INTRODUCTION
Over 1.6 million individuals in the United States are diagnosed with invasive cancer each year. Currently, 23% of all deaths in the United States are due to cancer, ranking second only to heart disease as the leading cause of mortality in this country. Over the past 10 years, however, cancer death rates have decreased. Death rates have continued to decline for the four top cancer sites (lung, colorectum, breast, and prostate). This reduction in overall cancer death rates translates to the avoidance of over 1 million deaths from cancer.
The surgeon is intimately involved in the care of cancer patients, since the majority will require surgical therapy at some time. Surgeons are often the first specialists to see newly diagnosed cancer patients or are often called upon to make the diagnosis in patients suspected to have cancer. As such, they will be responsible for orchestrating the patient’s care, including coordination with medical oncologists and radiation oncologists. It is imperative that they have an in-depth knowledge of the different types of cancer and the different modalities available for treatment.
TUMOR NOMENCLATURE
Neoplasms are defined as benign or malignant according to the clinical behavior of the tumor. Benign tumors have lost normal growth regulation but tend to be surrounded by a capsule and do not invade surrounding tissues or metastasize.
Benign tumors are generally designated by adding the suffix –oma to the name of the cell of origin. Examples include lipoma and adenoma. The term cancer normally refers to malignant tumors, which can invade surrounding tissues or metastasize to distant sites in the host. The nomenclature of malignant tumors is typically based on the cell’s embryonal tissue origins. Malignant tumors derived from cells of mesenchymal origin are called sarcomas. These include cancers that derive from muscle, bone, tendon, fat, cartilage, lymphoid tissues, vessels, and connective tissue. Neoplasms of epithelial origin are called carcinomas. These may be further categorized according to the histologic appearance of the cells. Tumor cells that have glandular growth patterns are called adenocarcinomas, and those that resemble squamous epithelial cells are called squamous cell carcinomas. Cancers composed of undifferentiated cells that bear no resemblance to any tissues are designated as “poorly differentiated” or “undifferentiated” carcinomas.
Beyond the type of cancer, it is important to classify tumors by their behavior and prognosis in order to determine appropriate therapy as well as evaluate different treatment modalities. Grading of a tumor is a histologic determination and refers to the degree of cellular differentiation. Separate pathologic grading systems exist for each histologic type of cancer. Depending on the type of tumor, these systems are based on nuclear pleomorphism, cellularity, necrosis, cellular invasion, and the number of mitoses. Increasing grades generally denote increasing degrees of dedifferentiation. While the grade of the tumor typically has less prognostic value than its stage, tumor grade has great clinical significance in soft tissue sarcoma, astrocytoma, transitional cell cancers of the genitourinary tract, and Hodgkin and non-Hodgkin lymphoma.
Tumor staging establishes the extent of disease and has important prognostic and therapeutic implications in most types of cancer. Clinical staging is based on the results of a noninvasive evaluation, including physical examination and various imaging studies. Pathologic staging is based on findings in surgical tumor specimens and biopsies and allows for the evaluation of microscopic disease undetectable by imaging techniques. Pathologic staging may reveal more extensive tumor spread than the clinical evaluation and is the more reliable information. Clinicians must be careful when attempting to compare clinically and pathologically staged patients, as the two groups may have dramatically different outcomes.
As with grading, the staging systems vary with different tumor types. Two major staging systems are currently in use, one developed by the Union Internationale Contre le Cancer (UICC) and the other by the American Joint Committee on Cancer (AJCC). The UICC system is based on the TNM classification. T refers to the primary tumor and is based on the size of the tumor and invasion of surrounding structures. Tumors are characterized as T1 to T4 cancers, with the higher T stages for larger and more invasive tumors. N refers to regional lymph nodes, and classifications of N0 to N3 denote increasing degrees of lymph node involvement. Finally, M refers to distant metastatic disease, with M0 signifying no distant metastases and M1 and M2 indicating the presence of blood-borne metastatic disease. The AJCC system divides cancers into stages 0 to IV, with higher stages representing more widespread disease and a poorer prognosis. Regardless of the staging system or the tumor type, higher stages correlate with decreased survival.
Cancer epidemiology is the study of the distribution of cancer and its determinants among defined populations and is used to examine cancer etiology as well as the efficacy of prevention, detection, and treatment strategies. The most basic types of epidemiologic terms describe cancer rates or cancer deaths for specific populations over a certain period of time.
While absolute numbers of cancer cases may be useful for health care planning, they do not take into account the size or nature of the underlying population at risk. For this reason, the most commonly used population-based measures of cancer are incidence and mortality. Cancer incidence rates are defined as the number of new cancer cases diagnosed during a fixed time period divided by the total population at risk. Cancer mortality rates are defined similarly, with cancer deaths replacing new cancer cases. These rates are typically expressed as the number of events per 100,000 individuals per year.
Incidence and mortality rates are compared across populations or over time to identify causes as well as the effect of screening or treatment. However, other factors among populations may contribute to observed differences, and these must be taken into account. For most cancers, age is the strongest risk factor, and so comparison of cancer incidence between two populations must consider the age distributions of the two groups. Adjustment (or standardization) is the most common method used to account for such differences. Comparing age-adjusted cancer incidence rates ensures that any observed differences are not the results of differences in age distributions between the two populations. Incidence and mortality rates are often also adjusted for gender, race, or socioeconomic status.
Cancer incidence examines only those diagnosed with the disease during that time period; it does not include patients diagnosed earlier who are living with cancer. Cancer prevalence describes the number of people with the disease either at a single point in time (point prevalence) or within a defined period of time (period prevalence). Prevalence is more relevant to the public health burden of cancer because all prevalent cases involve accessing health care. The relationship among incidence, prevalence, and mortality is influenced by the fatality of the disease. If the disease is highly fatal and the interval between presentation and death is short, mortality rates will be similar to incidence rates. The number of deaths from cancer divided by the total number people diagnosed with the cancer is known as the cancer fatality rate, although this is somewhat of a misnomer because they are not technically rates (they do not include time as a parameter).
Examining the fatality of cancer is obviously important when comparing treatments meant to improve outcome. Overall survival (OS) is the most global endpoint and is defined as the proportion of people alive at a specified period after being diagnosed with the disease. Five years is conventionally used as the time period (ie, 5-year survival). However, overall survival may not always reflect the success of treatment. Over that period of time, some patients may die of disease, but others may die of other causes. In addition, some patients may have a local or regional recurrence that is successfully treated, while some may recur with distant metastases but not succumb to them. For this reason, survival rates in cancer are often qualified by the patient’s disease status.
Disease-free survival refers to the proportion of patients alive and without disease over a specific period of time. A patient who developed metastases but is still alive would be included in the overall survival rate but not the disease-free survival rate. Disease-free survival and overall survival may provide different pictures of the success of treatment. A therapy that improves disease-free survival but not overall survival may still be important if quality of life is improved. In some cancers, local or regional recurrences can be readily treated with minimal impact on overall survival. In these cases, disease-free survival may present an overly pessimistic picture of outcome. Therefore, it may be more relevant to compare distant disease-free survival, which refers to the proportion of people alive and without distant metastases, regardless of local recurrence. In some cases, it is difficult to assess the efficacy of a treatment by looking at overall survival or disease-free survival if there are deaths from competing causes. It may be more helpful to compare disease-specific survival, which is the percentage of people who have survived a disease since diagnosis or treatment and does not count patients who died from other causes.
It is important for the surgeon to understand the different methods for describing cancer survival as well as the differences between the definitions, because the appropriateness of the comparison will vary with the biology of the disease and the clinical question being asked.
ROLE OF THE SURGICAL ONCOLOGIST
The surgeon is often the first specialist to see a patient with suspected or newly diagnosed cancer and in many cases assumes responsibility for orchestrating the overall management of the cancer patient’s care. The role of the surgeon involves not only the curative resection of the tumor but also obtaining tissue for diagnosis and staging, providing palliation for incurable patients, and preventing cancer by the prophylactic removal of organs. With improving imaging technologies, expanded use of neoadjuvant therapies, molecular staging, and increasing knowledge of the genetic predisposition to cancer, the role of the surgical oncologist is continuously evolving. It is therefore imperative for surgical oncologists to remain current on the newest approaches to cancer therapy and be prepared to adapt to the changing role of surgery.
A tissue diagnosis is critical to the care of all cancer patients. Depending on the type of tumor and its location, the method of biopsy will vary. Common diagnostic techniques include needle aspiration biopsy, core needle biopsy, incisional biopsy, and excisional biopsy.
Fine-needle aspiration biopsy (FNAB) is a rapid and minimally invasive technique for the biopsy of palpable superficial tumors. Deeper, nonpalpable lesions may also be sampled by this technique when FNAB is combined with various imaging modalities, such as ultrasonography or computed tomography (CT). FNAB involves aspiration of cells from a suspicious mass, followed by cytologic examination of the stained smear. FNAB is particularly useful in the diagnosis of enlarged lymph nodes, breast lumps, thyroid masses, and lung nodules.
Advantages to FNAB include the simplicity of the procedure and the low rate of complications. However, there are limitations. FNAB cytology requires an experienced cytopathologist for accurate interpretation. Because cytology does not demonstrate architecture, it does not allow the cytopathologist to accurately grade tumors or to differentiate between in situ and invasive disease. If this information is necessary, FNAB may be inadequate. Sampling errors can lead to false-negative results, so a negative FNAB should be interpreted cautiously. In addition, though rare, false-positive results can occur, so confirmation may be needed before definitive surgical intervention. For example, a mastectomy should never be performed on the basis of an FNAB of a breast lump without confirming the diagnosis by either preoperative core biopsy or frozen section analysis at the time of surgery.
Core needle biopsy utilizes a needle that removes a sliver of tissue for analysis. This technique provides more histologic information than FNAB because it allows the pathologist to see the histologic architecture of the sample rather than just the cellular characteristics. False-positive results are extremely rare. Although less so than with FNAB, sampling errors may occur, and a negative result must be weighed against clinical judgment. Core biopsies are frequently used for prostate, breast, and liver masses. Again, ultrasound and radiographic imaging may enable the clinician to sample deep-seated or nonpalpable masses. The technique may also be used during surgery to biopsy suspicious masses encountered at operation.
When a larger tumor sample is necessary for accurate grading or staging, or a needle biopsy provided inadequate information, an incisional or excisional biopsy is required. Excisional biopsy is the surgical removal of an entire gross lesion, while incisional biopsy involves sampling a representative portion of a suspicious lesion. In general, excisional biopsy is recommended whenever it is possible to excise the entire lesion without damage to surrounding structures. Incisional biopsy should be considered whenever a core biopsy fails to make the diagnosis, but removing the tumor might compromise the subsequent operation (eg, a large [> 5 cm], deep soft tissue mass for which sarcoma is a possibility) or preclude delivery of neoadjuvant therapy.
Although biopsy techniques are usually simple, the surgeon must adhere to some specific principles when performing a biopsy for a suspected malignancy. The positioning of the needle tract or scar should be such that if further surgery is required, the biopsy site will be easily included in the excised specimen. Excisional biopsies of the breast should consider the possibility of a subsequent mastectomy, and the excision of skin or subcutaneous lesions on the extremities should be oriented in a way that allows for the following wide excision and lymphatic mapping if malignancy is discovered. Meticulous hemostasis is imperative, as the formation of a wound hematoma may make subsequent operation more difficult. The surgeon should carefully orient the pathologic specimen to allow the pathologist to evaluate margins in the context of the preresection anatomy, which may prove important in curative surgical procedures.
Once a diagnosis is made, the next step is typically to determine the extent of the cancer, or staging. This step begins with a complete history and physical examination, looking for signs or symptoms of advanced or metastatic disease. Laboratory or imaging studies may follow to determine not only the extent of the primary tumor but the presence of regional or distant metastases. Patients with signs or symptoms of metastatic disease should undergo appropriate workup of their symptoms. For some tumor types, routine staging examinations are indicated. However, for many asymptomatic patients newly diagnosed with cancer, a full battery of staging studies is not necessary and not only will increase the cost of treatment but may lead to false-positive findings, unnecessary biopsies, and inappropriate changes in therapy.
Surgeons are often called upon to perform operations that provide staging information for various types of cancer. Such procedures are necessary when the clinical extent of the disease has a direct bearing on the choice of treatment modalities. Examples include laparoscopy for gastric or pancreatic cancer, a staging laparotomy for ovarian cancer, or mediastinoscopy for lung and esophageal cancer. Staging procedures can often help to avert highly morbid procedures in cases where there is little chance for cure.
Surgical resections with curative intent can be divided into three categories: resection of a primary lesion, resection of isolated metastases, and resection of metastatic deposits. In each case, the clinician must strive to reach a balance between the chance for cure and the morbidity of the procedure. Each situation must be evaluated individually, and the patient’s wishes must be paramount.
The guiding principle of cancer surgery is to remove the entire tumor with adequate margins so as to prevent local recurrence and potentially distant recurrence. What constitutes an adequate margin varies among tumor types. Various tumors require different disease-free margins in order to achieve optimal chances for a cure. For a tumor that appears adherent or fixed to adjacent structures, en bloc resection is mandatory, and any attachment should be considered malignant in nature. Appropriate preoperative imaging of the tumor is often necessary to be prepared in the operating room for the possible resection of small or large bowel, bladder, or other adjacent organs.
It is important that the surgeon have knowledge of other modalities that may be integrated into the management plan to allow for a less extensive surgical procedure. Radiation and chemotherapy are commonly used in combination with surgery and are referred to as adjuvant therapies if used after complete resection with no demonstrable local or systemic disease. While their use has in some cases diminished the extent of resection necessary for local control (breast, sarcoma, head, and neck), it is important to note that these modalities do not compensate for inadequate margins in controlling local disease. Every attempt should be made to achieve widely negative margins surgically, even if it requires a second operation, rather than assuming radiation will “clean up” residual disease.
If these modalities are used in the preoperative setting, they are called neoadjuvant therapies. In many cases, neoadjuvant therapy has dramatically improved outcomes, as with pediatric rhabdomyosarcoma or locally advanced or inflammatory breast cancer. In some cases, neoadjuvant therapy can convert an unresectable tumor to resectable, while in other cases, it can decrease the extent of surgery necessary to obtain control or decrease the likelihood of positive margins. Neoadjuvant therapy is commonly used in the treatment of esophageal cancer, rectal cancer, pancreatic cancer, breast cancer, and sarcoma. It is important the surgeon consider the possibility of neoadjuvant therapy when performing a biopsy, staging the patient, or planning surgery.
The regional lymph nodes represent the most prevalent site of metastases for solid tumors, and in most cases involvement of the regional nodes represents the most important prognostic factor. Removal of the regional lymph nodes not only provides important prognostic information that may help guide adjuvant therapy, but provides regional control, preventing regional recurrence and the associated complications. For this reason, the removal of the regional lymph nodes is often performed at the time of resection of the primary cancer. More controversial is whether the removal of regional lymph nodes can improve survival. These controversies concern both the extent and the timing of the procedure. For example, the extent of lymphadenectomy at the time of gastrectomy for stomach cancer has been hypothesized to have an impact on improving overall survival. This has not, however, been borne out in prospective randomized trials. It may be that extended lymphadenectomy results in more accurate staging of patients at a cost of increased morbidity and minimal effect, if any, on overall survival. The relative benefit of nodal dissection may also vary with the efficacy of adjuvant therapies, such as chemotherapy or radiation therapy. How extensive a lymph node dissection to perform at the time of definitive resection varies with tumor type and in many cases remains controversial.
For many nonvisceral solid tumors, such as melanoma or breast cancer, elective node dissections were performed in clinically node negative patients at the time of their primary tumor resection. Unfortunately, this exposed many node-negative patients to the morbidity of a node dissection, and a clear survival benefit could not be demonstrated in prospective randomized studies. This practice has been replaced with lymphatic mapping and sentinel lymph node biopsy. Mapping agents (a radioactive tracer or a blue dye) are injected around the site of the tumor prior to surgery. These travel to the lymph nodes that first receive drainage from the site of the primary, and thus are the most likely to harbor cancer. Only the sentinel nodes (those nodes that are blue or radioactive) are removed, and carefully examined for micrometastases. This has dramatically improved our ability to stage the regional lymph nodes, helping to guide adjuvant therapies, while minimizing morbidity. Classically, node negative patients could safely avoid further surgery, while node positive patients underwent a completion lymph node dissection. This approach has also changed recently. In breast cancer, the American College of Surgeons Oncology Group (ACoSOG) Z0011 trial, demonstrated that a subset of breast cancer patients with positive sentinel lymph nodes did not benefit from completion lymph node dissection. A similar trial, the Multicenter Selective Lymphadenectomy Trial II (MSLT-II), is examining a similar question among sentinel lymph node positive melanoma patients.
The surgeon plays a much more limited role when the patient has metastatic disease; nonetheless, the resection of “isolated” metastases in patients with solid malignancies is sometimes a consideration when technically feasible. The selection of candidate patients for surgical resection requires a thorough evaluation of the extent of known disease, likelihood of additional metastatic disease, length of time between the primary and the distant recurrence (disease-free interval), medical status of the patient, and feasibility of resecting the metastatic site with a negative margin. Ultimately, this process identifies a small subset of patients who would be surgical candidates. Although there are no prospective randomized trials documenting the survival benefit of surgical resection of metastatic disease, there is considerable retrospective evidence indicating that this approach can result in long-term benefit. The resection of lung metastases in patients with osteogenic or soft tissue sarcomas has been associated with an approximately 20%-25% overall survival rate greater than 5 years. There is also a large body of retrospective evidence documenting the benefit of resecting colorectal metastases to the liver, resulting in a 25%-40% overall 5-year survival rate, depending on the extent of liver involvement. A similar benefit has been demonstrated in an aggressive surgical approach to metastatic melanoma. Another question in stage IV disease is when to resect the primary tumor. Typically, resection of the primary cancer when the patient already has metastatic disease was only done for palliation, or to prevent future complications. This approach is changing, however. For some cancers, such as colon cancer, resection of the primary tumor in the face of metastatic disease to prevent obstruction or bleeding, is less necessary due to the improved efficacy of systemic agents. Conversely, in some cancers, such as renal cell carcinoma and possibly breast cancer, there is evidence that resection of the primary tumor improves may improve outcome in stage IV patients. One of the roles of the surgical oncologist is to know when it is appropriate to offer this option.
Surgical intervention is sometimes required in the patient with unresectable advanced cancer for palliative indications such as pain, bleeding, obstruction, malnutrition, or infection. The decision to operate must balance several factors, including the likelihood of adding significantly to the quality of life of the patient, the expected survival of the individual, the potential morbidity of the procedure, and whether there are alternative methods of palliation.
Malnutrition is a common problem in the cancer patient, especially one with advanced, unresectable disease. Commonly, the surgeon is involved in placement of vascular access for hyperalimentation, or if the gastrointestinal tract is functional, the placement of gastrostomy or jejunostomy tubes for enteral nutrition. Occasionally, the surgeon is involved in palliating pain due to a metastatic lesion compressing upon an organ or adjacent nerves. Examples include cutaneous or subcutaneous melanoma metastases, a large ulcerating breast cancer, or a recurrent intra-abdominal sarcoma mass. The surgeon must assess the relative risk-to-benefit ratio in resecting a symptomatic mass knowing that it will not impact the overall survival of the patient. If the quality of life of the individual can be improved at an acceptable operative risk, then the surgical intervention is warranted.
Finally, the surgeon may be called upon to manage oncologic emergencies. Acute hemorrhage and obstruction of a hollow viscus represent the most common potential oncologic emergencies. In these cases, surgeons may have to emergently intervene in the care of a cancer patient, or in some instances, use nonsurgical approaches (such as stents or angiography).
With our improved understanding of inherited genetic mutations and the identification of patients who are predisposed to cancer, surgical therapy has expanded beyond the therapy of established tumors and into the prevention of cancer. Prophylaxis is not a new concept in surgical oncology. Patients with chronic inflammatory diseases are known to be at high risk of subsequent malignant transformation. This typically prompts close surveillance and surgical resection at the first identification of premalignant changes. One of the earliest examples of this is the recommendation for total proctocolectomy for subsets of patients with chronic ulcerative colitis.
The ability to perform genetic screening for relevant mutations has allowed for prophylactic surgery to be implemented prior to the onset of symptoms or histologic changes. Familial adenomatous polyposis (FAP) syndrome, defined by the diffuse involvement of the colon and rectum with adenomatous polyps, almost always predisposes to colorectal cancer if the large intestine is left in place. With the identification of the gene responsible for FAP, the adenomatous polyposis coli (APC) gene, members of families in which an APC mutation has been identified can have genetic testing prior to polyps becoming evident and be considered for prophylactic proctocolectomy. Medullary thyroid cancer (MTC) is a well-established component of multiple endocrine neoplasia syndrome type 2A (MEN2A) or type 2B (MEN2B). Mutations in the RET protooncogene are present in almost all cases of MEN2A and 2B. Family members of MEN patients can be screened for the presence of a RET mutation, and those with the mutation should undergo total thyroidectomy at a young age (6 years for MEN2A, infancy for MEN2B). The role of prophylactic mastectomies has been greatly expanded with the identification of BRCA1 and BRCA2, which can be associated with a lifetime probability of breast cancer of between 40% and 85%. Other prophylactic operations are listed in Table 44–1. However, potential benefits of prophylactic surgeries must be weighed against quality-of-life issues and the morbidity of the surgery. A detailed discussion must be held with each patient considering prophylactic surgery regarding the risks and benefits, so today’s surgical oncologist needs a clear understanding of genetics and inherited risk.
| Prophylactic Surgery | Potential Indications |
|---|---|
| Bilateral mastectomy | BRCA1 or BRCA2 mutation |
| Atypical hyperplasia or lobular carcinoma in situ | |
| Familial breast cancer | |
| Bilateral oophorectomy | BRCA1 mutation |
| Familial ovarian cancer | |
| Hereditary nonpolyposis colorectal cancer | |
| Hysterectomy for endometrial cancer | |
| Colon resection for colon cancer | |
| Thyroidectomy | RET protooncogene mutation |
| Multiple endocrine neoplasia type 2A (MEN2A) | |
| Multiple endocrine neoplasia type 2B (MEN2B) | |
| Familial non-MEN medullary thyroid carcinoma (FMTC) | |
| Total proctocolectomy | Familial adenomatous polyposis (FAP) or antigen-presenting cell (APC) mutation |
| Ulcerative colitis | |
| Hereditary nonpolyposis colorectal cancer (HNPCC) germ-line mutation |
CYTOTOXIC CHEMOTHERAPY
The goal of chemotherapeutic regimens is to deliver pharmacologic agents systemically to eradicate all tumor cells. The ideal tumor drug would kill cancer cells without harming normal tissues. No such agent exists, and most drugs affect normal cells to some extent. The success of chemotherapy relies on the normal cell’s greater capacity for repair and survival relative to tumor cells.
Even a single cancer cell can potentially reproduce to form a lethal tumor. For this reason, the goal of curative chemotherapy must be the complete eradication of all tumor cells. Tumor burden is important in chemotherapy. A large cancer may harbor more than 109 tumor cells. If a tolerable dose of an effective drug killed 99.99% of these cells, the tumor burden would still be 105 cells. The remaining cells, while clinically undetectable, are likely to continue to grow and lead to a clinical recurrence of cancer. For this reason, most chemotherapy protocols rely on repeated administrations of drugs in order to achieve maximal cell killing. Tumor cells may avoid the cell-killing effects of a particular drug because of their stage in the cell cycle, residence in an area protected from the drug (central nervous system), or an inherent resistance to the drug.
Drug resistance plays a large role in chemotherapy failures. Several mechanisms of tumor resistance are known. The multidrug resistance (MDR) gene encodes a protein that actively pumps drugs out of tumor cells. This gene confers resistance on a variety of antitumor drugs, including the antibiotics and plant-derived compounds. Other tumor mechanisms of resistance include the alteration of target enzymes, increased production of a target enzyme to overwhelm the drug, and an increased capability for DNA repair. Tumor resistance to a given chemotherapeutic agent can often be overcome by the administration of multiple drugs.
Hematologic malignancies are typically treated by chemotherapy, radiation, or both, with surgery used primarily for diagnosis and staging. On the other hand, surgery is the primary treatment for nonhematologic malignancies, although there are some exceptions. Anal cancer is cured in approximately 80% of patients with the Nigro protocol—5-FU/mitomycin-C and radiation therapy—as first-line treatment. Testicular cancer, even when metastatic, is curable with bleomycin/etoposide/cisplatin in approximately 85% of patients.
Although all visible tumor may be removed at the time of surgery, microscopic tumor deposits may still be present locally or may have spread to distant locations. Chemotherapy is most effective against very small tumors and microscopic tumor deposits. Therefore, adjuvant chemotherapy is often given to improve the likelihood of cure after surgical resection.
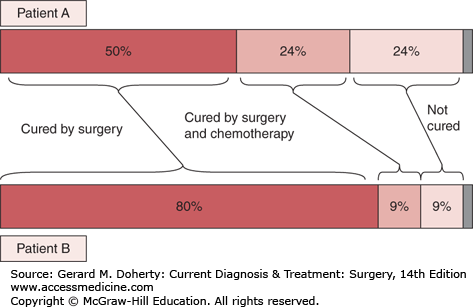
The benefit gained from adjuvant chemotherapy can be thought of in terms of absolute benefit or relative benefit (Figure 44–1). For example, after colectomy for stage III colon cancer, the chance of cure is approximately 50%. This can be increased to approximately 70% by adjuvant 5-FU/leucovorin. This represents a 40% relative benefit (40% more patients are cured with chemotherapy than without chemotherapy) but a 20% absolute benefit (20% of the patients who take the chemotherapy will have altered their outcome). Another way to look at this is that with a 20% absolute benefit, 80% of patients experience the inconvenience and side effects of chemotherapy without gaining any improvement themselves. The decision to receive adjuvant chemotherapy is a balance between the expected benefit of treatment, the patient’s comorbid conditions and general health, and the patient’s wishes.
Figure 44–1.
Benefits of adjuvant chemotherapy. For 100 patients treated with adjuvant chemotherapy, some will be cured by surgery alone (dark red bar), some will die of other causes (gray bar), and some will die of their cancer (light red bar). Adjuvant therapy will prevent a cancer death in a portion of those patients (medium red bar). Adjuvant chemotherapy will result in a relative benefit of 50% for both patient A and patient B, meaning treatment will reduce the likelihood of dying of cancer by 50%. However, the absolute benefit is different for both patients. For patient A, who has a high likelihood of dying of disease, the absolute benefit is 24%. For patient B, who has a good prognosis, the absolute benefit is only 9%.
Neoadjuvant chemotherapy is usually given to facilitate surgical resection by shrinking the primary tumor, or it may convert an unresectable tumor into a resectable tumor. In some cases, this treatment has been shown to prolong survival. Another advantage to neoadjuvant chemotherapy is that it allows the oncologist to observe the primary tumor to determine if it is sensitive to a particular chemotherapeutic regimen. During the course of cancer treatment, it is important to define the progress and outcomes resulting from therapy. The terms complete and partial response are often used as endpoints to evaluate the efficacy of a particular therapeutic regimen. A complete response is defined as the absence of demonstrable cancer. A partial response refers to a reduction of tumor mass by greater than 50%. The patient’s response to neoadjuvant chemotherapy can be an important predictor of outcome.
The majority of patients who are receiving chemotherapy have metastatic disease that is not curable. For these patients, treatment with chemotherapy is intended to prolong survival, improve quality of life, or both. Response rates range from 20% to 75% depending on the tumor type and chemotherapy regimen. However, even a complete remission is rarely durable. Most partial or complete remissions last only months.
As with all therapies, the decision to use chemotherapy must balance the potential benefits with the risks, toxicities, and the patient’s general health and condition. There is little to be gained by treating an asymptomatic patient if no prolongation of survival is expected. A detailed discussion must be held with each individual patient; some patients are more willing than others to tolerate the side effects of chemotherapy. Since the disease is not curable, treatment with single agents, which are less toxic than combination chemotherapy, are often considered, with more willingness to reduce doses for toxicity.
With all forms of curative chemotherapy, the goal is elimination of all tumor stem cells. Cells that are incapable of further division cannot cause progression of a tumor, and the sterilization of a tumor cell is as good as a kill. Chemotherapeutic drugs are generally classified as cell cycle-specific (CCS) drugs, which are toxic to actively proliferating cells, or cell cycle-nonspecific (CCNS) drugs, which are capable of killing cells that are not dividing during drug exposure. These two classifications are not absolute, and many drugs may overlap between the two categories.
In order to achieve maximal cell killing, most therapeutic protocols use combination chemotherapy. Agents with differing mechanisms of action and different toxic side effects are used, allowing for relatively high doses of multiple agents. This method of combining agents helps to combat tumor cell resistance and increase the tumor cell killing while avoiding the compounding of toxic effects.
These agents exert their effects by the transfer of alkyl groups to various cellular components, most importantly by the alkylation of DNA. Alkylators can cause DNA strand breaks, cross-linking of DNA strands, or miscoding of DNA during replication. The alkylating agents are considered cell cycle–nonspecific agents but tend to have their greatest effect on proliferating cells. Normal cells are able to avoid many of the lethal affects of alkylating agents because of their ability to repair DNA. The alkylating agents are effective in treatment of the hematologic malignancies and in a variety of solid tumors such as breast, melanoma, lung, and endometrial cancers. Included in this class are the nitrosoureas (eg, carmustine, semustine, lomustine), cyclophosphamide, chlorambucil, mechlorethamine, dacarbazine, and procarbazine.
The platinum analogs are similar to the alkylating agents. They bind DNA to form interstrand and intrastrand cross-links, leading to inhibition of DNA synthesis and transcription. The mechanisms of cancer cell resistance are also similar to those of alkylating agents: decreased cellular uptake of the drugs, increased activity of DNA repair enzymes, and increased thiol-containing proteins. In addition, resistance to both cisplatin and carboplatin has been associated with a deficiency of mismatch repair (MMR) genes. It is not known why this mechanism of resistance appears to be specific to cisplatin and carboplatin, but the efficacy of the newest platinum analog, oxaliplatin, is not affected by MMR gene deficiency.
Rapidly dividing cells require increased synthesis of nucleic acid precursors. This increased synthesis can be exploited pharmacologically by the antimetabolites. These drugs are analogs of nucleic acids or nucleic acid precursors. The antimetabolites may be incorporated into the nucleic acids of a cell and serve as a false messenger. Antimetabolites can shut down the cellular synthetic machinery by binding to and inhibiting enzymes important in the production of nucleic acids. Since this class of drugs affects all rapidly proliferating cells, they are relatively toxic to normal tissues that have a high rate of cell turnover. Antimetabolites are most effective in the hematologic malignancies but are also used in the treatment of solid tumors such as breast and gastrointestinal cancers. They include methotrexate, mercaptopurine, thioguanine, fluorouracil, and cytarabine.
A variety of antitumor drugs are derived from natural plants (and are also known as plant alkaloids). Vincristine, vinblastine, docetaxel, and paclitaxel work by binding tubulin and poisoning the assembly of microtubules in the mitotic spindle. This leads to mitotic arrest in metaphase, and these compounds are effective only on rapidly dividing cell populations. The plant alkaloids are most useful for hematologic malignancies and breast, renal, testicular, and head and neck cancers.
These plant derivatives exert their antitumor effects by binding to and inhibiting various forms of the enzyme topoisomerase. Topoisomerases are responsible for the maintenance of DNA structure and are also important in the cleavage and religation of DNA strands. Inhibition of these enzymes leads to DNA strand breakage and structural damage. The topoisomerase inhibitors are also cell cycle–specific agents and have their greatest activity against rapidly proliferating cells. Examples include etoposide, teniposide, and topotecan. These drugs are used in the treatment of hematologic malignancies and lung, bladder, prostate, and testicular cancers.
Most of the drugs in this class are derived from the soil fungus Streptomyces. All the antibiotics exert their antitumor effects by interference with the synthesis of nucleic acids. Most of the drugs in this class intercalate in DNA, blocking DNA synthesis and inducing strand breakages. The antibiotics are considered cell cycle–nonspecific, and they have antitumor activity against a wide variety of solid tumors. Included in this class of drugs are doxorubicin, dactinomycin, plicamycin, mitomycin, and bleomycin.
Most side effects from chemotherapeutic regimens are the result of toxicities to rapidly dividing normal cell populations—particularly bone marrow and epithelial cells. Bone marrow suppression is an adverse effect of many of these drugs, resulting in neutropenia, thrombocytopenia, and even anemia. Mucosal ulcerations and alopecia also occur in patients treated with cell cycle–specific agents. Intractable nausea and vomiting is another common side effect that can severely affect quality of life. Testicular or ovarian failure can result from chemotherapy, leading to sterility. Many of these drugs also are powerful teratogens and should be avoided in pregnant patients. Finally, many of the alkylating agents have been implicated in the development of secondary cancers, especially hematologic malignancies.
REGIONAL THERAPY
Systemic chemotherapy is limited by toxicity to the host. Regional delivery of chemotherapeutic agents via arterial cannulation allows for high levels of drugs in the region of the primary tumor while decreasing systemic toxicity.
Isolated limb perfusion (ILP) is a technique for the delivery of chemotherapeutic agents to an extremity with locally advanced cancer and is of benefit primarily in the treatment of extremity melanoma and sarcoma. In this approach, a tourniquet is applied to the extremity to occlude venous outflow. The major artery perfusing the limb is then isolated, cannulated, and perfused with hyperthermic chemotherapeutic agents using a pump oxygenator as for cardiopulmonary bypass. The perfusion is done in the operating room and lasts for approximately 1 hour. The cannula is then removed. Most protocols involve only a single treatment. Melphalan, an alkylating agent, is the most common agent used today in the treatment of both sarcomas and melanomas. In patients with extensive in-transit melanoma confined to an extremity, isolated limb perfusion can provide regional control and palliation. In patients with unresectable extremity sarcomas, preoperative limb perfusion may shrink the tumor and allow for a limb-sparing resection. While improving regional control, this therapy has yet to show a definitive survival benefit. An alternate approach is isolated limb infusion (ILI), which involves using minimally invasive techniques to access the vessels along with a tourniquet to minimize systemic uptake.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree