Thyroid and Parathyroid Surgery
LEARNING OBJECTIVES
After studying this chapter the reader will be able to:
• Identify anatomy relevant to surgery of the thyroid and parathyroid glands
• Correlate physiology to disease states requiring surgical intervention
• Identify procedural considerations
• Identify tissue layers involved in opening and closing sequences of thyroid and parathyroid surgery
• Identify specific instrumentation and supplies applicable to thyroid and parathyroid surgery
• List pharmacologic and hemostatic agents utilized in thyroid and parathyroid surgery
• Compare instruments and equipment used in open, laparoscopic, and endoscopic procedures
Overview
The first documented thyroid surgery occurred in the twelfth century (History box). Today surgeons continue to perform surgical procedures on the thyroid gland for various underlying causes. Surgery ranges from lobectomy and isthmectomy for papillary microcarcinomas (lesions smaller than 1 cm) to near-total and total thyroidectomies for symptomatic multinodular goiters. Technologic advances have added new approaches to thyroid and parathyroid surgery, such as minimally invasive surgery (MIS) techniques.
Surgical Anatomy
Thyroid Gland
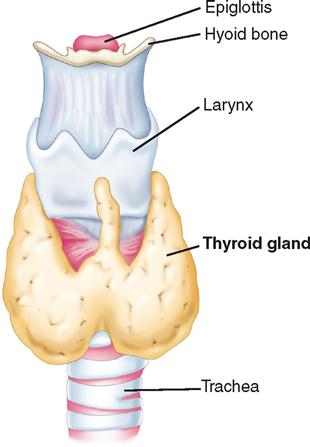
The thyroid gland (Figure 7-1) is a highly vascular organ that lies in the anterior portion of the neck, deep to the paired strap muscles, resting on the midline of the trachea. It is attached to the cricoid and trachea by the ligament of Berry. The inferior border of the thyroid varies and may be located above or below the sternal notch. It consists of right and left lobes united by a middle portion, the isthmus, and it weighs approximately 20 g. The isthmus is situated near the base of the neck, between the second and fourth tracheal rings, and the lobes lie beside the larynx, trachea, and esophagus. The pyramidal lobe, a long, thin projection of thyroid tissue protruding cephalad from the isthmus, is found in about 30% of patients at surgery; it is the vestige of the embryonic thyroglossal duct and migrates from the foramen cecum at the base of the tongue. If the migratory tract fails to degenerate, a fistula or cyst may be present (Loevner and Mukherji, 2008).
Blood supply to the thyroid is from the superior thyroid artery, which originates from the external carotid artery, and from the inferior thyroid artery, which originates from the thyrocervical trunk of the subclavian artery. The points at which these two arteries enter the thyroid gland are referred to as “poles” of the thyroid and are important anatomic landmarks during surgery. The thyroid gland is drained by three pairs of veins (superior, middle, and inferior thyroid veins). Occasionally a single thyroid artery (thyroidea ima) may arise directly from the arch of the aorta or the innominate artery and rise in front of the trachea to enter the midline of the gland inferiorly.
The recurrent laryngeal nerve, a branch of the vagus nerve, innervates the intrinsic muscles of the larynx. The right recurrent laryngeal nerve loops under the subclavian artery and ascends in an oblique direction lateral to the tracheoesophageal groove. The recurrent laryngeal nerve contains both motor and sensory fibers. The motor component innervates the abductor muscles of the true vocal cords. The sensory component supplies sensation to the larynx below the vocal cords. During surgery, care is taken to identify and protect this nerve. Immediate hoarseness occurs if the nerve is divided on one side. If the recurrent nerve is injured bilaterally, acute paralysis of both vocal cords may obstruct the airway and require emergency tracheotomy. Injury to the external branch of the superior laryngeal nerve, which innervates the cricothyroid muscle, results in difficulty shouting or singing high notes.
The thyroid gland produces three hormones: thyroxine (T4) and triiodothyronine (T3) (together known as the thyroid hormones [TH]) and calcitonin. T3 and T4 cannot be synthesized without iodine. Calcitonin increases calcium storage in the bone and decreases blood calcium levels. The primary function of thyroid hormones is to regulate energy metabolism, but they also play an important role in growth and development. Thyroid-stimulating hormone (TSH) is synthesized by the anterior pituitary, and stimulates the production and release of thyroid hormones and the uptake of iodine.
Parathyroid Gland
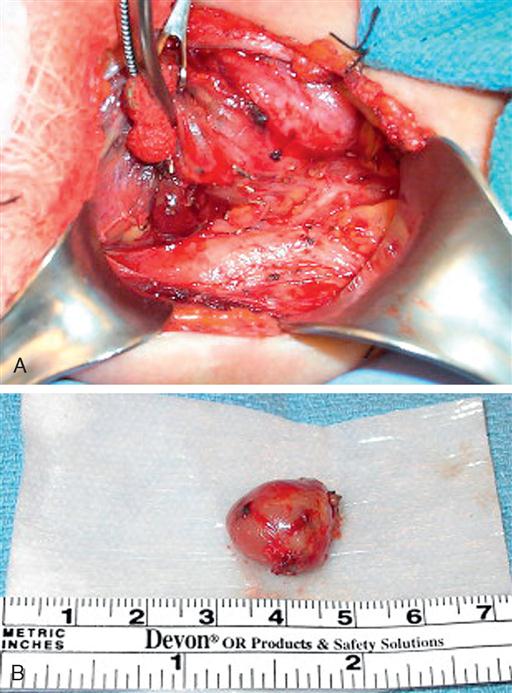
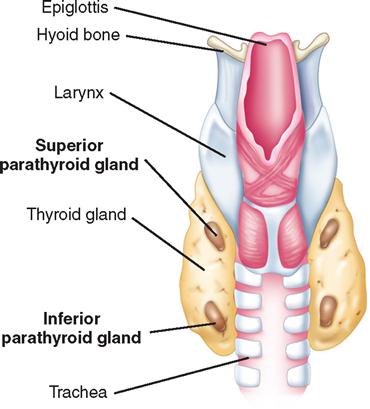
The parathyroid glands usually consist of four small, mahogany-to-yellow, flat, ovoid masses (Figure 7-2) (Gauger and Doherty, 2004). Normal parathyroid glands are generally small, lobular, and yellow in color, about the size of a lentil bean. It can be challenging to differentiate the gland from a lobule of fat, a thyroid nodule, or lymph node. Thyroid adenomas generally, although not always, tend to be larger, asymmetric, and more of a red-brown hue than normal parathyroids (Figure 7-3). The upper pair of glands lies in proximity to the posterior portion of the superior pole of the thyroid; the lower pair usually (about 60% of the time) lies near the posterior lateral aspect of the lower pole of the thyroid. Ectopic parathyroid glands, more common in the inferior parathyroid glands, may be found in the superior mediastinum, especially within the thymus and as low as the pericardium. Parathyroids may also be buried under the strap muscle, in the carotid sheath, or embedded in the thyroid beneath the capsule (Loevner and Mukherji, 2008). Each parathyroid gland measures approximately 3 mm × 3 mm × 3 mm and generally weighs less than 50 mg; the upper glands are generally smaller than the lower ones. Both the upper and lower parathyroid glands receive their blood supply from the inferior thyroid artery. As the blood supply to the superior parathyroid stems from that of the supply to the inferior parathyroid, disrupting supply to the inferior parathyroid necessarily compromises the supply to the upper parathyroid.
The parathyroid glands secrete parathyroid hormone (PTH), an antagonist to calcitonin. Both PTH and calcitonin work together to maintain calcium homeostasis by increasing calcium removal from storage in bone and increasing absorption of calcium by the intestines.
Surgical Technologist Considerations
Surgery on the neck is performed with the patient in supine position and the head on a specific headrest. Large thyroid nodules may cause a tracheal shift and present a challenge to the anesthesia provider for intubation and airway management. It may be prudent to have the “difficult airway” cart available.
Grossly enlarged thyroid gland may be more difficult to excise, and intraoperative bleeding is more common and profuse. Hemorrhage may cause tracheal compression and airway compromise. It is wise for the surgical technologist to have a tracheal tray in the operating room for this reason.
General surgery instruments are used for neck surgery, with the addition of special instruments from both vascular and otorhinolaryngology sets. The surgical technologist should also have vessel loops, suture boots, and a nerve stimulator for the procedure.
There may be several specimens on these procedures. When setting up, the scrub should have several containers to keep the specimens separated and labels to properly identify each specimen as it is handed off to the circulator.
Thyroid Assessment.
In addition to palpation of the thyroid gland for size, contour, consistency, lymph nodes, fixation, tenderness, and bruits (Wilson and Giddens, 2009), scans are used to clarify thyroid anatomy. CT and MRI scans are used to assess a thyroid with suspected malignancy for encroachment of the tumor outside the thyroid capsule into the adjacent tissue of the neck. Scans are the most effective means to evaluate local invasion of surrounding tissue.
While palpable thyroid nodules are present in 3% to 5% of healthy adults, ultrasound and autopsy studies show rates of more than 50%. Although most nodules are benign, 7% to 29% will be cancerous. Consequently, the most common endocrine malignancy is thyroid cancer, estimated to be the thirteenth most common cause of any new malignant neoplasm in the United States for 2007, ranking seventh in women (25,480 cases; 3.8%), while causing just over 1% of new cases in men. Prevalence figures are likely to increase further, as the yearly incidence of thyroid cancer has been growing exponentially from 7800 cases in 1974 to 33,550 cases in 2007 (Tuttle, 2007). Thus thyroid cancer is poised to become one of the most common cancer diagnoses in living cancer patients (Grebe, 2009).
PREOPERATIVE TESTING
Thyroid Isotope Scan (Thyroid Scintigraphy).
A thyroid nodule, the most common endocrine problem in the United States, is any abnormal growth of thyroid cells. Most nodules are discovered during routine physical examination, because they rarely are symptomatic. Imaging of the normal thyroid with radionuclide agents shows normal size, shape, position, and function of the thyroid, with no areas of decreased or increased uptake. Nodules that are hot demonstrate increased uptake of radionuclide agent; this may indicate benign adenoma or localized toxic goiter. Cold nodules are hypofunctioning or nonfunctioning and may indicate cyst, nonfunctioning adenoma or goiter, lymphoma, localized area of thyroiditis, or carcinoma. Two commonly used agents in thyroid imaging are radioactive iodine and technetium 99m-pertechnetate (99mTcO4−).
Ultrasonic Scan.
Ultrasonic scans are the treatment of choice to evaluate thyroid nodules and are useful to determine the size and number of nodules within the thyroid as well as to monitor size progression during follow-up. Involvement of the vessels in the neck or mediastinum, although rare, can occur. It is critical that the surgeon and operative team know this before surgery. Radiologic evaluation frequently dictates the extent of the surgery. Several new ultrasonic techniques have been applied to the thyroid. The three-dimensional (3D) ultrasound has proven to be more accurate. Tissue harmonic imaging (THI) uses the first harmonic of the transmitted frequency for image formation and results in images with less “noise.” These new techniques improve image clarity but do not improve lesion characterization.
Ultrasonography also helps to facilitate accurate needle placement and sampling of the target nodule during fine needle aspiration (FNA). Ultrasound-guided FNA with cytologic evaluation remains the mainstay for palpable and incidentally found thyroid nodules (Loevner and Mukherji, 2008).
Fine Needle Aspiration.
FNA is the most useful diagnostic tool currently available in the management of thyroid nodules. It is the accepted standard of care for all patients who have palpable nodules with normal functioning thyroids, has high accuracy and low risk, and is cost-effective (Lansford and Teknos, 2006). The procedure is usually performed under ultrasound guidance, but may be performed in the surgeon’s office. Several samples are usually taken from various parts of the nodule. Pathologic examination of FNA specimens reveals a benign nodule in 70% of biopsies, a nondiagnostic or inadequate biopsy 15% of the time, a suspicious nodule in 10% of cases, and malignancy in 5% of biopsies (American Thyroid Association, 2005). A suspicious nodule usually implies a follicular neoplasm, which can be benign or malignant, and requires removal for histologic evaluation.
Patient preparation for FNA should include the following information:
♦ Local anesthesia may or may not be used.
♦ Once the prick of the needle is felt, no talking, swallowing, or moving is allowed.
FNA may be an emotionally stressful procedure for the patient. Vials of ammonia should be kept in the room in case the patient feels faint. Lowering the head (using Trendelenburg’s position or sitting with the head down) is also important to treat vasovagal syncope. Postprocedure education should include (1) the necessity of refraining from using aspirin or aspirin-containing medications or nonsteroidal antiinflammatory drugs (NSAIDs) for the next 24 hours, and (2) the expectation of seeing a half-dollar–size bruise at the FNA site. There are no restrictions on food or activity.
Parathyroid Assessment.
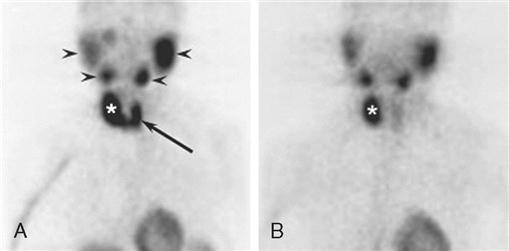
Elevation of serum PTH level in association with hypercalcemia is diagnostic of hyperparathyroidism in most cases. PTH normal levels are between 10 and 65 pg/ml (values vary with specific laboratory); the concentration of serum ionized calcium (4.5 to 5.6 mg/dl) is usually measured at the same time (Pagana and Pagana, 2009). Causes of primary hyperparathyroidism are single adenoma (80% to 85%), four-gland hyperplasia (10% to 15%), and rarely parathyroid carcinoma (less than 1%). Preoperative localization studies with a parathyroid sestamibi scan (Figure 7-4) or high-resolution ultrasonography can identify parathyroid adenomas with high sensitivity (70% to 80%). Sensitivity decreases significantly for hyperplasia (less than 50%).
Hyperparathyroidism causes an increase in the level of serum calcium and a decrease in the level of serum phosphate. Nursing diagnoses and plans of care are based on these imbalances and the severity of associated symptoms. Some patients are asymptomatic while others have symptoms that manifest themselves as disturbances in the central nervous system or cardiovascular, renal, gastrointestinal, or musculoskeletal system (Box 7-1).
Assessment includes determining whether the patient is apathetic or emotionally irritable; whether there is muscle weakness and atrophy, back or joint pain, nausea, vomiting, constipation, peptic ulcer disease, or cardiac dysrhythmia; and whether there is renal damage, stones, or disease (Canobbio, 2006). If any of these are present, the plan of care is adjusted. Otherwise, perioperative patient education and nursing management of the patient undergoing parathyroidectomy are essentially the same as those for thyroidectomy. In the early postoperative period for both thyroidectomy and parathyroidectomy, the patient is closely observed for any signs of hypocalcemia. The serum calcium level reaches its lowest level in 48 to 72 hours after surgery and returns to normal within the following 2 to 3 days. Symptoms include numbness and tingling of extremities and around the lips. Hyperactive tendon reflexes and a positive Chvostek’s sign (tapping on the facial nerve elicits contraction of facial muscles) can be demonstrated on physical examination. Tetany may develop and is exhibited by carpopedal spasms, tonic-clonic convulsions, and laryngeal stridor, which can be fatal.
Planning
The entire surgical team has mutual accountability for patient safety (Banschbach, 2008). The perioperative nurse and surgical technologist should know the OR team members who will participate in the procedure as well as the roles each team member will assume (Haynes et al, 2009). Careful planning, assessment, and tailoring the plan of care to the surgery and specific patient needs reduce inherent risks (Risk Reduction Strategies). The surgical team should consider a patient who is not at optimal weight at high risk for pressure ulcer development and plan on padding pressure areas to prevent skin and tissue damage. Intraoperatively, warm saline should be provided for irrigation and a forced-air warming device used. Patients with hypothyroidism are at increased risk of becoming hypothermic (Hegarty et al, 2009).
A rapidly progressive and potentially fatal complication for patients with hyperthyroidism is thyroid storm (thyrotoxic crisis) (Kuwajerwala et al, 2007). The perioperative staff must recognize and differentiate between uncomplicated thyrotoxicosis and thyroid storm (Box 7-2) and be prepared to act quickly. The use of a rapid response team (RRT) for patients in crisis impacts treatment and patient outcomes (Rapid Response Team box) (Steel and Reynolds, 2008). Thyroid storm can occur in patients who have been partially controlled or whose hyperthyroidism is untreated. Thyrotoxic crisis can be precipitated by a stressful event, such as surgery. By planning a quiet, calm atmosphere and helping the patient relax, the perioperative staff can reduce the risk of thyroid storm. Collaborating with the surgical and anesthesia team, the perioperative staff can plan for appropriate interventions to assist in reducing body temperature and heart rate, provide oxygen and intravenous solutions, and administer medications as prescribed in the event thyrotoxic crisis occurs (Surgical Pharmacology).