FIGURE 22-1 Bipolar right ventricular endocardial voltage maps from the patient in the case example are displayed in the right anterior oblique (RAO), left anterior oblique (LAO), and left lateral projections (A to C). A large confluent area of low bipolar voltage containing widespread isolated late potentials (black dots) is seen consistent with scarring. This is present in the anterior right ventricle and extends to the basal and mid septum. The unipolar right ventricular endocardial voltage map is displayed (D) and is adjusted for left ventricular septal wall thickness, rather than right ventricular free wall, with normal peak to peak amplitude defined as >8.3 mV. This suggests a much larger area of basal septal involvement.
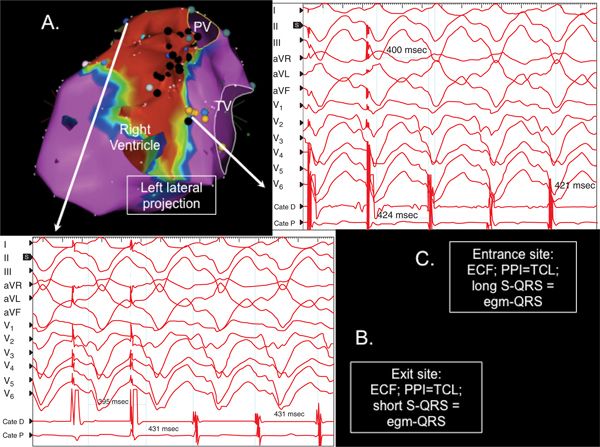
Unipolar voltage analysis suggested a significantly larger area of intramural septal substrate (Figure 22-1D). Seven distinct VTs were inducible with programmed stimulation, only two of which were of RBBB configuration (Figure 22-2). Due to frequently changing morphologies as well as hemodynamic intolerance, only one of the VTs was mappable: VT 2, which had a cycle length of 431 ms. Entrainment mapping showed this VT to be a large macroreentrant circuit with an exit site on the infundibular septum of the RV and an entrance site on the inferoseptal RV (Figure 22-3). Central isthmus circuit components were seen on the septal RV in confluent area of endocardial scarring containing widespread isolated and fractionated potentials. Ablation here terminated VT, but other VTs were still inducible. Extensive substrate ablation was then performed from both sides of the interventricular septum, targeting these potentials and sites of good pace-maps with long stimulus to QRS delay. Following this, VT 3 was still inducible with a right bundle right inferior axis morphology and precordial transition pattern break in V2, strongly suggestive of a preaorticepicardial exit. Percutaneous pericardial access was obtained, and epicardial substrate mapping showed preserved voltages over the LV and RV free walls with the expected low-voltage area over the interventricular groove due to pericoronary fat. However, at the anteroseptal LV and at the LV summit, on either side of the left anterior descending artery, a cluster of isolated and fractioned potentials were seen, and pace-maps here, while not perfect, recreated the precordial and limb lead vectors of VT 3 (Figure 22-4). Extensive ablation here (after coronary angiography defined the LAD to be >5 mm from lesion sites) and in the preaortic region of the LV endocardium rendered this VT noninducible. Rapid VT, similar to VT 5, was still inducible with triple extra stimuli at the end of the procedure.
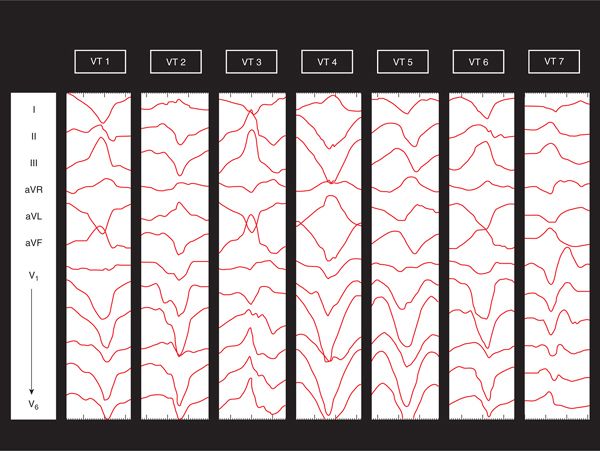
FIGURE 22-2 The seven ventricular tachycardia (VT) morphologies induced in this patient are displayed. VT 3 and VT 7 have left bundle branch block morphology. Only VT 2 was mappable.
FIGURE 22-3 Entrainment mapping of VT 2 from the patient in the case example, proving this VT to be due to a large loop reentrant circuit located in septal substrate. (A) This figure displays the endocardial right ventricular bipolar voltage map showing confluent septal scarring. (B) Exit site response is demonstrated, showing entrainment with concealed fusion (ECF), postpacing interval (PPI) equaling the tachycardia cycle length (TCL), and short stimulus to QRS (S-QRS) interval equaling the electrogram to QRS (egm-QRS). (C) Entrance site response is shown with concealed entrainment, PPI=TCL, and long S-QRS equaling the egm-QRS.
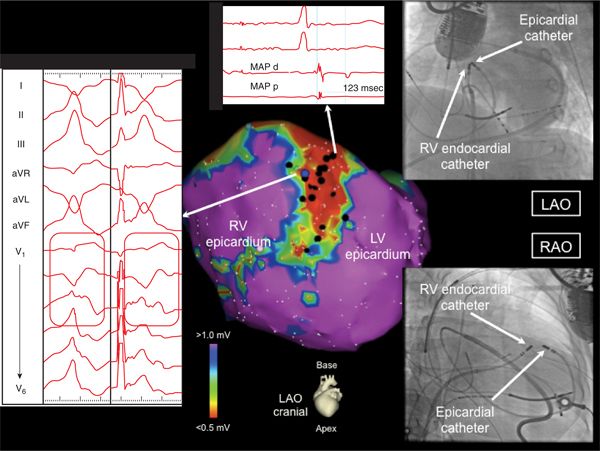
FIGURE 22-4 The epicardial voltage map from the patient in the case example is displayed. A small patch of midanteroseptal scarring is displayed with networks of isolated late potentials (one is highlighted) in addition to low bipolar voltage (<0.5 mV), distinguishing it from epicardial pericoronary fat. VT 3 was pacemapped to this region as shown. The fluoroscopic proximity of the endocardial and epicardial catheters mapping VT 3 is shown. Only epicardial pacemapping was able to recreate the V1-V3 precordial transition pattern break as shown.
After this procedure, no ICD shocks occurred over short-term follow-up.
CASE EXPLANATION
This patient with NICM due to cardiac sarcoidosis highlights some of the considerable challenges in dealing with VT in this context. The initial consideration of establishing a secondary cause for the NICM is well demonstrated as some etiologies other than idiopathic dilated cardiomyopathy (DCM) may have specific management or prognostic considerations. In the case of cardiac sarcoidosis, immunosuppression may be considered although even with treatment, prognosis is guarded with a considerable proportion of patients progressing to death or cardiac transplantation. Establishment of the diagnosis may require, in addition to a detailed clinical assessment, evaluation with biomarkers, serology, cardiac magnetic resonance imaging (MRI), PET scanning, and endomyocardial biopsy.
As seen in this patient, multiple VT morphologies are the rule in the setting of cardiac sarcoidosis, and indeed with any NICM, with the majority generally being unmappable by entrainment. Given the usually widespread, disparate regions of patchy, often subepicardial inflammation and fibrosis, both LBBB and RBBB VT configurations are seen. This patient displayed a less common pattern of confluent septal scarring, primarily manifest from the RV side of the septum with a patchier continuation to the epicardial aspect of the septum in the preaortic LV summit region. Such confluent scarring forms the electrophysiologic milieu necessary for the establishment of large-loop reentrant VT circuits, such as those that were proved by entrainment mapping in this patient. After ablating mappable tachycardias, extensive substrate ablation is necessary to target the usually widespread regions of endocardial, epicardial, and intramural scarring seen in these patients. Even after successful VT ablation, the prognosis for transplant-free survival is guarded in cardiac sarcoidosis and is worse than in other etiologies of NICM due to progressive heart failure and recurrent VT.1,2
EPIDEMIOLOGY AND CLINICAL SIGNIFICANCE
Although sustained monomorphic VT is an uncommon presentation in NICM, sudden death due to presumed ventricular arrhythmias is well described. The risk of this rises with worsening LV systolic function as the burden of ventricular fibrosis increases and the capacity to hemodynamically tolerate VT declines. The search for better risk stratifying tools remains the focus of much current investigation as this will lead to better targeted ICD therapy. Monitoring studies in patients suffering sudden death have demonstrated that monomorphic VT is the antecedent arrhythmia in many of these cases.3 Although sudden death can be effectively prevented with ICDs, such devices are not a cure for VT, and multiple shocks can confer considerable morbidity on patients and possibly even increase mortality.4–6 Antiarrhythmic drug therapy has not proven to be an effective management option to eliminate shocks and comes with a significant risk of adverse events, particularly with the use of amiodarone.7
ETIOLOGY AND PATHOPHYSIOLOGY OF VT IN NICM
In the context of NICM and in direct comparison to the postinfarct situation, catheter ablation of scar-related VT can be a difficult procedure with poorer outcomes in general.2 Compared to other contexts, VT in NICM is more likely to be focal and to involve the His-Purkinje system.8 These latter VTs include typical and atypical forms of bundle-branch reentry and are very important to recognize as they are readily amenable to catheter ablation.9 Myocardial reentrant VT is seen when the interstitial and replacement fibrosis typically present in the early stages of NICM has progressed to the point of forming more confluent regions of scarring that allow for the fixed and functional barriers of VT circuits to be formed. Unlike a mature infarct scar, which extends inward from the endocardium, the scarring in NICM may lie deep to the myocardium surface, where it can be effectively shielded from currently available mapping and ablation technologies.
ENDOCARDIAL ELECTROPHYSIOLOGIC SUBSTRATE
Although patients with NICM often have diffuse interstitial and replacement fibrosis as well as variable degrees of myocyte hypertrophy and myofibril disarray,10 those presenting with monomorphic VT generally have confluent areas of scar which form the substrate for reentrant and triggered arrhythmias.8 Remarkably, in a theme echoed across the various etiologies of nonischemic left and right ventricular cardiomyopathies, the fibrosis is generally centered around the basal, periannular regions.11,12 However, it is important to highlight
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree