Breast Surgery
LEARNING OBJECTIVES
After studying this chapter the reader will be able to:
• Describe the surgical anatomy of the breasts
• Correlate physiology to conditions requiring surgical intervention
• Identify risk factors associated with breast cancer
• Be familiar with the staging of breast cancers
• Identify special instruments and equipment used in breast surgery
• List the pharmacologic and hemostatic agents utilized in breast surgery
• Review diagnostic techniques used in breast surgery
• Describe the various surgical procedures for breast surgery
• Identify strategies that protect the patient from potential sources of surgical site infection
Overview
Most surgical procedures on the breast are performed to establish a definitive diagnosis or to treat breast cancer. Changing hormone levels from puberty throughout the remainder of life affect breast tissue in its physical and microscopic characteristics. In association with these changes, numerous aberrations and tumors can occur.
The occurrence of breast changes, benign or malignant, are some of the most emotionally upsetting health problems confronting women. Breast cancer is the most common cancer in women (Weaver, 2009); it accounts for nearly one of every three cancers diagnosed. The probability of developing breast cancer increases with age. Estimates are that one in eight women in the United States will develop breast cancer during her life. If the cancer is detected early, there is a 97% 5-year survival rate. Breast cancer risk increases if a woman’s mother, sister, or daughter had breast cancer, especially if the cancer developed before menopause. Early menarche (before 12 years of age) and a late natural menopause (after 50 years of age) are associated with a slightly increased risk for developing breast cancer. Further, a woman who has cancer in one breast is at increased risk for cancer in the other breast (American Cancer Society, 2009). Heightened public awareness, an increased number of women practicing self-examination, and early detection of breast masses by mammography have started to slow the annual increase in breast cancer mortality.
Surgical Anatomy
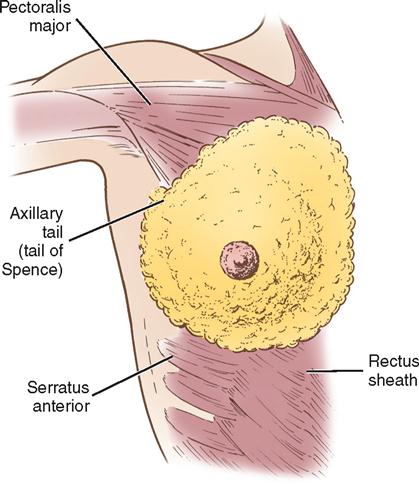
The breasts are bilateral mammary glands that lie on the pectoralis major fascia of the anterior chest wall. They are surrounded by a layer of fat and are encased in an envelope of skin. The breasts extend from the second to the sixth rib and horizontally from the lateral edge of the sternum to the anterior axillary line. The largest part of the mammary gland rests on the connective tissue of the pectoralis major muscle and laterally on the serratus anterior (upper outer quadrant of the breast), with a normal globular contour occurring as a result of fascial support (Cooper’s ligaments). An elongation of mammary tissue normally extends laterally on the pectoralis major toward the axilla and is known as the tail of Spence (Figure 8-1).
Each breast is made up of 12 to 20 glandular lobes separated by connective tissue. Each lobe drains by a single lactiferous duct that opens on the nipple. The nipple, located at about the fourth intercostal space, forms a conical projection into which the ducts open independently of each other on the surface. A pigmented circular area called the areola surrounds the nipple. Smooth muscle fibers of the areola contract to allow for nipple projection.
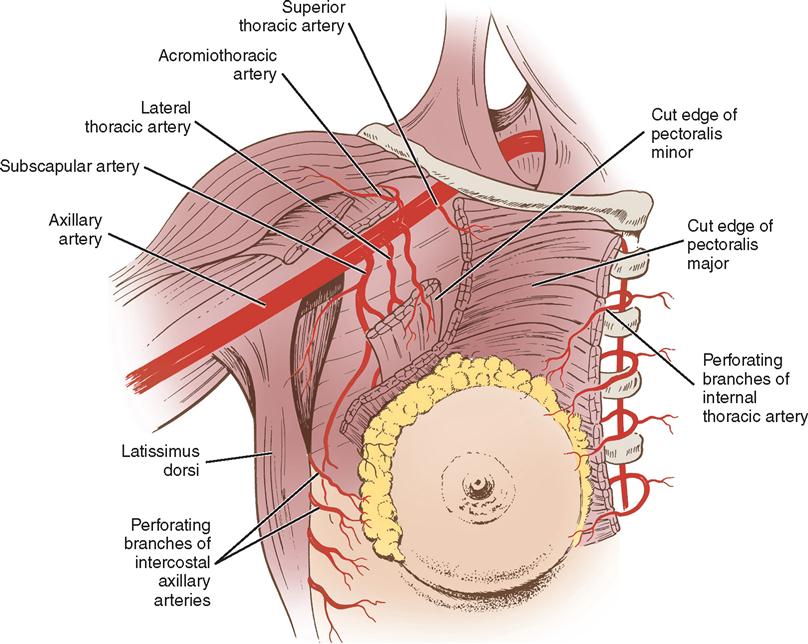
Three major arterial systems (Figure 8-2) supply the mammary glands with blood. The two main sources are branches of the internal mammary and lateral branches of the anterior aortic intercostal arteries, all of which form an extensive network of anastomoses over the breast. The third source is the pectoral branch, deriving from a branch of the axillary artery. The veins that mainly drain the breasts follow the course of the arteries. Superficial veins frequently dilate during pregnancy.
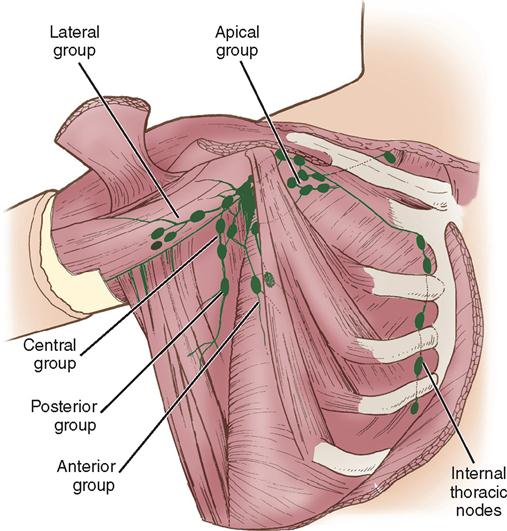
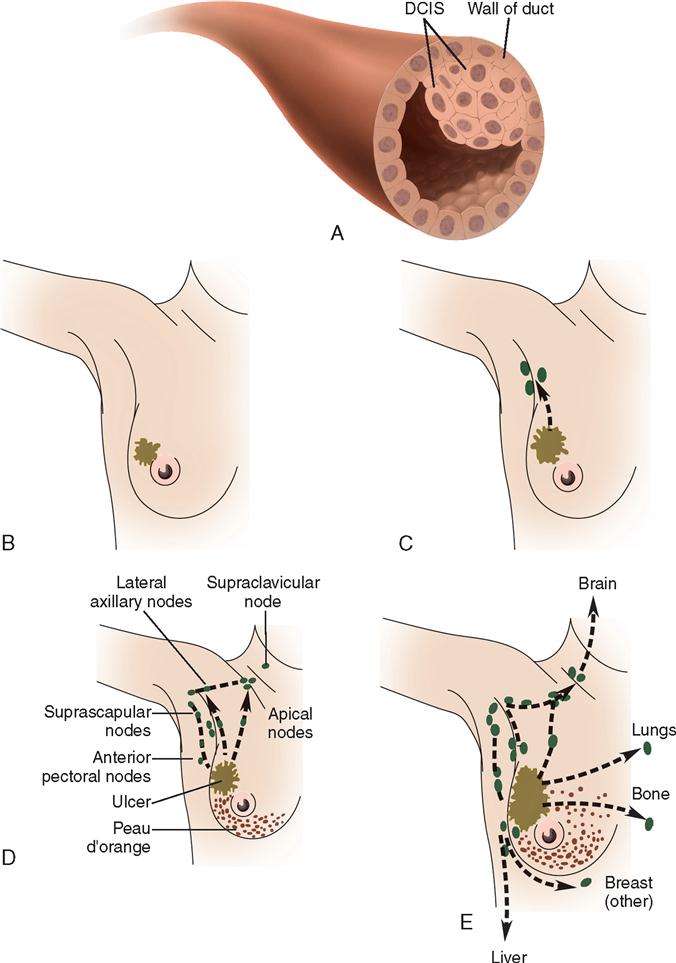
Lymph drainage generally follows the course of the vessels. Lymphatics drain into two main areas represented by the axillary nodes and the internal thoracic chain of nodes (Figure 8-3). The internal thoracic nodes are few, but are responsible for most lymph drainage from the inner half of the breast. Thus the lymph system can also be a channel for the spread of malignant disease from the breast to associated areas of the chest wall or to the axilla.
The breast’s sensory nerve supply is primarily threefold: the anterior cutaneous branches of the upper intercostal nerves, the third and fourth branches of the cervical plexus, and the lateral cutaneous branches of the intercostal nerves.
The mammary glands are affected by three types of physiologic changes: (1) those related to growth and development, (2) those related to the menstrual cycle, and (3) those related to pregnancy and lactation. The mammary glands are present at birth in both males and females. Hormonal stimulation, however, produces the development and function of these glands in females. Estrogen promotes growth of the ductal structures, whereas progesterone promotes lobular development. Occasionally, developmental errors of the breast occur. Additional nipples or extramammary tissue in the axilla or over the upper abdomen may be present. Absence of one or both nipples may also occur and may be associated with absence of the underlying pectoral muscle and chest wall.
BENIGN LESIONS OF THE BREAST
Fibrocystic change in the breast is an all-encompassing term used to describe many different breast changes. Examples of benign lesions that are generally considered when fibrocystic changes are discussed are multiple lesions of fibrous disease, intraductal papillomas, cysts, and solid masses, such as fibroadenomas (Table 8-1). These changes affect almost all women at some time in their lives. Frequently pain is present, which calls attention to the problem. Pain, fluctuations in size, and multiple lesions are common features that help differentiate these generally benign lesions from cancer.
TABLE 8-1
Typical Presentation of Benign Breast Disorders
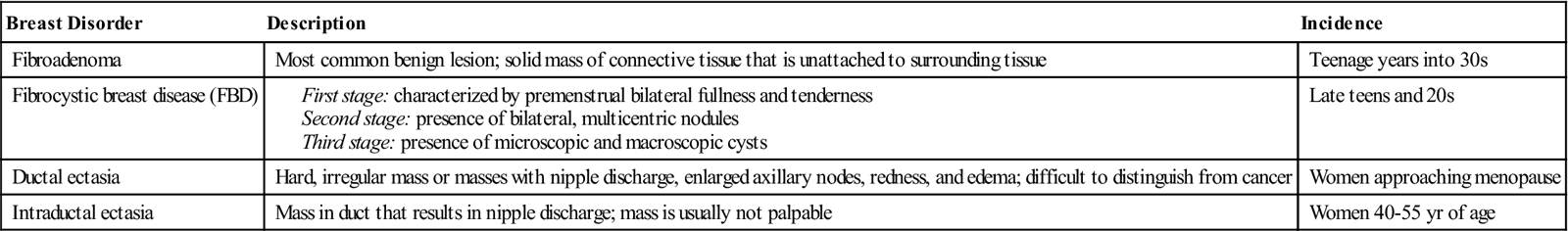
Data from American Cancer Society: Noncancerous breast conditions, 2009, Atlanta, Author; Ignatavicius DD, Workman ML: Medical-surgical nursing: patient-centered collaborative care, ed 6, St Louis, 2010, Saunders.
Nipple discharge is more commonly associated with benign lesions than with cancer. A postmenopausal woman who has some duct ectasia or who has borne children can manually produce nipple discharge. Discharge is usually significant only if it is spontaneous and persistent. Chronic unilateral nipple discharge, especially if bloody, should prompt an investigation for occult carcinoma.
BREAST CANCER
Breast cancer affects primarily women, although it can occur in the mammary gland of men. Until it can be prevented, early detection is the best hope for cure. All women should practice monthly self-examination to detect palpable lesions, and they should immediately report any changes or masses to a physician. External physical changes, such as dimpling of the skin, can also indicate the presence of a benign or malignant pathologic process. The older the patient, the more likely it is that a mass is malignant. The most common form of breast cancer is infiltrating ductal carcinoma (Table 8-2).
TABLE 8-2
Types of Invasive Breast Cancer
| Breast Cancer Type | Percent of Breast Cancers | Specific Features |
| Ductal carcinoma | ≈80 | Can appear as microcalcifications, can vary widely in appearance, can have features of other histologic subtypes of breast cancer |
| Lobular carcinoma | 10-15 | Begins in milk (lobules) glands of breast, high rate of bilaterality, grows as single file of malignant cells around ducts and lobules, poorly defined mass |
| Medullary carcinoma | 1-5 | Forms distinct boundary between tumor tissue and normal tissue, well circumscribed, frequent phenotype of BRCA1 hereditary breast cancer |
| Tubular | 2 | Infrequently metastasizes to lymph nodes, slow growing, long-term survival approaches 100% |
| Inflammatory carcinoma | ≈1 | Rapidly growing, often with metastasis present at diagnosis, first manifestations are breast skin edema and redness and warmth with dimples or ridges |
Data from Imaginis: What is breast cancer? 2008, available at www.imaginis.com/breasthealth/breast_cancer2.asp. Accessed June 1, 2009; Mulholland M et al: Greenfield’s surgery: scientific principles & practice, ed 4, Philadelphia, 2006, Lippincott Williams & Wilkins; Brunicardi FC et al: Schwartz’s principles of surgery, ed 8, New York, 2005, McGraw-Hill.
The cause of breast cancer remains unknown. Many factors, including environmental, dietary (National Cancer Institute, 2007), and familial influences, have been suggested as contributors to its development (Table 8-3). The belief that breast cancer spreads by direct extension from its initial site in the breast to adjacent lymph nodes may not always be correct. Breast cancer may be a systemic condition at the time of diagnosis (History box). Distant metastases may have already occurred without adjacent lymph node involvement at the time of its palpable detection. This could explain why radical breast surgery of the past, which involved removal of the affected breast and all axillary and thoracic lymph nodes, did not greatly lower mortality. Survival from breast cancer is best when detected early, reducing axillary lymph node involvement and improving long-term survival. Tumor size can usually be correlated with involvement of lymph nodes. The larger a tumor is, the more likely it is that lymph nodes are involved.
TABLE 8-3
Risk Factors for Breast Cancer
| Factors | Comments |
| HIGH INCREASED RISK (RELATIVE RISK >4.0) | |
| Female gender | 99% of all breast cancers occur in women. |
| Age >65 yr | Incidence increases with age and peaks in sixth decade. |
| Genetic factors | Inherited mutations of BRCA1 and/or BRCA2 increase risk. |
| Family history | Two or more first-degree relatives with breast cancer at an early age increases risk. |
| History of previous breast cancer | Risk for developing cancer in opposite breast is 5 times greater than average population at risk. |
| Breast density | Dense breasts contain more glandular and connective tissue. |
| MODERATE INCREASED RISK (RELATIVE RISK 2.1-4.0) | |
| Family history | One first-degree relative with breast cancer moderately increases risk. |
| Biopsy-confirmed atypical hyperplasia | Overactive growth of cells increases risk. |
| Ionizing radiation | Women who received frequent low-level radiation exposure to thorax have increased risk, especially if exposure occurred during periods of rapid breast formation. |
| High postmenopausal bone density | High estrogen levels over time both strengthen bone and increase breast cancer risk. |
| LOW INCREASED RISK (RELATIVE RISK 1.1-2.0) | |
| Reproductive history | Childless women have an increased risk, as do women who bear their first child near or at age 30. |
| Menstrual history | Risk for breast cancer rises as interval between menarche and menopause increases. |
| Women who undergo bilateral oophorectomy before age 35 have only 40% of risk for breast cancer compared to women who undergo natural menopause. | |
| Oral contraceptives | There is a slight increase in breast cancer risk in women taking oral contraceptives. |
| Hormone replacement therapy (HRT) | Recent and long-term use of hormone replacement therapy slightly increases risk. |
| Obesity | Postmenopausal obesity (especially increased abdominal fat), increased body mass, insulin resistance, and hyperglycemia have been reported to be associated with increased risk for breast cancer. |
| OTHER RISK FACTORS | |
| Alcohol | The equivalent of two drinks per day may increase risk by 21%. |
| High socioeconomic status | Breast cancer incidence is greater in women of higher education and socioeconomic background. This relationship is possibly related to lifestyle differences, such as age at first birth. |
| Jewish heritage | Women of Jewish Ashkenazic heritage have higher incidences of BRCA1 and BRCA2 genetic mutations. |
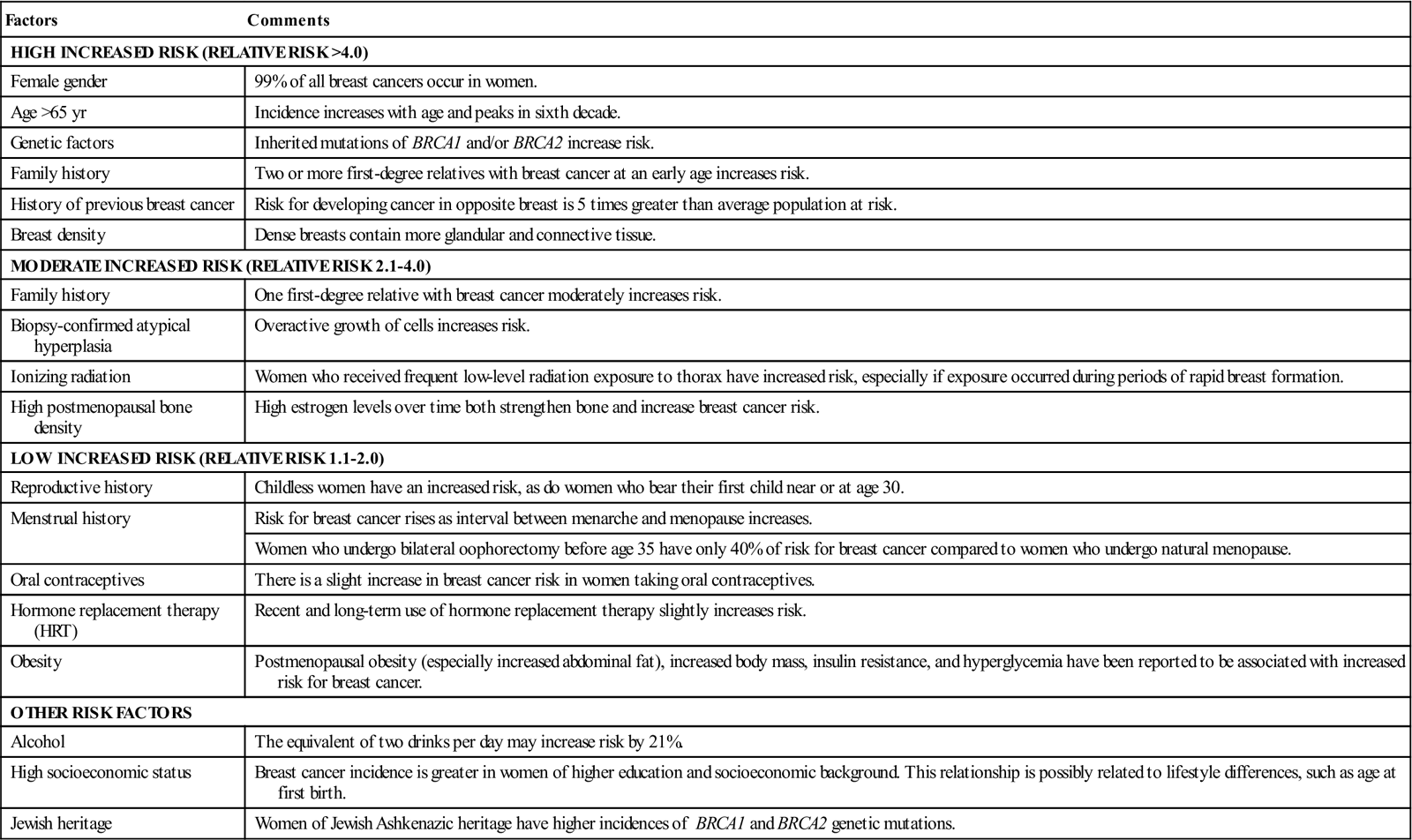
Modified from American Cancer Society: Breast cancer facts and figures 2007-2008, Atlanta, 2009, Author; Ignatavicius DD, Workman ML: Medical-surgical nursing: patient-centered collaborative care, ed 6, St Louis, 2010, Saunders.
Less radical surgery is the treatment of choice today. Surgical excision of the tumor, the use of radiation therapy alone, or a combination of surgery, chemotherapy, and radiation therapy are current treatment recommendations. The use of adjuvant chemotherapy is particularly recommended for premenopausal women with axillary-node metastasis. Studies show that similar therapy can benefit node-negative breast cancer patients. New studies and new therapeutic options are continually being developed and tested. Clinical use of accelerated partial breast irradiation (APBI) is the subject of ongoing clinical trials. In APBI the radiation is focused on the area of greatest risk for tumor recurrence, the lumpectomy site, instead of whole breast irradiation (WBI). Such focused radiation treatment therapies reduce the traditional 6-week radiation treatment plan to 1 week (Fearmonti et al, 2007).
Minimally invasive cryoablation technology to freeze and destroy core biopsy–proven fibroadenomas has been approved by the U.S. Food and Drug Administration (FDA) and is now performed safely in an office setting (Bland et al, 2009). Another breast surgery alternative is focused ultrasound (FUS) with magnetic resonance guidance (MRgFUS), which allows for imaging and tissue temperature monitoring while controlling the FUS beam direction during ablation of breast lesions (Bland et al, 2009).
SCREENING TECHNOLOGIES
Imaging methodologies, such as mammography and ultrasonography, have helped to detect breast masses too small for clinical detection (Hulvat et al, 2009). The American Cancer Society recommends clinical breast exams by a physician every 3 years for women ages 20 to 39 years and annual clinical breast exams and mammograms for women 40 and older (Table 8-4). The American College of Physicians recommends that women who are not at high risk for breast cancer should have regular mammograms starting at age 50. High-risk women, such as those who have a family history of breast cancer or who have detected a lump in the breast, should start annual mammograms at age 40 (Zuckerman, 2007).
TABLE 8-4
American Cancer Society Breast Cancer Screening Guidelines for Asymptomatic Women*
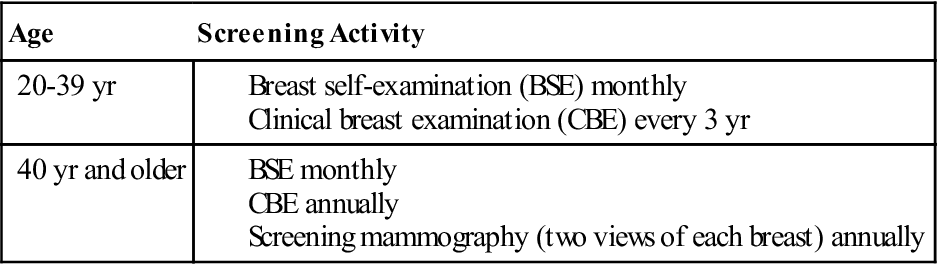
*Asymptomatic women who are identified to be at higher risk need to have an individualized screening plan that may differ from these guidelines.
From American Cancer Society: Breast cancer facts and figures 2007-2008, Atlanta, 2009, Author.
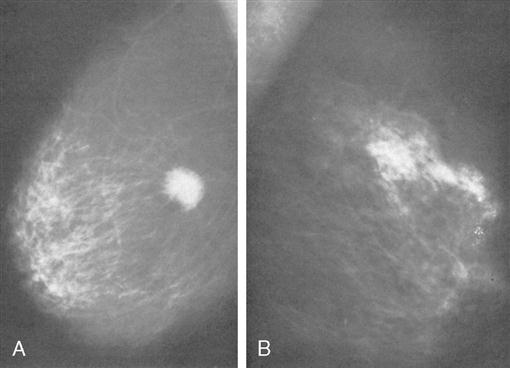
The most common screening mechanism for asymptomatic women is x-ray (film-screen) mammography (Figure 8-4). In mammography, the entire breast is visualized as x-ray beams are directed in several planes through it. Mammograms detect abnormal-appearing densities, irregular or spiculated margins, microcalcifications, and clusters of calcium deposits that are clinically nonpalpable. These masses may be as small as 1 cm in diameter (Mulholland et al, 2006). Often, previous mammograms are used for comparison. Screening mammography has led to identification of more nonpalpable breast masses. The accuracy of mammography depends on careful x-ray technique and breast size, structure, and density. Radiation dosage varies with individuals and techniques. As a result of improved radiologic techniques, radiation exposure in a mammogram is very low. The benefits of this screening mechanism far outweigh the minute risks of radiation exposure. Advances in computer-assisted detection allow the computer to analyze the mammogram, placing asterisks and triangles on small potential problem areas, which a radiologist then reviews. If screening mammography reveals a suspicious area, the patient is asked to return for diagnostic mammography (explained in the following paragraphs).
Digital mammography takes an electronic image of the breast and stores it directly in a computer. Digital mammography uses less radiation than film mammography and improves image storage and transmission, as images are stored and sent electronically. Radiologists also can use software to interpret digital mammograms. One obstacle to greater use of digital mammography is its cost, currently about 1.5 to 4 times more than film systems. Digital screening mammograms are more accurate than film screening mammograms, with a 70% detection rate compared with 55% for film screens. Women most likely to benefit from digital screening are those younger than 50 with dense breast tissue and who are premenopausal or perimenopausal (Hulvat et al, 2009).
When there is a palpable mass or other abnormality identified on screening mammography, a diagnostic imaging evaluation is done (Mikula, 2008). Additional diagnostic mammographic views are obtained, and the radiologist assigns a BI-RADS (Breast Imaging Reporting and Data System) score (Box 8-1). An ultrasound may then be ordered.
Ultrasonography differentiates between solid and cystic lesions. As a screening methodology, its sensitivity and specificity are less definitive than mammography. Ultrasonography can be useful with dense or dysplastic breasts and in pregnant or lactating women.
Magnetic resonance imaging (MRI) is another technique used as an adjunct to mammography in the detection of breast lesions. Breast MRI can image dense breast tissue, which shows poorly with conventional mammography. There is interest in the use of MRI to improve selection for breast-conserving therapy (BCT). In 2007 the American Cancer Society recommended that MRI be used for women at high risk. Such women include those who have known breast cancer–associated BRCA1 or BRCA2 gene mutations, have a first-degree relative with a BRCA1 or BRCA2 gene mutation, have a lifetime risk of breast cancer of 20% to 25% or greater according to risk assessment tools, had radiation therapy to the chest between 10 and 30 years of age, or have certain rare syndromes. The 2008 study by Martinez-Cecilia found that preoperative MRI improved tumor staging and changed the surgical approach for 13% of study patients; MRI discovered an additional malignant lesion in 8% of patients, and showed a tumor larger than originally believed in 16 patients, altering the planned surgery.
Molecular breast imaging (MBI) involves injection of a short-lived radiotracer, which is absorbed by breast tissue and preferentially so by breast tumors. MBI is especially useful in detecting breast cancer in women with dense breasts and who are at higher risk of developing the disease. This new technique has shown good promise as a helpful adjunct to screening mammography (U.S. Department of Health and Human Services, 2008).
Positron emission tomography (PET) scans with the glucose analog 2 are also being used for breast cancer detection. This scan is about 88% accurate for large breast tumors. For lesions less than 1 cm, the sensitivity of PET is 57% (Hulvat et al, 2009).
DNA-based genetic testing for BRCA1 and BRCA2 is not reommended for women without family histories that suggest risk for these gene mutations. A recent meta-analysis estimated that the lifetime risk for breast cancer was 47% to 66% in carriers of BRCA1 and 40% to 57% in carriers of BRCA2. Other studies have shown even higher estimates. Managing breast cancer risk for women with these mutations includes frequent screening and prophylactic surgeries (Nelson, 2009).
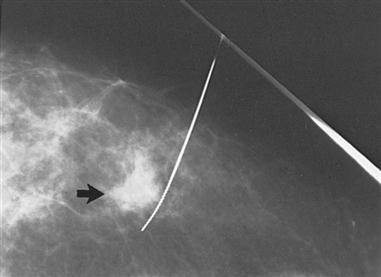
In some instances, such as when a lesion previously detected by mammogram is too small to palpate, mammograms are repeated immediately before surgery. The lesion is localized by the insertion of a needle or a wire within a needle (the procedure is often referred to as “needle” or “wire” localization). The wire is placed within the suspect area, and the distal end is left on the outside of the skin. The needle may be left in place or removed after insertion of the wire (Figure 8-5). The needle or wire is then taped in place, and the patient sent to the OR for surgical biopsy. Wire localization and surgical biopsy occur on the same day. After biopsy, the specimen is sent to the radiology department for mammographic validation of correct surgical excision of the questionable breast tissue. The tissue is then sent for pathologic examination.
DIAGNOSTIC TECHNIQUES
Once a mass is identified, the physician has multiple techniques to establish a diagnosis. During a fine needle aspiration biopsy (FNAB), the physician anesthetizes a small area of the breast with lidocaine. A 22- or 25-gauge needle attached to a 20-ml syringe is inserted into the mass, and a small amount of the contents is aspirated. Cytologic examination of the aspirate can assist in microscopic evaluation of the mass. FNAB yields greater diagnostic accuracy if the physician has been thoroughly trained in the technique. In a review of 31,340 procedures, FNAB sensitivity ranged from 65% to 98%. False-positive rates were less than 1%. As diagnosis of malignancy by FNAB is extremely reliable, treatment options can then be discussed with the patient and definitive surgery performed without the need for a surgical biopsy (Mulholland et al, 2006).
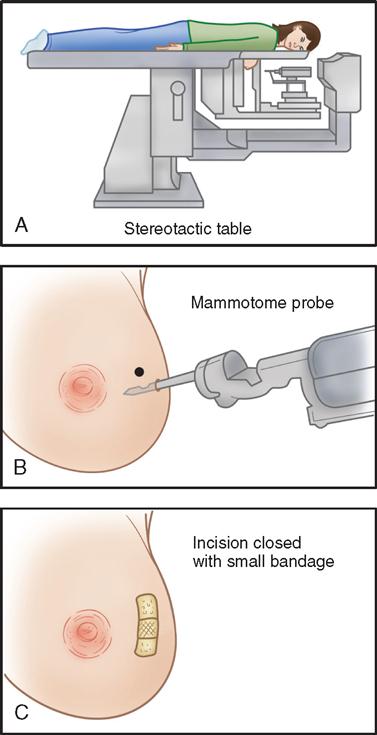
Advances in instrumentation now allow for simultaneous biopsy and removal of mammographic densities that are up to 20 mm in size using digital stereotactic imaging and minimally invasive instruments to locate and remove tissue for diagnosis. The patient is assessed preoperatively for neck or back problems. In addition, the patient should not be receiving anticoagulant therapy. Masses located near the patient’s areola, high in the axilla, or near the chest wall are not appropriate for this technique. The patient is placed on a specially designed table (Figure 8-6) in the prone position with the affected breast through the table’s 10-inch aperture to the work area below. The patient’s head is turned away from the physician. Padding is placed under bony prominences to improve comfort. When the suspicious area in the breast is located through stereotactic imaging, its coordinates are transferred to the table’s automated instrument. After preparing the skin with an antiseptic solution while the patient is administered a local anesthetic, the physician makes a small incision in the breast. The physician positions the disposable device to remove the identified tissue. Additional biopsies can be made if indicated, and coagulation can be used if necessary. A titanium vessel clip can be placed at the base of the biopsy specimen as a point of reference for future evaluations. A postoperative dressing is then applied to the area. The benefits to the patient include small incisions for cosmetic results, decreased disfigurement, shortened time between detection and diagnosis, and elimination of the need for more involved surgical intervention (Stephan, 2009).
SURGICAL TREATMENT OPTIONS
Surgical treatment ranges from minimally invasive breast biopsy, lumpectomy, and wide excision of the tumor mass; to modified radical mastectomy involving the breast and axillary lymph nodes; to salpingo-oophorectomy (Research Highlight). The goal of surgery is removal of the cancerous mass with a margin of normal tissue and a good cosmetic result. When a specimen of breast tissue is sent to the laboratory, it is inked to mark the relationship between the tumor and the surgical margins of the excision. The pathologist evaluates these margins on all sides of the tumor for malignant cells. If a margin is positive, it indicates that malignant cells may remain in the breast. Additional surgery is usually required until the margins contain only normal tissue (Re-excision for Close Margins, 2008).
The choice of procedure depends on the size and site of the mass, the characteristics of the cells, the stage of the disease, and the patient’s choice. A breast cancer diagnosis is usually staged to measure the extent of the disease and to classify patients for possible treatment modalities (Figure 8-7). The TNM (T = tumor; N = node; M = metastasis) classification has been adopted as a mechanism to clinically stage this disease. Staging results are used in designing a specific treatment plan. Radiation therapy, chemotherapy, or hormonal therapy may be used with surgery or as alternative treatment methods. An intravenous (IV) access port may be surgically placed for later infusion of chemotherapeutic drugs, fluids, or nutrition as well as to withdraw blood and laboratory specimens (Ambulatory Surgery Considerations).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree