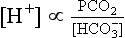24 If the [H+] and the PCO2 are known, the bicarbonate can be calculated. Indeed, blood gas analysers (Fig 24.1) are programmed to provide this on all samples, as the ‘standard bicarbonate’ i.e. under standard conditions. Other parameters usually included are the PO2, and the base excess, another way of assessing the metabolic component. The most important information available for the interpretation and classification of an acid-base disorder is provided by the patient’s clinical history. The predicted compensatory responses in [HCO3–] or PCO2 when [H+] changes as a result of primary acid–base disorders are shown in Table 24.1. Table 24.1 Primary acid–base disorders and compensatory responses A practical approach to the interpretation of blood gas results is shown in Figure 24.2. The steps in classifying the acid–base disorder are:
Acid–base disorders
diagnosis and management
Specimens for blood gas analysis
Interpreting results
Primary disorder
Compensatory response
↑PCO2 (Respiratory acidosis)
↑HCO3–
↓PCO2 (Respiratory alkalosis)
↓HCO3–
↓HCO3– (Metabolic acidosis)
↓PCO2
↑HCO3– (Metabolic alkalosis)
↑PCO2
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine