CHAPTER 4 The Neural Bases of Acupuncture: Central Mechanisms
BASIC CONCEPTS: BRAIN, ORGANS, AND ACUPUNCTURE
New Imaging Techniques and Understanding the Brain
A vast amount of clinical experience with traditional acupuncture has been gained over thousands of years.1–6 On the other hand, over the last several decades, Western medicine has progressed rapidly in both techniques and physiologic understanding of disease. The mechanisms of treatment have benefited from numerous newly developed medical techniques, such as X-ray computed tomography (XCT) and magnetic resonance imaging (MRI). With these developments it is now possible to visualize the inner organs of our living human body as well as the brain in vivo. More recently the development of functional imaging devices such as positron emission tomography (PET)7–9 and functional MRI (fMRI)10–12 has brought about a revolution in our understanding of the brain. PET and fMRI now allow us to image minute changes in glucose utilization as well as oxygen consumption in different parts of the brain. These techniques allow us to observe the brain’s energy metabolism and oxygenation status with a spatial and temporal resolution never before possible.9 Most importantly these technical advances allow us to investigate cortical responses of the brain as a function of acupuncture stimulation, such as the correlation of a specific stimulation with a specific cortical response.13–15 Direct observation of the human brain in vivo while the body is receiving acupuncture stimulation opens a new era in acupuncture research. In addition this information on cortical activation provides clues about how acupuncture works, that is, the nature of the mechanisms involved in acupuncture treatment. These remarkable developments have the potential to finally unravel the millennia-old enigma of acupuncture.
Neural Substrates of Pain Signal Processing
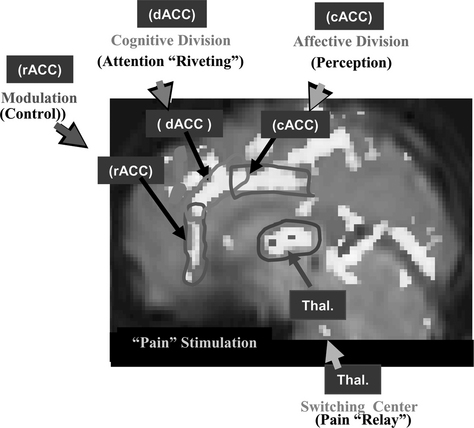
Let us examine the analgesic effect of acupuncture, one of the commonest modalities in acupuncture treatment. First it is necessary to study the basic pain mechanism, from which will follow an explanation of how AA works. In an experiment, pain stimulation was induced by immersion of the index finger into a bath of hot water (about 52° C) for a period of 30 seconds.16 The activation of cortical areas caused by pain stimulation was obtained by fMRI and is shown in Figure 4-1. Three areas are notably involved with pain signal processing and these are indicated by three rectangles (the dorsal anterior cingulate cortex [dACC], the caudal anterior cingulate cortex [cACC], and the rostral anterior cingulate cortex [rACC]) to illustrate more completely the details of the activation pattern. These three areas were chosen because they are believed to be the most significant cortical areas concerned with pain processing, that is, dACC for pain signal “riveting or attention-focusing,” cACC for “perception” of the emotional component of pain, and rACC for “modulation or control” of pain.
The thalamic areas, believed to be the major components of pain signal relay and attention selection centers, are also activated. A most intriguing aspect of this study is that the time-differential activation pattern provides important clues about how various parts of the cingulate cortex are involved in the perception and modulation of pain and in pain processing.16 For instance the rACC is activated during the last minutes of observation, suggesting that some form of the pain modulation process (e.g., the release of endogenous opioids, which initiate the inhibitory process by a stress effect resulting from sustained pain) is taking place at this late period because of sustained pain, as discussed by Zubieta et al.17 Considering the fact that all the cingulate cortices, dACC, cACC, and rACC, together with the thalamus, are activated as a result of pain stimulation, one can hypothesize that the main part of the pain signal processing is a central process rather than a peripheral process. Pain signal relay, attention focusing, pain perception, and modulation or control are all processed in the brain’s center, the cortical and subcortical centers of the brain, including the ACC and the thalamus. This dynamic pain processing pathway appears to provide key information for understanding pain relief mechanisms, such as AA.
ACUPUNCTURE ANALGESIA AND ITS NEURAL SUBSTRATES
Observation of Acupuncture Analgesia by Functional Magnetic Resonance Imaging
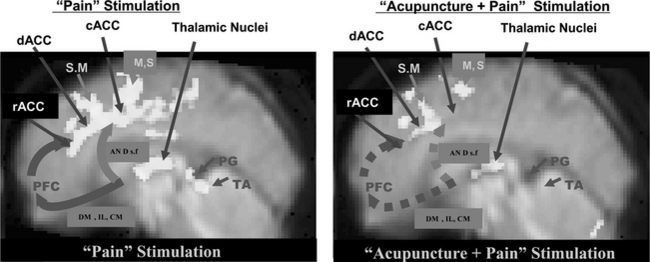
Meridian (Homeostatic) Acupoint
The pain and pain-relief mechanisms involved in AA are complex, and science has yet to provide a convincing physiological explanation of them. Although acupuncture has been used for many centuries,1–6 scientific evidence for the physiology and efficacy of pain treatment has not been established. Possible mechanisms for pain relief in AA have been studied in the West since 1965, beginning with the pioneering work of Melzack and Wall.3 Although rigorous scientific explanations are rare and other evidence is mostly anecdotal, acupuncture has been reported to successfully treat many classes of disease, and be especially effective for the control of pain.1–6,18 As discussed in the previous section, brain imaging tools such as PET and fMRI have made it possible to visualize directly brain function in vivo. fMRI experiments using the new data-processing technique known as dynamic regression analysis are providing the opportunity to study physiologically modulated cortical activation due to “pain stimulation” as well as “pain stimulation after the administration of acupuncture.”16 For the AA study, thermal stimulation was applied in the same way as in the pain study (i.e., immersing the index finger into a bath of hot water with a temperature of 52° C for a duration of 30 seconds). This stimulation resulted in a sequence of different sensations: feelings of heat, unpleasantness, extreme pain, and unbearable pain. Acupuncture stimulus was applied (or administered) to the meridian (homeostatic) acupoints H5 deep fibular (peroneal, Taichong) with manual twirling or rotating of the needle for 30 seconds (approximately one rotation per second), resting for 30 seconds without twirling the needle, and then repeating five times without removing the needle, after which the needle was removed for the remainder of the data acquisition period.19,20 Application of the pain stimulation was continued for 4 minutes after completing the acupuncture stimulation. From this experiment some new observations were made about the relationship between cortical activation and pain as well as the pain-relief mechanism of acupuncture. The experimental data suggest that the cortical areas related to pain signal relaying, attention-focusing or riveting, and perception are the anterior cingulate gyrus and the thalamic areas, as mentioned earlier.16 The experimental results obtained from “pain” and “acupuncture + pain” stimulation are illustrated in Figure 4-2. The activation pattern resulting from pain stimulation alone showed activation of various cortical areas, including the cingulate cortex, the thalamus, and the motor areas (see Figure 4-1). On the other hand, the “acupuncture + pain” experiment revealed a significant decrease in activation of the ACC and the thalamic areas as well as the motor areas. Most of the activations seen in the cingulate cortex and the thalamic nuclei with pain alone become diminished after acupuncture, suggesting that the administration of acupuncture indeed desensitizes or blocks pain perception.20
“Sham” Acupoints: Comparison of Meridian (Homeostatic) and “Sham” Acupoint Stimulation
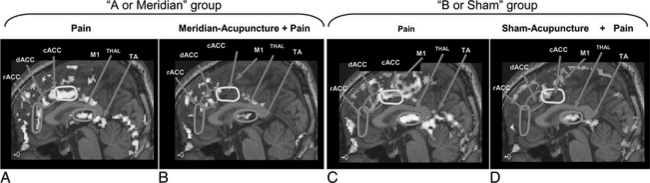
As shown in the previous study on “pain” and “pain with administration of acupuncture,” there appears to be substantial deactivation of the cortical areas when acupuncture is administered compared to when it is not, especially in areas involved with pain signal processing. One of the most interesting aspects of acupuncture, however, is the point specificity, that is, how accurately the acupoint must be localized and how the outcome of the acupuncture treatment is affected by uncertainty in localization. This question of point specificity was studied by comparing the result of traditional “meridian” acupoint stimulation with the result of stimulating “sham” points.19,20 Please note that the “meridian” acupoint Liv 3 Taichong used in these experiments is one of the 24 homeostatic acupoints (HAs) H5 deep fibular (peroneal) described in Chapter 5. All 24 of these HAs are in fact major meridian acupoints in the traditional acupuncture system (see Appendix II). “Sham” points are deliberately chosen as much as a few centimeters away from the traditional meridian acupoints. This study was a comparison of two sets of experiments, namely, one with “pain” versus “meridian acupuncture + pain” and the other with “pain” versus “sham acupuncture + pain.” Figure 4-3 shows the results.19,20
When the results of “pain” versus “sham acupuncture + pain” (see Figure 4-3, C and D) are compared with the traditional “pain” versus “meridian acupuncture + pain” results (see Figure 4-3, A and B), there appears to be very little difference, which suggests that sham and meridian acupuncture might share a common mechanism. This finding also brings into question the point specificity of acupuncture, at least in the case of AA.20
The similarity of the activation patterns in the two cases of meridian and sham acupuncture supports the hypothesis that the effect of acupuncture, at least for AA, is simply the effect of the stress-induced hypothalamic-pituitary-adrenal (HPA) axis response (the pathways are discussed more specifically below). That is, decreased activation may be due to “sustained pain stress” rather than to stimulation of a specific point as is taught in traditional acupuncture.17,21 Acupuncture or acupuncture-like sensory stimulation induces activation of the HPA axis and therefore activation of the endogenous central opiate circuitry, thereby reducing or inhibiting the ascending pain signals.
HYPOTHESIS OF ACUPUNCTURE MECHANISMS
Hypothesis of Acupuncture Mechanisms and Neural Substrates
Humoral, Neural, and Immune Responses Related to Acupuncture
As already described, acupuncture therapy has demonstrated efficacy in several clinical areas; among these is pain. Although the understanding of pain has progressed immensely in the last two decades,1–6,18 the underlying mechanisms of acupuncture in general and its analgesic effect in particular are still not clearly delineated. The leading hypotheses include the effects of local stimulation, neuronal gating, the release of endogenous opiates, and the placebo effect. Accumulating evidence suggests that the CNS is essential for the processing of these effects via its modulation of the autonomic nervous system (ANS), the neuroimmune system, and hormonal regulation. Because these processes are programmed into basic survival mechanisms, it appears vital to seek an understanding of the effects of acupuncture within a framework of neuroscience.
In view of the preceding results, we propose a model that incorporates the stress-induced HPA axis model of Maier et al22 and Akil et al,23 the cholinergic antiinflammatory action model of Tracey et al,24–26 and the recently observed neuroimaging results of Cho et al,19,20 Zubieta et al,17 and Petrovic et al.21 As already mentioned, over the last two decades there have been numerous developments in neuroscience concerning pain, the ANS, the immune system, and functional brain imaging.27–33 The underlying mechanisms of acupuncture, however, have appeared too diverse to be understood in a quantitative and rational manner and require further study. Because acupuncture therapy is claimed to be effective in many conditions, including acute pain, investigators have attempted to explain the underlying mechanism of acupuncture in many different ways: as an effect of local stimuli, a neural reflexive response, a humoral outflow response, and a placebo effect.1–6,34 In addition, physiologic studies using animal models and human subjects have also been developed to explain AA and these have generated increasing scientific support and enhanced the credibility of acupuncture as a treatment option.1–6
One clear fact is that the CNS controls the autonomic processes related to visceral reactivity; cognitive processes related to pain perception, learning, and memory; and other physiologic processes related to pain. Regardless of which pathways are being contemplated, whether it is supposed to work by neural, humoral, or neuroimmunologic interactions, it appears inevitable that the brain is involved in the behavioral effects of acupuncture. The CNS is precisely the network that is essential for information processing between afferent stimuli (inputs) and efferent responses (outputs), as well as control of stress hormones, the ANS, and even the immune system, all of which are essential for survival. On the other hand, recently developed brain imaging techniques, such as PET and fMRI, are beginning to shed light on the workings of the human brain, including acupuncture-related cortical responses.7–15 Because these techniques provide the opportunity to observe neuronal activities directly, both spatially (anatomically) and temporally (in a time-dependent manner), they allow quantitative analysis of the living brain while it is engaged in deliberate actions (e.g., acupuncture or acupuncture-like stimulation). As a result recent acupuncture research has been directed increasingly toward neuroimaging, with molecular science and pharmacokinetics as its bases.16–20
In this section, we briefly review how the scientific understanding of acupuncture has recently evolved based on neuroimmunophysiologic and molecular aspects, and evidence obtained by functional brain imaging.13–21,27–34 In addition, we propose a hypothesis, as well as a model, of the acupuncture mechanism that includes the stress-induced HPA-axis hypothesis,22,23 the neural-immune interaction of the cholinergic antiinflammatory activity hypothesis,24–26 and the recently obtained neuroimaging evidence.17,19–21