NEOPLASTIC DISEASES
BLADDER CANCER
 Definition
Definition
 Cancer arising in the urinary bladder is a carcinoma of urothelial (transitional cell) origin in the United States and Europe (90% of cases). Less frequently, urothelial carcinomas may originate in the renal pelvis, ureter, or urethra. In other parts of the world, bladder carcinomas of nonurothelial origin are more common.
Cancer arising in the urinary bladder is a carcinoma of urothelial (transitional cell) origin in the United States and Europe (90% of cases). Less frequently, urothelial carcinomas may originate in the renal pelvis, ureter, or urethra. In other parts of the world, bladder carcinomas of nonurothelial origin are more common.
 Who Should Be Suspected?
Who Should Be Suspected?
 Suspected patients are older than age 40 years, more commonly males with a history of cigarette smoking, who present with hematuria (painless, intermittent, grossly visible, and present throughout micturition), or irritative voiding symptoms (frequency, urgency, dysuria) that suggest carcinoma in situ (CIS) of the bladder.
Suspected patients are older than age 40 years, more commonly males with a history of cigarette smoking, who present with hematuria (painless, intermittent, grossly visible, and present throughout micturition), or irritative voiding symptoms (frequency, urgency, dysuria) that suggest carcinoma in situ (CIS) of the bladder.
 The association of pain with bladder cancer (located to the flank; suprapubic, hypogastric, and perineal; abdominal or right upper quadrant areas; bone pain; or headache/disordered cognitive function) can be signs of locally advanced or metastatic disease. Constitutional symptoms (fatigue, weight loss, anorexia, failure to thrive) are usually signs of advanced or metastatic disease and carry a poor prognosis.
The association of pain with bladder cancer (located to the flank; suprapubic, hypogastric, and perineal; abdominal or right upper quadrant areas; bone pain; or headache/disordered cognitive function) can be signs of locally advanced or metastatic disease. Constitutional symptoms (fatigue, weight loss, anorexia, failure to thrive) are usually signs of advanced or metastatic disease and carry a poor prognosis.
 The definitive diagnosis and staging of bladder cancer are by cystoscopy, beginning with a baseline evaluation of the bladder and uninvolved mucosa to record the number, size, location, appearance, and growth type (papillary or solid) of all lesions observed. Visible lesions can be biopsied or resected for histologic analysis.
The definitive diagnosis and staging of bladder cancer are by cystoscopy, beginning with a baseline evaluation of the bladder and uninvolved mucosa to record the number, size, location, appearance, and growth type (papillary or solid) of all lesions observed. Visible lesions can be biopsied or resected for histologic analysis.
 Laboratory Findings
Laboratory Findings
 Urinalysis: A positive dipstick test (detecting one to two red cells per highpower field [HPF]) should be confirmed by microscopic analysis (below). Infection should be ruled out by a urine culture prior to further workup of hematuria.
Urinalysis: A positive dipstick test (detecting one to two red cells per highpower field [HPF]) should be confirmed by microscopic analysis (below). Infection should be ruled out by a urine culture prior to further workup of hematuria.
 Urine sediment: Hematuria is significant if there are greater than three red cells per HPF, present throughout micturition. The presence of dysmorphic red cells or casts suggests a glomerular origin, whereas normally formed red cells likely originate from infections, tumors, or obstructions/calculi. The specimen should be maintained at room temperature and examined within 30 minutes of collection.
Urine sediment: Hematuria is significant if there are greater than three red cells per HPF, present throughout micturition. The presence of dysmorphic red cells or casts suggests a glomerular origin, whereas normally formed red cells likely originate from infections, tumors, or obstructions/calculi. The specimen should be maintained at room temperature and examined within 30 minutes of collection.
 Urine cytology: Urine cytologic analysis by fluorescence in situ hybridization (e.g., UroVysion™ FISH) can be a useful noninvasive aid both in the primary diagnosis of urothelial carcinoma and in monitoring tumor recurrence (occurring in about 70% of cases after initial treatments). UroVysion™ FISH is designed to detect certain numerical chromosomal abnormalities commonly associated with urothelial carcinoma (either amplifications of chromosomes 3, 7, and 17 or deletions of the 9p21 locus).
Urine cytology: Urine cytologic analysis by fluorescence in situ hybridization (e.g., UroVysion™ FISH) can be a useful noninvasive aid both in the primary diagnosis of urothelial carcinoma and in monitoring tumor recurrence (occurring in about 70% of cases after initial treatments). UroVysion™ FISH is designed to detect certain numerical chromosomal abnormalities commonly associated with urothelial carcinoma (either amplifications of chromosomes 3, 7, and 17 or deletions of the 9p21 locus).
 Urine biomarkers: Several urine-based biomarkers have been approved for diagnosis or surveillance of patients with a history of the disease. However, their sensitivity is low, and their use is not recommended for an initial workup of a suspected case.
Urine biomarkers: Several urine-based biomarkers have been approved for diagnosis or surveillance of patients with a history of the disease. However, their sensitivity is low, and their use is not recommended for an initial workup of a suspected case.
 Limitations on Interpretation of the UroVysion™ FISH Test for Bladder Cancer
Limitations on Interpretation of the UroVysion™ FISH Test for Bladder Cancer
 A positive result in the absence of clinical evidence of urothelial bladder cancer may indicate urothelial malignancies of other organs along the GU tract (kidney, ureter, prostate, or urethra).
A positive result in the absence of clinical evidence of urothelial bladder cancer may indicate urothelial malignancies of other organs along the GU tract (kidney, ureter, prostate, or urethra).
 A negative result in the presence of other signs or symptoms of urothelial carcinoma may suggest a false-negative test.
A negative result in the presence of other signs or symptoms of urothelial carcinoma may suggest a false-negative test.
Suggested Readings
Getzenberg RH. Urine-based assays for bladder cancer. Lab Med. 2003;34:613–617.
Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003;61:109–118.
PROSTATE CANCER
 Definition
Definition
 Prostate cancer is an adenocarcinoma of the prostate gland, most commonly occurring in the peripheral zone. There is a close association of the cancer with small clumps of cancer cells—carcinoma in situ or prostatic intraepithelial neoplasia (PIN)—although it has not been proven that PIN is the cancer precursor.
Prostate cancer is an adenocarcinoma of the prostate gland, most commonly occurring in the peripheral zone. There is a close association of the cancer with small clumps of cancer cells—carcinoma in situ or prostatic intraepithelial neoplasia (PIN)—although it has not been proven that PIN is the cancer precursor.
 Prostate cancer is generally so indolent that most men die of other causes before the disease becomes clinically advanced. However, globally it is the sixth leading cause of cancer deaths in men (second leading cause in the United States and first in the United Kingdom).
Prostate cancer is generally so indolent that most men die of other causes before the disease becomes clinically advanced. However, globally it is the sixth leading cause of cancer deaths in men (second leading cause in the United States and first in the United Kingdom).
 Who Should Be Suspected?
Who Should Be Suspected?
 Prostate cancer tends to develop in men over age 50. In the early stage of the disease, most men have no symptoms directly linked to the cancer, but because the gland surrounds the prostatic urethra, changes in urinary function can occur with disease progression.
Prostate cancer tends to develop in men over age 50. In the early stage of the disease, most men have no symptoms directly linked to the cancer, but because the gland surrounds the prostatic urethra, changes in urinary function can occur with disease progression.
 As a presenting symptom, a change in urinary function (frequency, urgency, nocturia, hesitancy) is the most common, but benign prostatic hyperplasia (BPH) figures into the differential diagnosis and is usually the cause.
As a presenting symptom, a change in urinary function (frequency, urgency, nocturia, hesitancy) is the most common, but benign prostatic hyperplasia (BPH) figures into the differential diagnosis and is usually the cause.
 Hematuria and hematospermia are uncommon symptoms but, if present, also are more likely to be caused by BPH. However, if occurring in older men, prostate cancer should be included in the differential diagnosis.
Hematuria and hematospermia are uncommon symptoms but, if present, also are more likely to be caused by BPH. However, if occurring in older men, prostate cancer should be included in the differential diagnosis.
 Bone pain, often in the vertebrae, pelvis, or ribs, if present, would indicate metastatic disease.
Bone pain, often in the vertebrae, pelvis, or ribs, if present, would indicate metastatic disease.
 Early Detection
Early Detection
 The two methods for early detection of suspected prostate cancer are the digital rectal examination for asymmetric areas of induration or nodules on the posterior and lateral aspects of the prostate gland and measurement of serum prostate-specific antigen (PSA). About 20% of early detections occur through a suspicious digital rectal examination and the remaining 80% through a suspicious PSA test. A definitive diagnosis of prostate cancer by either method of early detection is established by a positive biopsy.
The two methods for early detection of suspected prostate cancer are the digital rectal examination for asymmetric areas of induration or nodules on the posterior and lateral aspects of the prostate gland and measurement of serum prostate-specific antigen (PSA). About 20% of early detections occur through a suspicious digital rectal examination and the remaining 80% through a suspicious PSA test. A definitive diagnosis of prostate cancer by either method of early detection is established by a positive biopsy.
 Screening of unsuspected cases for prostate cancer via the PSA test is controversial. Because of the low specificity of elevated PSA levels for prostate cancer versus BPH or prostatitis, the benefits of screening are outweighed by the harms of unnecessary treatment. Screening is not recommended by the U.S. Preventive Services Task Force (Grade “D,” 2012) and the Centers for Disease Control and Prevention. The American Society of Clinical Oncology and the American College of Physicians discourage screening in those expected to live less than another 10–15 years. The American Urological Association recommends shared decision making in those from age 55 to 69 and no more often than every 2 years.
Screening of unsuspected cases for prostate cancer via the PSA test is controversial. Because of the low specificity of elevated PSA levels for prostate cancer versus BPH or prostatitis, the benefits of screening are outweighed by the harms of unnecessary treatment. Screening is not recommended by the U.S. Preventive Services Task Force (Grade “D,” 2012) and the Centers for Disease Control and Prevention. The American Society of Clinical Oncology and the American College of Physicians discourage screening in those expected to live less than another 10–15 years. The American Urological Association recommends shared decision making in those from age 55 to 69 and no more often than every 2 years.
 Laboratory Findings
Laboratory Findings
 PSA testing: PSA levels normally correlate with age and prostate size, averaging 1 ng/mL for men under age 50 and 3 ng/mL for men over age 60. A value of 4.0 ng/mL is widely used as a cutoff for prostate cancer. There are two effective methods of enhancing the specificity of the PSA test—use of an age-based reference range and calculation of the free versus total PSA ratio.
PSA testing: PSA levels normally correlate with age and prostate size, averaging 1 ng/mL for men under age 50 and 3 ng/mL for men over age 60. A value of 4.0 ng/mL is widely used as a cutoff for prostate cancer. There are two effective methods of enhancing the specificity of the PSA test—use of an age-based reference range and calculation of the free versus total PSA ratio.
 Age-based reference range: A PSA reference range based on age should be calculated for each laboratory performing PSA testing.
Age-based reference range: A PSA reference range based on age should be calculated for each laboratory performing PSA testing.
 PSA free versus total ratio: The risk of prostate cancer is increased if the ratio of free to total PSA is <25%.
PSA free versus total ratio: The risk of prostate cancer is increased if the ratio of free to total PSA is <25%.
 PSA velocity: An annual rate of change in the PSA level >2.0 ng/mL, while not an effective screening test, offers value in assessing preoperative mortality risk.
PSA velocity: An annual rate of change in the PSA level >2.0 ng/mL, while not an effective screening test, offers value in assessing preoperative mortality risk.
Suggested Readings
Berger AP, Cheli C, Levine R, et al. Impact of age on complexed PSA levels in men with total PSA levels of up to 20 ng/mL. Urology. 2003;62:840–844.
Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542–1547.
Crawford ED, DeAntoni EP, Etzioni R, et al. Serum prostate-specific antigen and digital rectal examination for early detection of prostate cancer in a national community-based program. The Prostate Cancer Education Council. Urology. 1996;47:863–869.
D’Amico A, Chen M, Roehl K, Catalona W. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–135.
CARCINOMA OF THE RENAL PELVIS AND URETER
 Definition
Definition
 Carcinomas of the renal pelvis and ureter are primary tumors of urothelial (transitional cell) origin. Primary tumors arising in the renal pelvis include urothelial carcinomas (>90% of cases), squamous cell carcinomas (8%), and adenocarcinomas (rare).
Carcinomas of the renal pelvis and ureter are primary tumors of urothelial (transitional cell) origin. Primary tumors arising in the renal pelvis include urothelial carcinomas (>90% of cases), squamous cell carcinomas (8%), and adenocarcinomas (rare).
 Who Should Be Suspected?
Who Should Be Suspected?
 Individuals with carcinoma of the renal pelvis or ureter are most likely to have hematuria (70–95% of cases) or flank pain (8–40%) stemming from obstruction of the ureter or ureteropelvic junction by a tumor mass. Other types of urinary tract symptoms (bladder irritation, constitutional symptoms) are less likely to be seen at diagnosis (<10%). Calculi or chronic infection may precede the squamous cell carcinomas.
Individuals with carcinoma of the renal pelvis or ureter are most likely to have hematuria (70–95% of cases) or flank pain (8–40%) stemming from obstruction of the ureter or ureteropelvic junction by a tumor mass. Other types of urinary tract symptoms (bladder irritation, constitutional symptoms) are less likely to be seen at diagnosis (<10%). Calculi or chronic infection may precede the squamous cell carcinomas.
 Laboratory Findings
Laboratory Findings
 Urine cytology: Examination of urinary sediment for malignant cells is a less reliable method for diagnosis of these cases than for bladder cancers because of the poor yield of low-grade tumors and the likelihood of synchronous bladder cancer (40–50% of cases).
Urine cytology: Examination of urinary sediment for malignant cells is a less reliable method for diagnosis of these cases than for bladder cancers because of the poor yield of low-grade tumors and the likelihood of synchronous bladder cancer (40–50% of cases).
Suggested Readings
Olgac S, Mazumdar M, Dalbagni G, et al. Urothelial carcinoma of the renal pelvis: a clinicopathologic study of 130 cases. Am J Surg Pathol. 2004;28:1545–1552.
Paonessa J, Beck H, Cook S. Squamous cell carcinoma of the renal pelvis associated with kidney stones: a case report. Med Oncol. 2011;28(Suppl 1):S392–S394.
LEUKOPLAKIA OF THE RENAL PELVIS
 Definition
Definition
 Leukoplakia of the renal pelvis is a visualized grayish patch observed on the mucosal surface epithelium of the renal pelvis (part of the kidney urothelium) and represents metaplastic squamous plaque (squamous metaplasia and keratinization).
Leukoplakia of the renal pelvis is a visualized grayish patch observed on the mucosal surface epithelium of the renal pelvis (part of the kidney urothelium) and represents metaplastic squamous plaque (squamous metaplasia and keratinization).
 Who Should Be Suspected?
Who Should Be Suspected?
 Candidates are typically middle-aged individuals with recurrent episodes of renal or ureteric colic. In 90% of cases, the lesion is unilateral.
Candidates are typically middle-aged individuals with recurrent episodes of renal or ureteric colic. In 90% of cases, the lesion is unilateral.
 Laboratory Findings
Laboratory Findings
 Urine cytology (cell block or Pap smear): The finding of sheets of desquamated keratinized epithelial cells in urine during an attack of renal colic is pathognomonic.
Urine cytology (cell block or Pap smear): The finding of sheets of desquamated keratinized epithelial cells in urine during an attack of renal colic is pathognomonic.
 Flow cytometry (DNA): High-grade (aneuploid) tumors can be detected in >90% of cases.
Flow cytometry (DNA): High-grade (aneuploid) tumors can be detected in >90% of cases.
Suggested Readings
Hertle L, Androulakakis P. Keratinizing desquamative squamous metaplasia of the upper urinary tract: leukoplakia—cholesteatoma. J Urol. 1982;127:631–635.
Smith BA Jr, Webb EA, Price WE. Renal leukoplakia: observations of behavior. J Urol. 1962;87:279–287.
Terry TR, Shearer RJ. Conservative surgical management of leukoplakia of upper urinary tract. J R Soc Med. 1986;79:544–545.
 DISORDERS
DISORDERS
BENIGN PROSTATIC HYPERPLASIA (BPH)
 Definition
Definition
 BPH is enlargement of the prostate resulting from hyperplasia of prostatic stromal and epithelial cells, compressing the periurethral region of the prostate and causing partial or complete obstruction of the urethra.
BPH is enlargement of the prostate resulting from hyperplasia of prostatic stromal and epithelial cells, compressing the periurethral region of the prostate and causing partial or complete obstruction of the urethra.
 Who Should Be Suspected?
Who Should Be Suspected?
 Candidates are men, generally older than 30 years, with moderate to severe lower urinary tract symptoms (frequency, nocturia, hesitancy, urgency, weak stream) that gradually progress with time.
Candidates are men, generally older than 30 years, with moderate to severe lower urinary tract symptoms (frequency, nocturia, hesitancy, urgency, weak stream) that gradually progress with time.
 A history and physical examination should include a digital rectal examination of the prostate. A urine culture and urinalysis for hematuria should be undertaken to rule out other or more serious disorders that could cause symptoms similar to those of BPH (urinary tract infection, bladder calculi, prostatitis, prostate cancer, or bladder cancer). On digital rectal examination, symmetric enlargement and firmness of the prostate are typical of BPH, whereas asymmetric areas are suggestive of prostate cancer.
A history and physical examination should include a digital rectal examination of the prostate. A urine culture and urinalysis for hematuria should be undertaken to rule out other or more serious disorders that could cause symptoms similar to those of BPH (urinary tract infection, bladder calculi, prostatitis, prostate cancer, or bladder cancer). On digital rectal examination, symmetric enlargement and firmness of the prostate are typical of BPH, whereas asymmetric areas are suggestive of prostate cancer.
 Laboratory Findings
Laboratory Findings
 Serum prostate-specific antigen (PSA): In 20% of BPH patients, serum PSA may be increased from the widely used prostate cancer cutoff value of 4.0– 10 ng/mL. In fact, BPH is a more common cause of elevated PSA levels than is prostate cancer.
Serum prostate-specific antigen (PSA): In 20% of BPH patients, serum PSA may be increased from the widely used prostate cancer cutoff value of 4.0– 10 ng/mL. In fact, BPH is a more common cause of elevated PSA levels than is prostate cancer.
 Serum creatinine: While not recommended by the American Urological Association in the management of patients with BPH, a high serum creatinine value may suggest a bladder outlet obstruction or underlying renal or prerenal disease and an increased risk for post–prostate surgery complications and mortality.
Serum creatinine: While not recommended by the American Urological Association in the management of patients with BPH, a high serum creatinine value may suggest a bladder outlet obstruction or underlying renal or prerenal disease and an increased risk for post–prostate surgery complications and mortality.
Suggested Readings
Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557.
Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology. 2001;58:5–16.
Madersbacher S, Alivizatos G, Nordling J, et al. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol. 2004;46:547–554.
CALCULI
 Definition
Definition
 A renal calculus (kidney stone) is a solid concretion/crystalline aggregate formed in the kidneys by supersaturation of dietary minerals in the urine, one or more of which nucleate seed crystals. Both the supersaturation and the crystalline aggregation processes are pH dependent.
A renal calculus (kidney stone) is a solid concretion/crystalline aggregate formed in the kidneys by supersaturation of dietary minerals in the urine, one or more of which nucleate seed crystals. Both the supersaturation and the crystalline aggregation processes are pH dependent.
 Calculi can be classified by their location and chemical composition.
Calculi can be classified by their location and chemical composition.
 Locations include the kidney (nephrolithiasis), ureter (ureterolithiasis), or bladder (cystolithiasis).
Locations include the kidney (nephrolithiasis), ureter (ureterolithiasis), or bladder (cystolithiasis).
 Varieties of chemical composition include calcium containing (primarily calcium oxalate but also calcium phosphate); struvite (magnesium ammonium phosphate); uric acid; and cystine.
Varieties of chemical composition include calcium containing (primarily calcium oxalate but also calcium phosphate); struvite (magnesium ammonium phosphate); uric acid; and cystine.
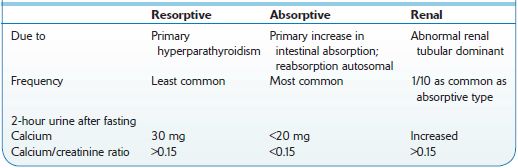
 Calcium oxalate or calcium phosphate calculi occur in 85% of male and 70% of female patients. Calcium oxalate crystals require an acid environment. Calcium phosphate crystals occur with hypercalciuria, hypocitraturia, and an alkaline environment (Figure 7-1). A comparison of idiopathic causes of hypercalciuria is presented in Table 7-1.
Calcium oxalate or calcium phosphate calculi occur in 85% of male and 70% of female patients. Calcium oxalate crystals require an acid environment. Calcium phosphate crystals occur with hypercalciuria, hypocitraturia, and an alkaline environment (Figure 7-1). A comparison of idiopathic causes of hypercalciuria is presented in Table 7-1.
 Struvite stones (staghorn calculi), occurring in 10–15% of patients, are generated by UTI urea-splitting bacteria, including Proteus species (>50% of cases; after ruling out Klebsiella, Pseudomonas, Serratia, and Enterobacter), and in patients with persistently alkaline urine. Although not producing symptoms unless inducing urinary tract obstruction or infection, this type of calculus can lead to renal failure over years if present bilaterally. Staghorn calculi should be cultured.
Struvite stones (staghorn calculi), occurring in 10–15% of patients, are generated by UTI urea-splitting bacteria, including Proteus species (>50% of cases; after ruling out Klebsiella, Pseudomonas, Serratia, and Enterobacter), and in patients with persistently alkaline urine. Although not producing symptoms unless inducing urinary tract obstruction or infection, this type of calculus can lead to renal failure over years if present bilaterally. Staghorn calculi should be cultured.
 Cystine stones are rare, occurring in patients with homozygous congenital familial cystinuria, and characterized by bilateral obstructive staghorn calculi with associated renal failure.
Cystine stones are rare, occurring in patients with homozygous congenital familial cystinuria, and characterized by bilateral obstructive staghorn calculi with associated renal failure.
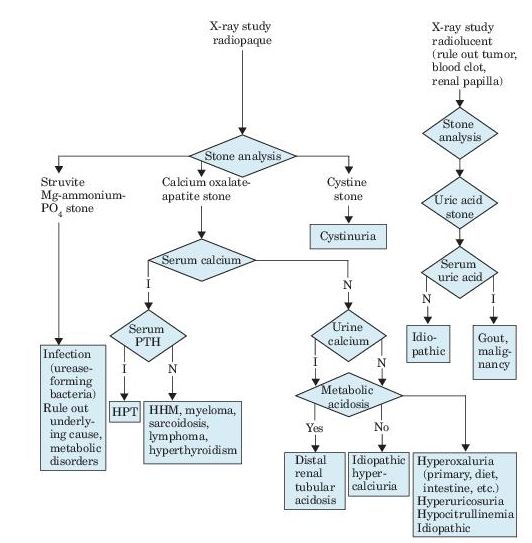
Figure 7–1 Algorithm for diagnosis of renal calculi, as revealed by flank pain, renal colic, hematuria, fever, and urinalysis findings. I, increased; N, normal; PTH, parathyroid hormone; HPT, hyperparathyroidism; HHM, humeral hypercalcemia of malignancy.
Table 7-1 Comparison of Types of Idiopathic Hypercalciuria
 Who Should Be Suspected?
Who Should Be Suspected?
 In adults, the most common symptom of calculi that obstruct the ureter or renal pelvis is excruciating, intermittent pain that radiates from the flank to the groin or to the genital area and inner thigh. The pain is commonly accompanied by urinary urgency, restlessness, hematuria, sweating, nausea, and vomiting.
In adults, the most common symptom of calculi that obstruct the ureter or renal pelvis is excruciating, intermittent pain that radiates from the flank to the groin or to the genital area and inner thigh. The pain is commonly accompanied by urinary urgency, restlessness, hematuria, sweating, nausea, and vomiting.
 The waves or paroxysms of pain usually last 20–60 minutes and is related to the passage of the stone down the ureter and the associated ureteral spasm.
The waves or paroxysms of pain usually last 20–60 minutes and is related to the passage of the stone down the ureter and the associated ureteral spasm.
 Flank pain is caused by upper ureteral or renal pelvic obstruction, whereas genital pain is caused by lower ureteral obstruction.
Flank pain is caused by upper ureteral or renal pelvic obstruction, whereas genital pain is caused by lower ureteral obstruction.
 The differential diagnosis of patients with flank pain includes renal bleeding, pyelonephritis, ectopic pregnancy, rupture or torsion of an ovarian cyst, dysmenorrhea, intestinal obstruction, diverticulitis, appendicitis, biliary colic and cholecystitis, and herpes zoster. The intestinal and hepatic causes of flank pain are not accompanied by hematuria nor is a herpes zoster infection (which is usually accompanied by a rash).
The differential diagnosis of patients with flank pain includes renal bleeding, pyelonephritis, ectopic pregnancy, rupture or torsion of an ovarian cyst, dysmenorrhea, intestinal obstruction, diverticulitis, appendicitis, biliary colic and cholecystitis, and herpes zoster. The intestinal and hepatic causes of flank pain are not accompanied by hematuria nor is a herpes zoster infection (which is usually accompanied by a rash).
 Precipitating causes in adults with calculi (20–30%) include destructive bone diseases (either destructive, e.g., metastatic tumors; or osteoporotic, e.g., immobilization, Paget disease, or Cushing syndrome); milk-alkali (Burnett) syndrome; hypervitaminosis D; sarcoidosis; RTA-type I (hypercalciuria, highly alkaline urine, normal serum calcium); hyperthyroidism; and gout (25% of primary cases, 40% of cases with marrow-proliferative disorders).
Precipitating causes in adults with calculi (20–30%) include destructive bone diseases (either destructive, e.g., metastatic tumors; or osteoporotic, e.g., immobilization, Paget disease, or Cushing syndrome); milk-alkali (Burnett) syndrome; hypervitaminosis D; sarcoidosis; RTA-type I (hypercalciuria, highly alkaline urine, normal serum calcium); hyperthyroidism; and gout (25% of primary cases, 40% of cases with marrow-proliferative disorders).
 Precipitating causes in children with calculi include infections (13–40%); hypercalciuria (idiopathic but also caused by distal RTA and therapy with furosemide, prednisone, or ACTH); oxaluria (3–13%); uric acid (4%); cystinuria (5–7%); hypocitraturia (10%); xanthine (an inborn error of metabolism); and adenine phosphoribosyltransferase deficiency.
Precipitating causes in children with calculi include infections (13–40%); hypercalciuria (idiopathic but also caused by distal RTA and therapy with furosemide, prednisone, or ACTH); oxaluria (3–13%); uric acid (4%); cystinuria (5–7%); hypocitraturia (10%); xanthine (an inborn error of metabolism); and adenine phosphoribosyltransferase deficiency.
 Laboratory Findings
Laboratory Findings
 Two 24-hour urine specimens should be collected and tested for daily volume and levels of magnesium, sodium, uric acid, calcium, citrate, and oxalate.
Two 24-hour urine specimens should be collected and tested for daily volume and levels of magnesium, sodium, uric acid, calcium, citrate, and oxalate.
 A urine culture should be performed to detect infecting microorganisms.
A urine culture should be performed to detect infecting microorganisms.
 Urine microscopy should be performed to detect the presence and level of red cells, white cells, urinary casts, and crystals.
Urine microscopy should be performed to detect the presence and level of red cells, white cells, urinary casts, and crystals.
 Calculi should be collected by urination through a stone screen, for chemical analysis.
Calculi should be collected by urination through a stone screen, for chemical analysis.
 Hematuria: Gross or microscopic, occurs in 80% of symptomatic patients and is the single most definitive predictor of a calculus in patients with unilateral flank pain. However, hematuria is not detected in 10–30% of patients with documented nephrolithiasis.
Hematuria: Gross or microscopic, occurs in 80% of symptomatic patients and is the single most definitive predictor of a calculus in patients with unilateral flank pain. However, hematuria is not detected in 10–30% of patients with documented nephrolithiasis.
 Renal function tests: Useful for interpretation of hypercalcemia.
Renal function tests: Useful for interpretation of hypercalcemia.
 Crystalluria: Diagnostically useful for cystine crystals (in familial cystinuria) or struvite crystals.
Crystalluria: Diagnostically useful for cystine crystals (in familial cystinuria) or struvite crystals.
 Cyanide-nitroprusside test: Positive (false positive may occur with sulfurcontaining drugs). Calcium oxalate, phosphate, and uric acid should arouse suspicion about possible causes, but they may occur in normal urine.
Cyanide-nitroprusside test: Positive (false positive may occur with sulfurcontaining drugs). Calcium oxalate, phosphate, and uric acid should arouse suspicion about possible causes, but they may occur in normal urine.
 Neutrophilia: Suggestive of infection, for example, in the finding of struvite crystals.
Neutrophilia: Suggestive of infection, for example, in the finding of struvite crystals.
Suggested Readings
Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–1152.
Elton TJ, Roth CS, Berquist TH, et al. A clinical prediction rule for the diagnosis of ureteral calculi in emergency departments. J Gen Intern Med. 1993;8:57–62.
Teichman JM, Long RD, Hulbert JC. Long-term renal fate and prognosis after staghorn calculus management. J Urol. 1995;153:1403–1407.
HEMATURIA
 Definition
Definition
 The term hematuria refers to the microscopic detection in urine of >2 RBCs per high-power field. It should not to be confused with hemoglobinuria, a term reserved for the presence of free hemoglobin in urine.
The term hematuria refers to the microscopic detection in urine of >2 RBCs per high-power field. It should not to be confused with hemoglobinuria, a term reserved for the presence of free hemoglobin in urine.
 Hematuria may be macroscopic (grossly visible as red or brown urine) or microscopic (detectable only by microscopy). It can be classified as glomerular or nonglomerular in origin. Centrifugation allows one to differentiate hematuria (RBCs in sediment) from hemoglobinuria (normal sediment, heme-pigmented supernatant), which can be tested for heme with a urine dipstick.
Hematuria may be macroscopic (grossly visible as red or brown urine) or microscopic (detectable only by microscopy). It can be classified as glomerular or nonglomerular in origin. Centrifugation allows one to differentiate hematuria (RBCs in sediment) from hemoglobinuria (normal sediment, heme-pigmented supernatant), which can be tested for heme with a urine dipstick.
 Who Should Be Suspected?
Who Should Be Suspected?
 Hematuria is common and, in many patients, particularly young adults, is transient and inconsequential. With increasing age, common causes can include inflammation or infection of the prostate or bladder and calculi. In patients over age 35, hematuria is associated with a higher risk of benign prostatic hyperplasia and renal or GU malignancies.
Hematuria is common and, in many patients, particularly young adults, is transient and inconsequential. With increasing age, common causes can include inflammation or infection of the prostate or bladder and calculi. In patients over age 35, hematuria is associated with a higher risk of benign prostatic hyperplasia and renal or GU malignancies.
 Patients on oral anticoagulants and those with a high international normalized ratio (INR) are at higher risk of hematuria. Even if present in such patients, it is necessary to investigate for alternative source(s) of the condition.
Patients on oral anticoagulants and those with a high international normalized ratio (INR) are at higher risk of hematuria. Even if present in such patients, it is necessary to investigate for alternative source(s) of the condition.
 Isolated hematuria occurs in patients with calculi, trauma, prostatitis, sickle cell trait or disease, tuberculosis, and Schistosoma haematobium infection. Acute cystitis or urethritis in women can cause gross hematuria. Hypercalciuria and hyperuricosuria are also risk factors for unexplained isolated hematuria.
Isolated hematuria occurs in patients with calculi, trauma, prostatitis, sickle cell trait or disease, tuberculosis, and Schistosoma haematobium infection. Acute cystitis or urethritis in women can cause gross hematuria. Hypercalciuria and hyperuricosuria are also risk factors for unexplained isolated hematuria.
 Benign familial or recurrent hematuria refers to asymptomatic, recurrent hematuria without proteinuria or other laboratory abnormalities. Persistent or recurrent hematuria, even if only microscopic, should be investigated, especially in patients over age 50. Other family members may be affected. The condition may clear spontaneously.
Benign familial or recurrent hematuria refers to asymptomatic, recurrent hematuria without proteinuria or other laboratory abnormalities. Persistent or recurrent hematuria, even if only microscopic, should be investigated, especially in patients over age 50. Other family members may be affected. The condition may clear spontaneously.
 Laboratory Findings
Laboratory Findings
 The single most important test in the evaluation of hematuria is the microscopic analysis of urine sediment, which can often distinguish glomerular from nonglomerular bleeding.
The single most important test in the evaluation of hematuria is the microscopic analysis of urine sediment, which can often distinguish glomerular from nonglomerular bleeding.
 Microscopy of centrifuged urinary sediment should be examined under high dry magnification. Note that <3% of normal persons have ≥3 RBC per HPF. RBCs or casts indicate that the blood is of glomerular origin. The most common causes of isolated glomerular hematuria are IgA nephropathy, hereditary nephritis (Alport syndrome), and thin basement membrane disease. The presence of clots rules out a glomerular origin—large thick clots suggest a bladder origin, whereas small stringy clots indicate upper urinary tract disease. The presence of WBCs suggests inflammation or infection.
Microscopy of centrifuged urinary sediment should be examined under high dry magnification. Note that <3% of normal persons have ≥3 RBC per HPF. RBCs or casts indicate that the blood is of glomerular origin. The most common causes of isolated glomerular hematuria are IgA nephropathy, hereditary nephritis (Alport syndrome), and thin basement membrane disease. The presence of clots rules out a glomerular origin—large thick clots suggest a bladder origin, whereas small stringy clots indicate upper urinary tract disease. The presence of WBCs suggests inflammation or infection.
 The urine dipstick can detect RBCs at a level equivalent to one to two RBCs per HPF, but results in more false-positive tests owing to a number of interfering factors (listed below), and so a positive dipstick test must be confirmed by microscopic examination of the urine. Proteinuria is also detected by dipstick, and a 2+ proteinuria in the presence of microscopic hematuria indicates glomerular disease.
The urine dipstick can detect RBCs at a level equivalent to one to two RBCs per HPF, but results in more false-positive tests owing to a number of interfering factors (listed below), and so a positive dipstick test must be confirmed by microscopic examination of the urine. Proteinuria is also detected by dipstick, and a 2+ proteinuria in the presence of microscopic hematuria indicates glomerular disease.
 Immunocytochemical staining for human Tamm-Horsfall protein is positive with >80% of RBCs of renal origin and <13.1% of RBCs of nonrenal origin.
Immunocytochemical staining for human Tamm-Horsfall protein is positive with >80% of RBCs of renal origin and <13.1% of RBCs of nonrenal origin.
 Imaging studies, urinary cytology, cystoscopy, or occasionally renal biopsy may be indicated in cases of persistent hematuria with no obvious etiology.
Imaging studies, urinary cytology, cystoscopy, or occasionally renal biopsy may be indicated in cases of persistent hematuria with no obvious etiology.
 Limitations on the Urine Dipstick Test
Limitations on the Urine Dipstick Test
 Causes of false-positive results
Causes of false-positive results
 Vaginal bleeding (menstruation)
Vaginal bleeding (menstruation)
 Viral illness
Viral illness
 Bacteriuria
Bacteriuria
 Certain foods (beets, blackberries, rhubarb)
Certain foods (beets, blackberries, rhubarb)
 Pigmenturia (myoglobin, porphyrin, hemoglobin)
Pigmenturia (myoglobin, porphyrin, hemoglobin)
 Drugs (rifampin, phenolphthalein, iodides, bromides, copper, oxidizing agents, permanganate)
Drugs (rifampin, phenolphthalein, iodides, bromides, copper, oxidizing agents, permanganate)
 Postejaculate semen
Postejaculate semen
 Red diaper syndrome
Red diaper syndrome
 Trauma
Trauma
 Vigorous exercise prior to collection
Vigorous exercise prior to collection
 pH > 9
pH > 9
 Factitious
Factitious
 Causes of false-negative results
Causes of false-negative results
 Reducing agents (high doses of vitamin C)
Reducing agents (high doses of vitamin C)
 pH < 5.1
pH < 5.1
Suggested Readings
Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348:2330–2338.
Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy—part I: definition, detection, prevalence, and etiology. Urology. 2001;57:599–603.
HEMOGLOBINURIA
 Definition
Definition
 Hemoglobinuria refers to the presence of free hemoglobin (Hb) in urine. The condition is often associated with hemolytic anemia, wherein intravascular red cell destruction increases levels of free plasma Hb. The excess Hb is filtered by the kidneys and excreted into the urine where it is visibly detected. The renal threshold for hemoglobinuria is 100–140 mg Hb/dL plasma.
Hemoglobinuria refers to the presence of free hemoglobin (Hb) in urine. The condition is often associated with hemolytic anemia, wherein intravascular red cell destruction increases levels of free plasma Hb. The excess Hb is filtered by the kidneys and excreted into the urine where it is visibly detected. The renal threshold for hemoglobinuria is 100–140 mg Hb/dL plasma.
 Although free Hb directly passing the glomeruli in the ultrafiltrate is relatively uncommon (usually, RBCs enter the urinary tract and undergo various amounts of lysis), nevertheless, conditions resulting in intravascular hemolysis have the potential of producing hemoglobinuria once all available plasma haptoglobin is bound by Hb. Hb is readily absorbed by the renal proximal tubules as dissociated dimers and catabolized to ferritin. In turn, ferritin is denatured to hemosiderin that can be found in urine in cases of severe, prolonged hemoglobinuria.
Although free Hb directly passing the glomeruli in the ultrafiltrate is relatively uncommon (usually, RBCs enter the urinary tract and undergo various amounts of lysis), nevertheless, conditions resulting in intravascular hemolysis have the potential of producing hemoglobinuria once all available plasma haptoglobin is bound by Hb. Hb is readily absorbed by the renal proximal tubules as dissociated dimers and catabolized to ferritin. In turn, ferritin is denatured to hemosiderin that can be found in urine in cases of severe, prolonged hemoglobinuria.
 Who Should Be Suspected?
Who Should Be Suspected?
 Candidates include patients with red urine but no red cells in urinary sediment, especially if there is a history suggesting intravascular hemolysis. The classic patient with hemolysis may have many of the following findings: rapid onset of pallor, anemia, jaundice, a history of pigmented (bilirubin) gallstones, splenomegaly, the presence of circulating spherocytic or fragmented red cells on the peripheral blood smear, and/or a positive direct antiglobulin test (Coombs test).
Candidates include patients with red urine but no red cells in urinary sediment, especially if there is a history suggesting intravascular hemolysis. The classic patient with hemolysis may have many of the following findings: rapid onset of pallor, anemia, jaundice, a history of pigmented (bilirubin) gallstones, splenomegaly, the presence of circulating spherocytic or fragmented red cells on the peripheral blood smear, and/or a positive direct antiglobulin test (Coombs test).
 Inciting causes of hemoglobinuria fall into several categories:
Inciting causes of hemoglobinuria fall into several categories:
 Hemolytic anemias with intravascular hemolysis
Hemolytic anemias with intravascular hemolysis
 Paroxysmal nocturnal hemoglobinuria
Paroxysmal nocturnal hemoglobinuria
 Paroxysmal cold hemoglobinuria
Paroxysmal cold hemoglobinuria
 Microangiopathic hemolytic anemias (thrombotic thrombocytopenic purpura/hemolytic uremic syndrome), prosthetic heart valves, severely damaged natural valves (especially aortic)
Microangiopathic hemolytic anemias (thrombotic thrombocytopenic purpura/hemolytic uremic syndrome), prosthetic heart valves, severely damaged natural valves (especially aortic)
 Severe autoimmune hemolytic anemias
Severe autoimmune hemolytic anemias
 Fava bean sensitivity, G6PD deficiency, and other hemoglobinopathies
Fava bean sensitivity, G6PD deficiency, and other hemoglobinopathies
 Severe hereditary spherocytosis
Severe hereditary spherocytosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


