GYNECOLOGIC DISORDERS
CANCER OF THE BREAST
 Definition
Definition
Cancer of the breast is a malignancy arising from the breast epithelium (carcinoma) and/or stroma (sarcoma).
 Clinical Presentation
Clinical Presentation
Breast cancer is the most common malignancy in women and a leading cause of cancer death. Risk factors include increased age and female gender, race, preexisting benign breast disease, family history of breast or ovarian cancer, exposure to ionizing radiation, and environmental factors.
 Laboratory Findings
Laboratory Findings
The diagnosis of breast cancer is made on mammographic and/or ultrasound findings followed by biopsy and histologic evaluation. Patients with a familial history of breast cancer may be screened for BRCA1 and BRCA2; however, <10% of all breast cancers are associated with genetic mutations (see Chapter 10).
The histologic types of breast carcinoma include infiltrating ductal carcinoma (see eBook Figure 8-1C), infiltrating lobular (see eBook Figure 8-2D) carcinoma, and mixed ductal/lobular carcinoma. In addition, there are sarcomas and mixed tumors, phyllodes tumor (see eBook Figure 8-3C).
Molecular subtypes include luminal subtypes A and B (the majority of ER-positive breast cancers), and HER2-enriched (often negative for ER and PR), basal subtypes (triple negative) (see eBook Figures 8-1 to 8-3).
At the time of diagnosis, immunohistochemical staining is performed on the tumor to determine estrogen (ER) and progesterone (PR) receptor expression for prognosis and human epidermal growth factor 2 (HER2) receptors to determine if the patient will respond to Herceptin. Grading is based on architecture, nuclear morphology, and the number of mitoses using a system such as the Scarff-Bloom-Richardson grading system. Staging is based on the TNM system from the American Joint Committee on Cancer and the International Union for Cancer Control.
CANCER OF THE CERVIX
 Definition
Definition
Squamous cell carcinoma of the cervix is one of the most frequent neoplasms that affect a woman’s reproductive system1 (see eBook Figure 8-4A). Cervical squamous cell carcinoma is the result of infection with various strains of the human papilloma virus (HPV), especially (but not exclusively) the oncogenic types 16 and 18. Persistent viral infection transforms the epithelial cells that undergo a progression of changes in the cervix particularly at the squamocolumnar junction, which can be identified on Pap testing and on biopsy. The progression from acute infection to dysplasia to invasive carcinoma may take 3–7 years. Regular screening for high-risk HPV and by Pap testing has decreased the incidence of this cancer worldwide. HPV vaccination should decrease the incidence further in coming years. Adenocarcinoma of the cervix is also caused by HPV transformation of the endocervical cells but is less common than SCC and is not easily detected by Pap testing (see eBook Figure 8-4B).
 Clinical Presentation
Clinical Presentation
Cervical carcinoma is usually seen in women in their 40s and 50s but may occur as early as the mid-20s if there is a history of early sexual activity and multiple partners. It is more likely in patients who have never been screened or who have not had a Pap test in the previous 5 years. Patients may be asymptomatic or present with abnormal or postcoital bleeding or vaginal discharge that may be watery, mucoid, or purulent and malodorous. The presence of pelvic or lower back pain suggests advanced disease. Suspicion should be high in the presence of an abnormal Pap test.
 Laboratory Findings
Laboratory Findings
Pap testing may be performed by conventional smear or liquid methodology (SurePath Liquid-Based Pap Test and ThinPrep Pap Test). Cytology is reported in the Bethesda system as negative, atypical squamous cells (ASCUS), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), squamous cell carcinoma, and atypical glandular cells (AGUS) (see eBook Figure 8-5A–E). A statement on the adequacy of the cellularity for testing is also made.2
ACOG recommends screening for cervical cancer with cytology (smear or liquid based) and high-risk HPV DNA testing as follows3:
 No screening for women younger than 21 years.
No screening for women younger than 21 years.
 Cytology alone should be performed for women aged 21–29 years.
Cytology alone should be performed for women aged 21–29 years.
 HPV and cytology cotesting performed every 5 years for women aged 30–65 years.
HPV and cytology cotesting performed every 5 years for women aged 30–65 years.
 For women with three negative cytology tests or two negative cotesting screens, no further screening is necessary after age 65 years.
For women with three negative cytology tests or two negative cotesting screens, no further screening is necessary after age 65 years.
 Women with a history of treated CIN 2, CIN 3, or adenocarcinoma in situ should continue routine age-based screening for at least 20 years.
Women with a history of treated CIN 2, CIN 3, or adenocarcinoma in situ should continue routine age-based screening for at least 20 years.
 For women who have undergone total hysterectomy and who have not had a history of CIN 2, CIN 3, or adenocarcinoma in situ in the previous 20 years, no screening is necessary.
For women who have undergone total hysterectomy and who have not had a history of CIN 2, CIN 3, or adenocarcinoma in situ in the previous 20 years, no screening is necessary.
 For women vaccinated against HPV, follow age-specific recommendations similar to unvaccinated women.
For women vaccinated against HPV, follow age-specific recommendations similar to unvaccinated women.
Follow-up of screening tests as follows:
 Negative cytology and negative HPV rescreen in 5 years
Negative cytology and negative HPV rescreen in 5 years
 ASCUS Pap and negative HPV rescreen in 3 years
ASCUS Pap and negative HPV rescreen in 3 years
 Negative cytology and positive HPV repeat cotesting in 12 months or test for HPV 16/18
Negative cytology and positive HPV repeat cotesting in 12 months or test for HPV 16/18
 If HPV 16/18 positive refer to colposcopy
If HPV 16/18 positive refer to colposcopy
 If HPV 16/18 negative repeat cotesting in 12 months
If HPV 16/18 negative repeat cotesting in 12 months
Referral of patients for colposcopic examination and biopsy should be performed for patients with positive HPV 16 or 18 and any cytology results higher than LSIL or any patient with atypical glandular cells. Patients should undergo biopsy of any visualized cervical lesions and endocervical curettage if no lesions are apparent (see eBook Figures 8-6 and 8-7). For patients with abnormal Pap tests (ASCUS and HSIL), positive high-risk HPV DNA test and negative biopsy additional tissue diagnosis should be attempted with conization (loop electrocautery excision).
For women diagnosed with invasive squamous cell carcinoma of the cervix, imaging studies (CT or MRI) are recommended to evaluate possible involvement of adjacent organs or metastases.
Testing options:
Many laboratories now offer reflex testing for HPV from the liquid pap vial based on the ACOG recommendations, making it easier for the clinician. In addition, PCR testing for GC, Chlamydia, and Trichomonas may also be performed on the same liquid pap vial.
Limitations of the Pap test:
 False-negative results in approximately 5–10% of cases.
False-negative results in approximately 5–10% of cases.
 Unsatisfactory cellularity occurs with fewer than 5,000 well-visualized, wellpreserved squamous cells in a liquid-based Pap, and 8,000 cells on a conventional smear are obtained.
Unsatisfactory cellularity occurs with fewer than 5,000 well-visualized, wellpreserved squamous cells in a liquid-based Pap, and 8,000 cells on a conventional smear are obtained.
 Sampling problems occur in up to 10% of samples collected; these have integrity issues and are considered unsatisfactory due to the presence of blood or mucous, inflammation, insufficient cells, or problems with the slide preparation. Malignant cells may not be present if the smear is repeated too soon after a previous abnormal smear.
Sampling problems occur in up to 10% of samples collected; these have integrity issues and are considered unsatisfactory due to the presence of blood or mucous, inflammation, insufficient cells, or problems with the slide preparation. Malignant cells may not be present if the smear is repeated too soon after a previous abnormal smear.
 The Pap test was designed to screen for squamous tumors. Other tumor types are less readily diagnosed (e.g., adenocarcinoma, lymphoma, and sarcoma).
The Pap test was designed to screen for squamous tumors. Other tumor types are less readily diagnosed (e.g., adenocarcinoma, lymphoma, and sarcoma).
 Human error in interpreting difficult cells; <3% of preventable cervical cancers are due to misread smears.
Human error in interpreting difficult cells; <3% of preventable cervical cancers are due to misread smears.
References
1. Saraiya M, Ahmed F, Krishnan S, et al. Cervical cancer incidence in a prevaccine era in the United States, 1998–2002. Obstet Gynecol. 2007;109:360–370.
2. Solomon D, Nayar R (eds). The Bethesda System for Reporting Cervical Cytology, 2nd ed. New York: Springer Science and Business Media, LLC; 2004.
3. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin No. 131: screening for cervical cancer. Obstet Genecol. 2012;120:1222–1238.
CANCER OF THE ENDOMETRIUM
 Definition
Definition
Endometrial carcinoma is the most common invasive gynecologic cancer in North America (cervical carcinoma is most common worldwide). There are two types recognized. Type 1 is estrogen or tamoxifen related, is usually a low-grade endometrioid type, and is preceded by endometrial intraepithelial neoplasia.
 Clinical Presentation
Clinical Presentation
Cancer of the endometrium is associated with PTEN mutations, obesity, and hereditary nonpolyposis colonic cancer syndrome. Concurrent ovarian carcinoma may occur in 10–20%. Type 2 is unrelated to estrogen or tamoxifen, is usually a highergrade papillary serous or mixed type, and is associated with p53 mutations without a preceding in situ component. The progression of type 2 disease is usually rapid, and the prognosis is poor1. Patients with endometrial carcinoma present with a history of abnormal vaginal bleeding, especially if postmenopausal.
 Laboratory Findings
Laboratory Findings
The diagnosis of endometrial carcinoma is made on endometrial biopsy or curettage (positive in 95% of patients) and rarely is identified on Pap test (see eBook Figure 8-8). A negative Pap test does not rule out carcinoma. Blood tests may show anemia if bleeding is chronic or severe, but otherwise are noncontributory.
Reference
1. Crum CP, Lee KR (eds). Diagnostic Gynecologic and Obstetric Pathology. Philadelphia, PA: Elsevier Saunders; 2006.
CANCER OF THE OVARY
EPITHELIAL OVARIAN CARCINOMA
 Definition
Definition
Cancer of the ovary may derive from the epithelium (95% of cases) or from the stromal supporting cells or germ cells. This section will deal with the epithelial carcinomas arising from the surface of the ovary that are contiguous with the peritoneum and include low-grade serous carcinomas, serous tumors of low malignant potential, high-grade serous carcinomas, mucinous carcinoma, endometrioid carcinomas, clear cell tumors, Brenner (transitional cell) tumors, and undifferentiated carcinomas.
 Clinical Presentation
Clinical Presentation
Patients may present with either acute symptoms such as bowel obstruction or pleural effusion or subacute symptoms such as adnexal mass, pain, bloating, urinary frequency, or early satiety. Patients with a positive family history of breast or ovarian cancer or who have BRCA1 or BRCA2 mutations or Lynch syndrome may be at increased risk (see Hereditary and Genetic Diseases, Chapter 10).
 Laboratory Findings
Laboratory Findings
The diagnosis of ovarian cancer is made on histologic examination of tissue or cytology of peritoneal or pleural fluid if present (see eBook Figure 8-9). Rarely abnormal glandular cells may be seen on Pap test, which on further workup are found to originate from the ovary.
Imaging is the most important tool for identifying an adnexal mass. Surgical excision of the intact mass with intraoperative frozen-section diagnosis is performed whenever possible as transabdominal FNA or biopsy of ovarian tumors has been shown to increase the risk of seeding the malignant cells into the peritoneum by rupture or incision of the mass.
Screening tests for ovarian carcinoma have been sought to aid in finding these patients before symptoms occur. These include the following:
 CA-125 is elevated in approximately 50% of patients with early-stage disease and in >80% of patients with advanced disease. It may also be elevated in normal women and in patients with endometriosis, leiomyoma, cirrhosis, PID, or other malignancies. Following serial CA-125 levels over time may be more beneficial as a screening tool.
CA-125 is elevated in approximately 50% of patients with early-stage disease and in >80% of patients with advanced disease. It may also be elevated in normal women and in patients with endometriosis, leiomyoma, cirrhosis, PID, or other malignancies. Following serial CA-125 levels over time may be more beneficial as a screening tool.
 Human epididymis protein 4 (HE4) is helpful in diagnosing recurrent or progressive disease or in the evaluation of a suspicious adnexal mass.
Human epididymis protein 4 (HE4) is helpful in diagnosing recurrent or progressive disease or in the evaluation of a suspicious adnexal mass.
 Carcinoembryonic antigen (CEA) is nonspecific. Levels may be elevated in malignancies (particularly mucinous carcinomas) of the ovary, GI tract, breast, pancreas, thyroid, and lung. It is also elevated in patients who smoke, or who have mucinous cystadenoma, cholecystitis, cirrhosis, pancreatitis, pneumonia, and diverticulitis and IFD.
Carcinoembryonic antigen (CEA) is nonspecific. Levels may be elevated in malignancies (particularly mucinous carcinomas) of the ovary, GI tract, breast, pancreas, thyroid, and lung. It is also elevated in patients who smoke, or who have mucinous cystadenoma, cholecystitis, cirrhosis, pancreatitis, pneumonia, and diverticulitis and IFD.
 CA 19-9 is a mucin protein that may be elevated in ovarian cancer but is also positive in gastric cancers. It may be used to follow recurrence in a patient with known CA19-9–positive ovarian cancer.
CA 19-9 is a mucin protein that may be elevated in ovarian cancer but is also positive in gastric cancers. It may be used to follow recurrence in a patient with known CA19-9–positive ovarian cancer.
 OVA1 is a panel that includes five serum biomarkers to assess the likelihood of malignancy in patients with an adnexal mass. Two markers are up-regulated (CA 125 II, beta-2 microglobulin) and three down-regulated (transferrin, transthyretin, apolipoprotein A1). An algorithm determines the patient’s risk for ovarian cancer. OVA1 is commercially available through Quest Diagnostics.
OVA1 is a panel that includes five serum biomarkers to assess the likelihood of malignancy in patients with an adnexal mass. Two markers are up-regulated (CA 125 II, beta-2 microglobulin) and three down-regulated (transferrin, transthyretin, apolipoprotein A1). An algorithm determines the patient’s risk for ovarian cancer. OVA1 is commercially available through Quest Diagnostics.
 Pathologic diagnosis of tumor type and grade forms the basis for treatment and prognosis. These tumors are staged according to the International Federation of Gynecology and Obstetrics (FIGO)/TNM system. For a complete review of the pathology of epithelial ovarian carcinomas, (see Crum and Lee).1 Diagnostic Gynecologic and Obstetric Pathology. Philadelphia, PA: W B Saunders Co, 2005.
Pathologic diagnosis of tumor type and grade forms the basis for treatment and prognosis. These tumors are staged according to the International Federation of Gynecology and Obstetrics (FIGO)/TNM system. For a complete review of the pathology of epithelial ovarian carcinomas, (see Crum and Lee).1 Diagnostic Gynecologic and Obstetric Pathology. Philadelphia, PA: W B Saunders Co, 2005.
OVARIAN GERM CELL TUMORS
 Definition
Definition
Ovarian germ cell neoplasms originate from the germ cells of the ovary and comprise 5% of the malignant ovarian neoplasms. These tumors may be malignant or benign and include teratomas (mature, dermoid, and immature), dysgerminomas, endodermal sinus (yolk sac) tumors, embryonal carcinomas, and nongestational choriocarcinoma (see eBook Figure 8-10).
 Clinical Presentation
Clinical Presentation
Patients presenting with ovarian germ cell neoplasms are usually between 10 and 30 years of age. They are more frequent in Asian/Pacific Islander and Hispanic women than in Caucasians. Presenting symptoms include effects of hCG production by the tumor (precocious puberty, abnormal vaginal bleeding), abdominal enlargement, ascites, or abdominal pain (including acute abdomen due to torsion).
 Laboratory Findings
Laboratory Findings
The definitive diagnosis requires histologic evaluation at the time of surgical excision. A presumptive diagnosis may be made with an adnexal mass on pelvic imaging (CT, MRI, or ultrasound) and elevation of an associated tumor marker. Tumor markers are also used to monitor patients post–surgical resection for recurrence. These include the following:
 hCG is increased in embryonal cell carcinomas, ovarian choriocarcinomas, mixed germ cell tumors, and some dysgerminomas.
hCG is increased in embryonal cell carcinomas, ovarian choriocarcinomas, mixed germ cell tumors, and some dysgerminomas.
 AFP is increased in endodermal sinus tumors, embryonal cell carcinomas, mixed germ cell tumors, and some immature teratomas.
AFP is increased in endodermal sinus tumors, embryonal cell carcinomas, mixed germ cell tumors, and some immature teratomas.
 Lactate dehydrogenase (LDH) is increased in dysgerminomas.
Lactate dehydrogenase (LDH) is increased in dysgerminomas.
 Pathologic diagnosis of tumor type and grade forms the basis for treatment and prognosis. Malignant germ cell neoplasms are staged according to the FIGO/TNM system. For a complete review of the pathology of ovarian germ cell tumors (see Crum and Lee).1 Diagnostic Gynecologic and Obstetric Pathology. Philadelphia, PA: W B Saunders Co, 2005.
Pathologic diagnosis of tumor type and grade forms the basis for treatment and prognosis. Malignant germ cell neoplasms are staged according to the FIGO/TNM system. For a complete review of the pathology of ovarian germ cell tumors (see Crum and Lee).1 Diagnostic Gynecologic and Obstetric Pathology. Philadelphia, PA: W B Saunders Co, 2005.
Reference
1. Crum CP, Lee KR (eds). Diagnostic Gynecologic and Obstetric Pathology. Philadelphia, PA: WB Saunders Co,; 2005.
OVARIAN SEX CORD-STROMAL NEOPLASMS
 Definition
Definition
Ovarian sex cord-stromal neoplasms are benign or malignant tumors that arise from the cells supporting the oocytes, including ovarian hormone–producing cells. These are rare tumors comprising <2% of all ovarian cancers. These include fibromas, thecomas, granulosa cell tumors, Sertoli or Sertoli–Leydig cell tumors, and gynandroblastoma.
 Clinical Presentation
Clinical Presentation
Patients with sex cord tumors present with abdominal distention, bloating, pain or pelvic symptoms, and a finding of adnexal mass on imaging. They also may exhibit hormonal manifestations including signs of estrogen excess (precocious puberty, abnormal uterine bleeding) or androgen excess (virilization). The risk for sex cord tumors may be decreased in current or past smokers, in women who have taken oral contraceptive pills, and in multiparous women.
 Laboratory Findings
Laboratory Findings
The diagnosis of sex cord-stromal tumor is made on tissue evaluation at the time of surgery (see eBook Figure 8-11). For any suspected ovarian malignancy, a complete oophorectomy must be performed to prevent potential spread of neoplastic cells. Presumptive diagnosis may be made in a patient with hormonal changes, an adnexal mass on imaging (transpelvic ultrasound), or bimanual exam and elevation of an associated tumor marker. These include the following:
 AFP is seen in embryonal carcinoma and polyembryoma and may be seen in immature teratoma, endodermal sinus tumors, mixed germ cell tumors, and Sertoli–Leydig cell tumors.
AFP is seen in embryonal carcinoma and polyembryoma and may be seen in immature teratoma, endodermal sinus tumors, mixed germ cell tumors, and Sertoli–Leydig cell tumors.
 hCG is seen in embryonal carcinoma, choriocarcinoma, and polyembryoma and may be seen in mixed germ cell tumors and dysgerminoma.
hCG is seen in embryonal carcinoma, choriocarcinoma, and polyembryoma and may be seen in mixed germ cell tumors and dysgerminoma.
 LDH is seen in dysgerminoma and endodermal sinus tumors and may be seen in embryonal carcinoma, choriocarcinoma, immature teratoma, and mixed germ cell tumors.
LDH is seen in dysgerminoma and endodermal sinus tumors and may be seen in embryonal carcinoma, choriocarcinoma, immature teratoma, and mixed germ cell tumors.
 Inhibin is seen in granulosa cell tumors where both inhibin A and inhibin B should be ordered, and it may be seen in Sertoli–Leydig cell tumors and gonadoblastoma.
Inhibin is seen in granulosa cell tumors where both inhibin A and inhibin B should be ordered, and it may be seen in Sertoli–Leydig cell tumors and gonadoblastoma.
 Estradiol may be seen in granulosa cell tumors, Sertoli–Leydig cell tumors, gynandroblastoma, immature teratoma, embryonal carcinoma, and dysgerminoma.
Estradiol may be seen in granulosa cell tumors, Sertoli–Leydig cell tumors, gynandroblastoma, immature teratoma, embryonal carcinoma, and dysgerminoma.
 Testosterone is elevated when virilization is present in Sertoli–Leydig cell tumors and may also be seen in granulosa cell tumors and gynandroblastoma.
Testosterone is elevated when virilization is present in Sertoli–Leydig cell tumors and may also be seen in granulosa cell tumors and gynandroblastoma.
 Androstenedione may be seen in gynandroblastoma and Sertoli–Leydig cell tumors.
Androstenedione may be seen in gynandroblastoma and Sertoli–Leydig cell tumors.
 DHEA may be seen in immature teratoma, gonadoblastoma, and Sertoli– Leydig cell tumors.
DHEA may be seen in immature teratoma, gonadoblastoma, and Sertoli– Leydig cell tumors.
 Müllerian inhibiting substance (MIS) appears to be a more specific tumor marker for granulosa cell tumors but is not yet available for clinical use.
Müllerian inhibiting substance (MIS) appears to be a more specific tumor marker for granulosa cell tumors but is not yet available for clinical use.
 Genetic testing is not helpful at this time. Sex cord-stromal neoplasms have no known association with BRCA1 or BRCA2. Studies have shown a somatic mutation in FOXL2, a gene that encodes a transcription factor, may be associated with granulosa cell tumors, and somatic mutations affecting the RNase IIIb domain of DICER1 may be associated with Sertoli–Leydig cell tumors.
Genetic testing is not helpful at this time. Sex cord-stromal neoplasms have no known association with BRCA1 or BRCA2. Studies have shown a somatic mutation in FOXL2, a gene that encodes a transcription factor, may be associated with granulosa cell tumors, and somatic mutations affecting the RNase IIIb domain of DICER1 may be associated with Sertoli–Leydig cell tumors.
 Pathologic diagnosis of tumor type and grade forms the basis for treatment and prognosis. Sex cord–stromal neoplasms are staged according to the FIGO/TNM system. For a complete review of the pathology of ovarian germ cell tumors (see Crum and Lee).1
Pathologic diagnosis of tumor type and grade forms the basis for treatment and prognosis. Sex cord–stromal neoplasms are staged according to the FIGO/TNM system. For a complete review of the pathology of ovarian germ cell tumors (see Crum and Lee).1
URINARY TRACT INFECTIONS
See Chapter 7
PELVIC INFLAMMATORY DISEASE*
CHORIOAMNIONITIS
 Definition
Definition
Pelvic inflammatory disease (PID) refers to infection of the upper genital tract of women. It may include the endometrium, myometrium, parametrium, uterine tubes, and ovaries. Other pelvic and abdominal organs may be secondarily infected (e.g., peritonitis, perihepatitis).
 Who Should Be Suspected?
Who Should Be Suspected?
PID is most commonly caused as a complication of STIs (85%); 15% of cases arise postoperatively or as a complication of childbirth. Factors for increased risk for PID include risk factors associated with STI: Age <25 years and young age at onset of sexual activity; new or multiple sex partners, especially partners with STI symptoms; unprotected sexual activity; and history of STI. Additional factors may include IUD use, douching, and bacterial vaginosis.
Clinical features:
 PID describes infection in any of various organs, including the uterus, ovaries, and adjacent abdominal organs. Clinical presentation depends on the primary sites and severity of infection.
PID describes infection in any of various organs, including the uterus, ovaries, and adjacent abdominal organs. Clinical presentation depends on the primary sites and severity of infection.
 Diagnosis should be strongly considered in women presenting with abdominal pain if physical examination reveals cervical or adnexal tenderness. Diffuse, subacute abdominal pain is typical. Abdominal tenderness, sometimes with subtle peritoneal signs, and onset during menses are commonly described. Abnormal uterine bleeding is reported frequently. Nonspecific symptoms, like fever and lower genital tract symptoms, may be reported; other causes should be investigated if symptoms related to the GI or urinary tract are predominant.
Diagnosis should be strongly considered in women presenting with abdominal pain if physical examination reveals cervical or adnexal tenderness. Diffuse, subacute abdominal pain is typical. Abdominal tenderness, sometimes with subtle peritoneal signs, and onset during menses are commonly described. Abnormal uterine bleeding is reported frequently. Nonspecific symptoms, like fever and lower genital tract symptoms, may be reported; other causes should be investigated if symptoms related to the GI or urinary tract are predominant.
 Laboratory Findings
Laboratory Findings
 There is no gold standard for the diagnosis of PID. Evaluation must consider findings on physical examination and laboratory testing. Additional testing may be required, including imaging studies, laparoscopy, and histopathology.
There is no gold standard for the diagnosis of PID. Evaluation must consider findings on physical examination and laboratory testing. Additional testing may be required, including imaging studies, laparoscopy, and histopathology.
 Serum pregnancy test should be performed to rule out ectopic pregnancy or other complication of pregnancy.
Serum pregnancy test should be performed to rule out ectopic pregnancy or other complication of pregnancy.
 A positive result of NAAT for Neisseria gonorrhoeae or Chlamydia trachomatis, with compatible clinical presentation, confirms a diagnosis of PID.
A positive result of NAAT for Neisseria gonorrhoeae or Chlamydia trachomatis, with compatible clinical presentation, confirms a diagnosis of PID.
 The quality and wet mount/Gram stain of cervical/vaginal fluid should be examined for increased WBCs or other abnormality. Abnormal secretions or increased WBCs (≥3 WBC per high-power field) support a diagnosis of PID.
The quality and wet mount/Gram stain of cervical/vaginal fluid should be examined for increased WBCs or other abnormality. Abnormal secretions or increased WBCs (≥3 WBC per high-power field) support a diagnosis of PID.
 Positive results for peripheral blood WBC, ESR, or CRP support a diagnosis of PID.
Positive results for peripheral blood WBC, ESR, or CRP support a diagnosis of PID.
 Supplemental tests: Inflammation of upper genital or adjacent organs, detected by laparoscopy, biopsy, peritoneal fluid analysis, or positive culture from normally sterile upper genital sites, confirms a clinical diagnosis of PID.
Supplemental tests: Inflammation of upper genital or adjacent organs, detected by laparoscopy, biopsy, peritoneal fluid analysis, or positive culture from normally sterile upper genital sites, confirms a clinical diagnosis of PID.
 Other tests: HIV and syphilis testing should be requested. Additional testing and microbiologic testing are performed on the basis of signs and symptoms in specific patients.
Other tests: HIV and syphilis testing should be requested. Additional testing and microbiologic testing are performed on the basis of signs and symptoms in specific patients.
Suggested Reading
Peipert JF, Boardman L, Hogan JW, et al. Laboratory evaluation of acute upper genital tract infection. Obstet Gynecol. 1996;87:730–736.
VAGINOSIS AND VAGINITIS (BACTERIAL VAGINOSIS, TRICHOMONIASIS, VULVOVAGINAL CANDIDIASIS)*
Definition
 Vaginitis is used to describe conditions associated with significant inflammation, whereas vaginosis is used when vaginal secretions do not show a marked increase in inflammatory cells. Symptoms attributed to vaginitis may also be due to primary cervicitis, urethritis, or inflammation to other related tissues.
Vaginitis is used to describe conditions associated with significant inflammation, whereas vaginosis is used when vaginal secretions do not show a marked increase in inflammatory cells. Symptoms attributed to vaginitis may also be due to primary cervicitis, urethritis, or inflammation to other related tissues.
 Changes in the amount or character of vaginal discharge are common presenting complaints of women seeking medical attention. Although there is normal variability in vaginal secretions, infectious and other pathologic causes are common and should be carefully evaluated.
Changes in the amount or character of vaginal discharge are common presenting complaints of women seeking medical attention. Although there is normal variability in vaginal secretions, infectious and other pathologic causes are common and should be carefully evaluated.
 Causes
Causes
 Complaints associated with noninfectious causes may be indistinguishable from those caused by genital tract infections. Common noninfectious causes include the following: lead to vaginal dryness and itching rather than an increase in vaginal secretions. Here, there are mixed nonspecific gram-negative rods with decreased lactobacilli; vaginal cytology shows an atrophic pattern.
Complaints associated with noninfectious causes may be indistinguishable from those caused by genital tract infections. Common noninfectious causes include the following: lead to vaginal dryness and itching rather than an increase in vaginal secretions. Here, there are mixed nonspecific gram-negative rods with decreased lactobacilli; vaginal cytology shows an atrophic pattern.
 Allergy and irritants. Many products, such as detergents, soaps, bubble bath, latex (e.g., condoms), and topical medications, may cause inflammation of the vaginal mucosa and changes in the character and volume of secretions. Clinical management requires elimination of the allergen or irritant.
Allergy and irritants. Many products, such as detergents, soaps, bubble bath, latex (e.g., condoms), and topical medications, may cause inflammation of the vaginal mucosa and changes in the character and volume of secretions. Clinical management requires elimination of the allergen or irritant.
 Atrophic vaginitis. This type of vaginitis is caused by estrogen deficiency and is usually associated with menopause but may be seen in the postpartum period or as a result of medication. Symptoms of estrogen deficiency
Atrophic vaginitis. This type of vaginitis is caused by estrogen deficiency and is usually associated with menopause but may be seen in the postpartum period or as a result of medication. Symptoms of estrogen deficiency
 Physiologic leukorrhea. Vaginal secretions may vary significantly in normal women, especially related to the menstrual cycle. The volume of vaginal secretion is typically greatest in mid-cycle. Significant symptoms and inflammation are not seen with physiologic leukorrhea; the odor, color, and viscosity of secretions are similar to the characteristics in the absence of leukorrhea.
Physiologic leukorrhea. Vaginal secretions may vary significantly in normal women, especially related to the menstrual cycle. The volume of vaginal secretion is typically greatest in mid-cycle. Significant symptoms and inflammation are not seen with physiologic leukorrhea; the odor, color, and viscosity of secretions are similar to the characteristics in the absence of leukorrhea.
 Bacterial vaginosis, trichomoniasis, and vulvovaginal candidiasis are the most common causes of clinically significant vaginosis/vaginitis and are described in detail below. Other infectious causes of vaginitis include the following:
Bacterial vaginosis, trichomoniasis, and vulvovaginal candidiasis are the most common causes of clinically significant vaginosis/vaginitis and are described in detail below. Other infectious causes of vaginitis include the following:
 Condyloma acuminata. Increased vaginal discharge, pruritus, and pain are common symptoms caused by anogenital warts.
Condyloma acuminata. Increased vaginal discharge, pruritus, and pain are common symptoms caused by anogenital warts.
 Foreign body or traumatic vaginitis. Foreign bodies, like a retained tampon, may cause a change in the normal vaginal flora and mild signs and symptoms of infection. Removal of the foreign body is usually all that is required for clinical management.
Foreign body or traumatic vaginitis. Foreign bodies, like a retained tampon, may cause a change in the normal vaginal flora and mild signs and symptoms of infection. Removal of the foreign body is usually all that is required for clinical management.
 Group A Streptococcus, Staphylococcus aureus, and other pathogens may cause acute vaginal infection with pain, edema, erythema, and purulent vaginal discharge. Gram staining and culture confirm the diagnosis.
Group A Streptococcus, Staphylococcus aureus, and other pathogens may cause acute vaginal infection with pain, edema, erythema, and purulent vaginal discharge. Gram staining and culture confirm the diagnosis.
 Diagnosis: General Aspects of Vaginosis
Diagnosis: General Aspects of Vaginosis
The initial diagnostic evaluation should include a detailed history and laboratory testing. (Note that symptoms may be caused by more than one infectious condition.) A detailed clinical history may provide information that is useful in distinguishing infectious vaginitis from other conditions that may cause changes in the character of vaginal discharge (e.g., urethritis, cervicitis, noninfectious inflammatory conditions). Important factors include the following:
 Menstrual history: Vaginal secretions may vary with pregnancy and menstrual cycle. Vulvovaginal candidiasis often occurs in the premenstrual period; trichomoniasis often occurs in the postmenstrual period.
Menstrual history: Vaginal secretions may vary with pregnancy and menstrual cycle. Vulvovaginal candidiasis often occurs in the premenstrual period; trichomoniasis often occurs in the postmenstrual period.
 Sexual history: Factors associated with an increased risk of STDs, including BV and trichomoniasis, include new sexual partner, exposure to multiple sexual partners, and history of STD.
Sexual history: Factors associated with an increased risk of STDs, including BV and trichomoniasis, include new sexual partner, exposure to multiple sexual partners, and history of STD.
 Recent and current medications: Antibiotics, estrogen and progestin drugs, and other medications may predispose to vaginitis through changes in the vaginal environment or flora.
Recent and current medications: Antibiotics, estrogen and progestin drugs, and other medications may predispose to vaginitis through changes in the vaginal environment or flora.
 Personal hygiene and potential irritants: Hygienic products and practices, frequent or recent douching, soaps and detergents, topical medications, and panty liners and other products may cause vaginal irritation, resulting in symptoms indistinguishable from infectious causes.
Personal hygiene and potential irritants: Hygienic products and practices, frequent or recent douching, soaps and detergents, topical medications, and panty liners and other products may cause vaginal irritation, resulting in symptoms indistinguishable from infectious causes.
 In addition to history and physical examination, the following tests are recommended: vaginal pH, microscopic examination of vaginal secretions (wet mount, Gram stain), and amine test. Additional testing, including testing for specific microorganisms, is recommended for patients in whom testing does not provide a diagnosis.
In addition to history and physical examination, the following tests are recommended: vaginal pH, microscopic examination of vaginal secretions (wet mount, Gram stain), and amine test. Additional testing, including testing for specific microorganisms, is recommended for patients in whom testing does not provide a diagnosis.
 Laboratory Tests
Laboratory Tests
Specific diagnosis requires laboratory testing (see Table 8-1).
 Vaginal pH: Secretions are collected, using a dry swab, from the vaginal sidewall halfway between the cervix and introitus. A narrow-range paper (pH 4.0–5.5) should be used.
Vaginal pH: Secretions are collected, using a dry swab, from the vaginal sidewall halfway between the cervix and introitus. A narrow-range paper (pH 4.0–5.5) should be used.
 Microscopic examination: Saline wet mount preparations are used for direct detection of yeast-like cells and pseudohyphae, trichomonads, and host cells. Vaginal secretions collected by swab are suspended in a drop of normal saline on a microscopic slide. Normal vaginal secretions show a predominance of SECs with a minimal number of PMNs. Note that although Candida species are common components of the normal vaginal microflora, visualization of many yeast-like cells or pseudohyphae is abnormal and characteristic of candidiasis. (Detection of yeast may be facilitated by addition of 10% KOH to the saline wet mount preparation.) “Clue” cells are squamous epithelial cells covered by coccobacillary organisms, resulting in fuzzy or indistinct cell borders.
Microscopic examination: Saline wet mount preparations are used for direct detection of yeast-like cells and pseudohyphae, trichomonads, and host cells. Vaginal secretions collected by swab are suspended in a drop of normal saline on a microscopic slide. Normal vaginal secretions show a predominance of SECs with a minimal number of PMNs. Note that although Candida species are common components of the normal vaginal microflora, visualization of many yeast-like cells or pseudohyphae is abnormal and characteristic of candidiasis. (Detection of yeast may be facilitated by addition of 10% KOH to the saline wet mount preparation.) “Clue” cells are squamous epithelial cells covered by coccobacillary organisms, resulting in fuzzy or indistinct cell borders.
 Gram stain: Gram stains are used for direct detection of bacteria, yeast, and host cells. Normal vaginal secretions show a predominance of SECs with a minimal number of PMNs. There is a predominance of gram-positive bacilli consistent with Lactobacillus species.
Gram stain: Gram stains are used for direct detection of bacteria, yeast, and host cells. Normal vaginal secretions show a predominance of SECs with a minimal number of PMNs. There is a predominance of gram-positive bacilli consistent with Lactobacillus species.
 Amine “whiff” test: A drop of 10% KOH may be added to vaginal secretions on a microscopic slide. The immediate release of a “fishy” (volatile amine) odor is typical of BV.
Amine “whiff” test: A drop of 10% KOH may be added to vaginal secretions on a microscopic slide. The immediate release of a “fishy” (volatile amine) odor is typical of BV.
 Culture: Culture of vaginal secretions may improve the sensitivity of detection for trichomoniasis, but special techniques are required for isolation of T. vaginalis. Culture is not recommended for routine evaluation of vulvovaginal candidiasis. Positive cultures for yeast must be interpreted with caution because C. albicans and other yeast may represent normal endogenous flora. Culture may be useful for patients with recurrent vulvovaginal candidiasis, or candidiasis resistant to standard therapy. Bacterial culture, including culture for G. vaginalis, is not reliable for the diagnosis of BV because no single organism can be specifically implicated in the pathogenesis of BV.
Culture: Culture of vaginal secretions may improve the sensitivity of detection for trichomoniasis, but special techniques are required for isolation of T. vaginalis. Culture is not recommended for routine evaluation of vulvovaginal candidiasis. Positive cultures for yeast must be interpreted with caution because C. albicans and other yeast may represent normal endogenous flora. Culture may be useful for patients with recurrent vulvovaginal candidiasis, or candidiasis resistant to standard therapy. Bacterial culture, including culture for G. vaginalis, is not reliable for the diagnosis of BV because no single organism can be specifically implicated in the pathogenesis of BV.
 Serology: Serologic testing does not play a significant role in the diagnosis of vaginitis.
Serology: Serologic testing does not play a significant role in the diagnosis of vaginitis.
 Molecular tests: Molecular diagnostic tests are increasingly available for the diagnosis of infectious vaginitis. For example, nucleic acid hybridization provided greater sensitivity for detection of agents associated with BV, trichomoniasis, and vulvovaginal candidiasis compared with standard methods.
Molecular tests: Molecular diagnostic tests are increasingly available for the diagnosis of infectious vaginitis. For example, nucleic acid hybridization provided greater sensitivity for detection of agents associated with BV, trichomoniasis, and vulvovaginal candidiasis compared with standard methods.
 HIV and syphilis serology and testing related to other STIs should be considered.
HIV and syphilis serology and testing related to other STIs should be considered.
 Diagnosis
Diagnosis
 Common presenting symptoms of vaginitis include change in the volume, character or odor of vaginal secretions; irritation of the genital mucosa, including erythema, burning, and itching; dysuria; and spotting.
Common presenting symptoms of vaginitis include change in the volume, character or odor of vaginal secretions; irritation of the genital mucosa, including erythema, burning, and itching; dysuria; and spotting.
 In premenopausal women, the volume of vaginal secretions is <5 mL/day. Secretions are typically odorless, transparent, and viscous and white to yellowish. Normal vaginal pH is 4.0–4.5. Microscopic examination demonstrates a predominance of normal squamous epithelial cells (SECs) and few PMNs; there is a predominance of gram-positive bacilli consistent with lactobacilli (long, slender, may form chains).
In premenopausal women, the volume of vaginal secretions is <5 mL/day. Secretions are typically odorless, transparent, and viscous and white to yellowish. Normal vaginal pH is 4.0–4.5. Microscopic examination demonstrates a predominance of normal squamous epithelial cells (SECs) and few PMNs; there is a predominance of gram-positive bacilli consistent with lactobacilli (long, slender, may form chains).
TABLE 8–1. Comparison of Various Causes of Vaginitis
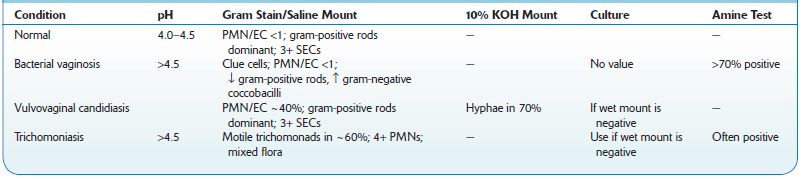
↓, decreased;↑, increased; PMN/EC, ratio of polymorphonuclear cells to epithelial cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


