (secondary adrenal insufficiency). See Figures 29-1 and 29-2 for the anatomical and functional structure of the adrenal gland and for the regulation of corticosteroids from the anterior pituitary, respectively.
FIGURE 29-1 The adrenal cortex contains 3 anatomically and functionally distinct compartments. The major functional compartments of the adrenal cortex are shown, along with the steroidogenic enzymes that determine the unique profiles of corticosteroid products. Also shown are the predominant physiological regulators of steroid production: angiotensin II (Ang II) and K+ for the zona glomerulosa and ACTH for the zona fasciculata. The physiological regulator(s) of dehydroepiandrosterone (DHEA) production by the zona reticularis are not known, although ACTH acutely increases DHEA biosynthesis.
FIGURE 29-2 Overview of the hypothalamic-pituitary-adrenal (HPA) axis and the immune inflammatory network. Also shown are inputs from higher neuronal centers that regulate corticotropin-releasing hormone (CRH) secretion. + indicates a positive regulator, – indicates a negative regulator, + and – indicates a mixed effect, as for NE (norepinephrine). In addition, arginine vasopressin stimulates release of ACTH from corticotropes.
b. Why is hydrocortisone chosen to treat this patient?
Hydrocortisone is the active form of cortisone (see Figure 29-4). Although some patients with primary adrenal insufficiency can be maintained on hydrocortisone and liberal salt intake, most also require a mineralocorticoid such as fludrocortisone acetate to maintain water and electrolyte balance.
c. What are the major considerations in maintaining the proper dose of hydrocortisone in this patient?
Glucocorticoid doses often must be adjusted upward in patients who are also taking drugs that increase the plasma clearance of glucocorticoids (eg, phenytoin, rifampin, or barbiturates). The stress of concurrent illness may also necessitate a dose adjustment. During minor illnesses, the glucocorticoid dose should be doubled. If nausea or vomiting precludes the retention of oral medications, the patient’s physician should be notified, and family members should be trained to administer parenteral dexamethasone and then seek medical attention immediately.
TOXICITIES OF ADRENOCORTICAL STEROIDS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree