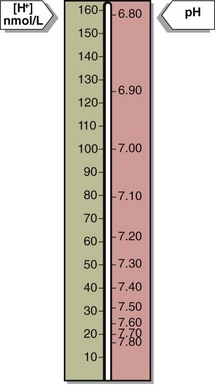
20 Blood hydrogen ion concentration [H+] is maintained within tight limits in health. Normal levels lie between 35 and 45 nmol/L. Values greater than 120 nmol/L or less than 20 nmol/L require urgent treatment; if sustained they are usually incompatible with life. The [H+] in blood may also be expressed in pH units. pH is defined as the negative log of the hydrogen ion concentration. (Fig 20.1).
Acid–base
concepts and vocabulary
H+ concentration
Basicmedical Key
Fastest Basicmedical Insight Engine