Thyroid hormone synthesis, action and metabolism
Synthesis and metabolism
Thyroxine (T4) and small amounts of tri-iodothyronine (T3) and reverse T3 (rT3) are all synthesised in the thyroid gland (Figure 8.1) by a process involving:
- Trapping of iodide from plasma by a sodium iodine symporter in the thyroid.
- Oxidation of iodide to iodine by thyroid peroxidase.
- Incorporation of iodine into tyrosyl residues on thyroglobulin in the colloid of the thyroid follicle. Mono-iodotyrosine (MIT) and di-iodotyrosine (DIT) are formed.
- Production of T3 and T4 by coupling iodotyrosyl residues in the thyroglobulin molecule.
- Splitting off of T4 and T3 from thyroglobulin following its reabsorption from the colloid.
- Release of T4 and T3 into the circulation.
These stages are shown diagrammatically in Figure 8.2.
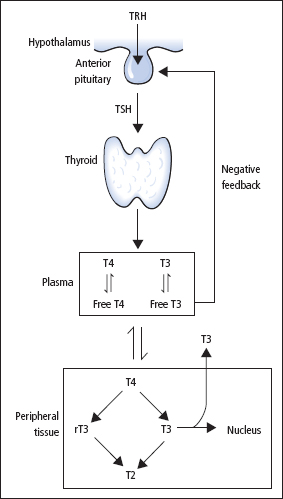
Thyroxine, a pro-hormone, is produced exclusively by the thyroid. The biologically active hormone is T3, and about 85% of plasma T3 is formed by outer-ring (5′) mono-deiodination of T4 in liver, kidneys and muscle (Figure 8.3). Thyroxine also undergoes inner-ring (5) mono-deiodination in non-thyroidal tissues, with the production of metabolically inactive rT3.
Plasma transport and cellular action
Thyroid hormones are transported in plasma almost entirely bound, reversibly, to plasma proteins. Thyroxine-binding globulin (TBG) is the major binding protein, binding about 70% of plasma T4 and 80% of plasma T3. Transthyretin (also called thyroxine-binding pre-albumin) and albumin also bind thyroid hormone such that more than 99.8% of thyroid hormones circulate bound to these three proteins.
Figure 8.1 The structure of the thyroid hormones T4, T3 and reverse T3.
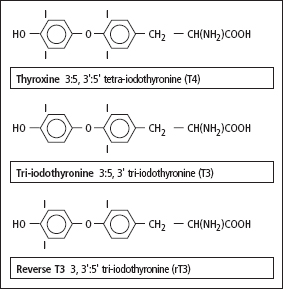
Figure 8.2 The synthesis of T3 and T4 in the thyroid gland. The metabolism of T4 is described in Figure 8.3. DIT and MIT are hormonally inactive.

Approximately 0.05% of plasma T4 and 0.2% of plasma T3 are free (i.e. unbound to protein). Only the free fractions can cross the cell membrane and affect intracellular metabolism. After binding to high-affinity binding sites on the plasma membrane, the hormones are actively transported into cells by specific energy-dependent transporters. In the cell T4 is metabolised to T3 which then binds to specific nuclear receptors that in turn activate T3-responsive genes. These gene products modify a wide range of cell function including basal metabolic rate and the metabolism of lipids, carbohydrates and proteins. High concentrations of thyroid hormone increase the basal metabolic rate and stimulate breakdown of protein and lipids. Low concentrations of thyroid hormone result in low metabolic rate, weight gain and poor physical and mental development in the child.
Regulation of thyroid function
The most important regulator of thyroid homeostasis is TSH (or thyrotrophin; Figure 8.3). The production of TSH is controlled by a stimulatory effect of the hypothalamic tripeptide, TRH (or thyroliberin), mediated by a negative feedback from circulating FT3 and FT4. It is thought that the hypothalamus, via TRH, sets the level of thyroid hormone production required physiologically, and that the pituitary acts as a ‘thyroid-stat’ to maintain the level of thyroid hormone production that has been determined by the hypothalamus.
Dopamine, somatostatin and glucocorticoids also appear to be involved in inhibiting the release of TSH, and these agents together with the interleukins may be important modifiers of TSH release in non-thyroidal illness (NTI).
Figure 8.3 Regulation of thyroid hormone synthesis and metabolism.
Investigations to determine thyroid status
Measurement of TSH and thyroid hormones should be performed to determine the patient’s thyroid status.
Thyrotrophin (reference range 0.5–4.5 mU/L)
The measurement of [TSH] in a basal blood sample by immunometric assay provides the single most sensitive, specific and reliable test of thyroid status in both overt and subclinical thyroid disease. In primary hypothyroidism, [TSH] is increased, while in primary hyperthyroidism, [TSH] is below 0.01 mU/L. There are exceptions to this, and both raised and undetectable [TSH] may be found in some euthyroid patients (Table 8.1).
Free T4 (reference range 10–21 pmol/L); free T3 (reference range 2.6–6.2 pmol/L)
Free thyroid hormone concentrations are independent of changes in the concentration and affinity of thyroid hormone-binding proteins and theoretically provide a more reliable means of diagnosing thyroid dysfunction than measurement of total hormone concentrations. Several assay techniques have been developed to measure free hormone concentrations. These methods produce results that show good agreement in most ambulant patients; however, in patients with NTI, results may not always correlate well with one another due to various assay artefacts.
Total T4 (reference range 70–150 nmol/L); total T3 (reference range 1.2–2.8 nmol/L)
Since more than 99% of T4 and T3 circulate in plasma bound to protein, any change in the concentration of these binding proteins, particularly [TBG], will be reflected in the concentrations of total T4 and total T3. However, [FT4] and [FT3] remain normal if pituitary–thyroid homeostasis is maintained. In practice [FT4] and [FT3] are more widely used than [total T4] and [total T3].
Table 8.1 Causes of an abnormal plasma [TSH] in some clinically euthyroid patients
| Undetectable plasma [TSH] | Increased plasma [TSH] |
| Pregnancy, during the first 20 weeks | Subclinical hypothyroidism |
| Treated hyperthyroid patients – first 6 months | During recovery stage of NTI1 |
| Ophthalmic Graves disease | |
| Various kinds of NTI* | |
| Non-toxic multinodular goitre | |
| Treatment with dopaminergic drugs | |
| Treatment with high-dose glucocorticoids |
The thyroid status of patients with subclinical hypothyroidism and need for treatment is controversial.
* NTI = non-thyroidal illness; discussed on p.125.
Selective use of thyroid function tests
Many laboratories measure basal [TSH] as the initial test of thyroid function. This strategy is not infallible, but it will detect both overt and subclinical primary thyroid disease. A normal [TSH] virtually excludes primary thyroid dysfunction. If an abnormal result is obtained, thyroid hormone measurements must be made to confirm that thyroid dysfunction is present, and to determine the severity of the disease.
Initial measurement of both TSH and FT4 together provides a more satisfactory method of assessing thyroid status, since in some situations a single TSH result may be misleading (Tables 8.1 and 8.2). If this strategy is followed, a significant number of cases will arise in which one test will be abnormal while the complementary test will be normal. It is thus essential to understand and appreciate the factors that can affect the results of thyroid function tests.
Table 8.2 Situations in which first-line TSH is not ideal since it may give misleading results in some patients
| First-line TSH not ideal | First-line TSH acceptable |
| Symptomatic patients – first presentation | Monitoring patients stabilised on thyroxine |
| Optimising treatment of hypothyroidism and hyperthyroidism – early months | Screening asymptomatic ‘at risk’ groups |
| Screening and monitoring in pregnancy | |
| Diagnosis and monitoring hypopituitarism | |
| Diagnosis of TSH secreting tumour and end organ resistance (rare) |
Screening and surveillance
Screening in the healthy asymptomatic population is not warranted. Thyroid function tests should be done however on:
- the elderly patient
- women who present to their primary care physician at the menopause
- patients presenting with diabetes or an autoimmune disorder
- any patient with features of a thyroid disorder.
Table 8.3 Suggested screening protocols for patients at high risk of thyroid dysfunction
| Annual check |
| Patients with autoimmune disease |
| Turner‘s and Down’s syndrome |
| Post-neck irradiation |
| Type 2 diabetes |
| 6-monthly check |
| Amiodarone or lithium |
| At presentation |
| At menopause if vague symptoms |
| Elderly with vague symptoms |
Patients with a high risk of developing a thyroid disorder should also be monitored annually using serum [TSH]. Such patients include type 1 and type 2 diabetes, Down’s and Turner’s syndrome, and post-neck irradiation. Patients taking lithium or aminodarone should be assessed before commencing treatment and 6-monthly thereafter (Table 8.3).
Interpreting results of thyroid function tests
Figures 8.4 and 8.5 provides a guide to the interpretation of thyroid function tests.
Hyperthyroidism
Overt primary hyperthyroidism (FT4 and FT3 high; TSH low)
[TSH] is nearly always below 0.01 mU/L, due to feedback inhibition on the pituitary. Free and total T4 and T3 concentrations are nearly always increased in overt hyperthyroidism. In a very small percentage of hyperthyroid patients, [total T4] and [FT4] are both normal, whereas both [total T3] and [FT3] are increased; this condition is known as T3 hyperthyroidism or T3 thyrotoxicosis.
Figure 8.4 Interpretation of thyroid function tests in suspected hyperthyroidism.

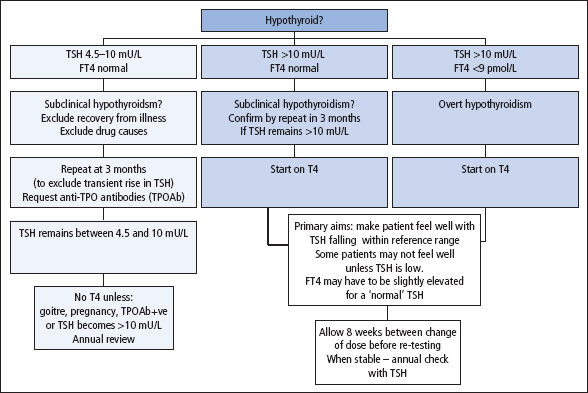
Figure 8.5 Interpretation of thyroid function tests in suspected hypothyroidism.
Patients with a hormone profile suggestive of hyperthyroidism may get an isotope scan (technetium Tc-99m pertechnate) to identify the cause. A diffuse increased uptake of isotope is consistent with Graves disease, whilst the presence of ‘hot nodules’ with surrounding areas of reduced isotope uptake would be consistent with nodular hyperthyroidism. Poor uptake of isotope across the gland is consistent with thyroiditis.
Subclinical hyperthyroidism (FT4 and FT3 normal; TSH low)
Thyroid disease presents as a spectrum of clinical and biochemical features of varying severity. The clinical diagnosis of mild thyroid disorders is often difficult since the patient may have few if any clinical features and the only biochemical abnormality may be an abnormal plasma [TSH]. The combination of a persistent abnormality in [TSH], together with normal thyroid hormone concentrations, is known as ‘subclinical thyroid disease’. This description is unsatisfactory, since it rests solely on the results of chemical investigations. Many clinically euthyroid patients with multinodular goitre or with exophthalmic Graves disease have ‘subclinical hyperthyroidism’, that is, [TSH] below 0.01 mU/L and [FT4] and [FT3] in the upper part of their respective reference ranges. Before assigning this diagnosis, however, other causes of a low TSH should be excluded, including NTI, pregnancy and drugs that suppress TSH (dopaminergic drugs, high dose glucocorticoids). The tests should be repeated 1–2 months later and if the abnormalities persist the patient should be referred to an endocrinologist to establish the diagnosis and give optimal treatment (Figure 8.4).
Management of hyperthyroidism
The therapeutic objective is to maintain [FT4] in the upper half of the reference range after treating with anti-thyroid drugs, radioiodine or subtotal thyroidectomy. Monitoring every 4–6 weeks for the first few months is required. Measurement of [TSH] is not a reliable guide of thyroid status during the first 4–6 months of treatment for hyperthyroidism, since [TSH] may still be suppressed even when plasma thyroid hormone concentrations have become abnormally low. [TSH] can be used to determine the adequacy of treatment after normal thyrotroph responsiveness has returned.
After radioactive iodine treatment, the likelihood that patients will eventually develop hypothyroidism is high. Annual follow-up of these patients is essential. Patients treated by subtotal thyroidectomy may have temporary disturbances of thyroid function tests in the early post-operative period but in the long-term are more likely to remain euthyroid than are patients treated with radioactive iodine. Annual follow-up is still required however.
Patients with subclinical hyperthyroidism who are not treated should be monitored every 6–12 months.
Hypothyroidism
Overt primary hypothyroidism (FT4 low; TSH high)
[TSH] is invariably increased, often to more than 20 mU/L, as feedback inhibition of the pituitary (Figure 8.3) is diminished. [FT4] and [total T4] are usually low. FT3 and total T3 measurements are of no value here, since normal concentrations are often observed.
Subclinical primary hypothyroidsm (FT4 normal; TSH high)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


