OUTLINE
I. OPHTHALMIC SOLUTIONS
A. Definition: “Ophthalmic solutions are sterile solutions, essentially free from foreign particles, suitably compounded and packaged for instillation into the eye” (1).
B. Special cautions As stated in the definition, ophthalmic solutions must be sterile and free from particulates. Because of the inherent danger of causing serious eye infection and even loss of eyesight through the use of contaminated ophthalmic preparations, the greatest care must be used in preparing these solutions. Unfortunately, there have been a number of accidents in recent years in which preparations that were intended to be sterile were compounded in pharmacies and were not processed properly and so were not sterile. In one case, the preparations were ophthalmic solutions, and several patients were injured and two patients lost eyes (2). Ophthalmic solutions should be compounded extemporaneously only if the following conditions apply:
1. There are no available commercial product alternatives.
2. The compounder possesses the appropriate knowledge and technique.
3. The necessary equipment and supplies are available to make sterile solutions.
C. Desired properties
1. Sterility and clarity are not just desired properties; they are absolute requirements for ophthalmic solutions.
2. When the solution is dispensed in a multidose container that is to be used over a period of time longer than 24 hours, a preservative must be added to ensure microbiologic safety over the period of use. If the solution is to be used during or following a surgical procedure on the eye, the solution should not contain a preservative because this may be irritating or damaging to exposed ocular tissues (1). In this case, if a compounded solution is needed, a sterile, freshly prepared solution should be used.
3. Although solutions with the same pH as lacrimal fluid (7.4) are ideal, the outer surfaces of the eye tolerate a larger range, 3.5 to 8.5 (1). The normal useful range to prevent corneal damage is 6.5 to 8.5 (3). The final pH of the solution is often a compromise, because many ophthalmic drugs have limited solubility and stability at the desired pH of 7.4. Buffers or pH-adjusting agents or vehicles can be added to adjust and stabilize the pH at a desired level (1).
4. Solutions that are isotonic with tears are preferred. An amount equivalent to 0.9% NaCl is ideal for comfort and should be used when possible. The eye can tolerate within the equivalent range of 0.6% to 2% NaCl without discomfort (1). There are times when hypertonic ophthalmic solutions are necessary therapeutically or when the addition of an auxiliary agent, required for reasons of stability, supersedes the need for isotonicity. There are several methods useful for calculating isotonicity. These are described and illustrated in Chapter 11, Isotonicity Calculations.
5. As with all pharmaceutical solutions, ophthalmic solutions must be chemically and physically stable. This subject is discussed in Chapter 27, Solutions, and in Chapter 37, Compatibility and Stability of Drug Products and Preparations.
6. The active ingredient(s) should be present in the most therapeutically effective form. This goal must often be compromised for reasons of solubility or stability of the active ingredient or comfort for the patient. For example, though most drugs are most active in their undissociated form, they are least soluble in this form. They may also be less stable at pH values that favor the undissociated form (1).
7. Ophthalmic solutions should be free of chemicals or agents that cause allergy or toxicity to the sensitive membranes and tissues of the eye. Auxiliary agents, such as preservatives and antioxidants, should be added with care, because many patients are sensitive to these substances. Before adding an auxiliary agent, check with the patient about allergies and sensitivities.
D. Active ingredients and components
1. The easiest way to compound an ophthalmic solution is to use sterile water or sterile isotonic saline solution to dissolve or dilute a manufactured sterile, solid drug or concentrated, aqueous solution of the drug. If the active ingredient is not available in a sterile form, a nonsterile but high-quality pure powder may be used. Use of each type of ingredient is illustrated in the sample prescriptions at the end of this chapter.
2. In deciding whether other components are needed, the compounder must use knowledge of chemistry, pharmaceutics, microbiology, and therapeutics. Possible auxiliary agents include buffers, tonicity adjustors, preservatives, antioxidants, and viscosity-inducing agents.
3. Buffers
a. Formulas for a variety of ophthalmic buffering vehicles are given in Chapter 18, Buffers and pH Adjusting Agents. The most widely used ophthalmic buffer solutions are Boric Acid Vehicle and Sorensen’s Modified Phosphate Buffer.
b. Boric Acid Vehicle
(1) Boric Acid Vehicle is a 1.9% aqueous solution of boric acid. This concentration is approximately isotonic with tears, but it is not iso-osmotic with red blood cells. This is because the membrane on red blood cells is permeable to boric acid. Therefore, although boric acid is a common ingredient in ophthalmic preparations, it may not be used parenterally.
(2) Boric Acid Vehicle has a pH of approximately 5. Though this vehicle does not possess large buffer capacity, it will stabilize the pH of a drug solution close to pH 5. This, of course, depends on the pH generated by the drug itself and on the buffer capacity of the drug.
(3) Because Boric Acid Vehicle does not have strong buffering capacity, it is useful when extemporaneously compounding ophthalmic solutions of drugs that are most stable at acid pH. Boric Acid Vehicle will stabilize the pH of the solution at approximately 5 for the short expiratory periods used for compounded solutions. At the same time, its weak buffer capacity is easily overcome by the natural buffers in lacrimal fluid, so its acidic solutions are not uncomfortable when instilled in the eye.
(4) Boric acid is available as crystals and powder. The crystals are preferred for making Boric Acid Vehicle, because they give more crystal-clear solutions than does the powder.
(5) According to the USP XXI, Boric Acid Vehicle is useful for making ophthalmic solutions of the salts of the following drugs: benoxinate, cocaine, dibucaine, phenylephrine, piperocaine, procaine, proparacaine, tetracaine, and zinc (4). King’s Dispensing of Medication adds ethylmorphine, neostigmine, ethyl hydrocupreine, and phenacaine to the list (5).
(6) A modified Boric Acid Vehicle can be made for drugs that are especially sensitive to oxidation. The antioxidants sodium bisulfite and sodium metabisulfite in a concentration of 0.1%, or the chelating agent disodium edetate in a concentration of 0.1%, may be added to retard oxidation. This modified vehicle is useful for drugs prone to oxidation. Examples include physostigmine and epinephrine (5).
c. Sorensen’s Modified Phosphate Buffer
(1) Sorensen’s Phosphate Buffer is made using two stock solutions: one acidic, containing NaH2PO4, and one basic, containing Na2HPO4. The formulas for the stock solutions are given in Table 18.3 of Chapter 18, Buffers and pH Adjusting Agents. Each solution is 1/15 or 0.067 M. The stock solutions are mixed in an appropriate ratio to give a desired pH. Table 18.3 also shows the volume ratios to mix to give a desired pH.
(2) When mixed as directed, these buffer solutions are not isotonic. If an isotonic buffer is desired, a solute must be added for tonicity adjustment. Examples of possible solutes include sodium chloride, sodium nitrate, and dextrose. The choice of a tonicity adjusting solute depends on the compatibility of the solute with the other ingredients in the formulation. Table 18.3 shows the weight in grams of sodium chloride that must be added to 100 mL of buffer solution to give an isotonic buffer. If a different solute is desired, the amount of this solute can be calculated using its sodium chloride equivalent (see section III of Chapter 11 for sample calculations).
(3) Sorensen’s Modified Phosphate Buffer has a significant buffer capacity and should not be used outside the pH range of 6.5 to 8.0.
(4) According to the USP XXI, Sorensen’s Modified Phosphate Buffer is useful for making ophthalmic solutions of the salts of the following drugs: pilocarpine, eucatropine, scopolamine, and homatropine (4). King’s Dispensing of Medication adds atropine, ephedrine, and penicillin to this list (5).
d. An alternative way of adding a buffering vehicle is to use a manufactured artificial tears product that contains an appropriate buffer. Caution must be exercised in using these products, because they may also contain other ingredients, such as viscosity-inducing agents and preservatives that could cause compatibility problems. Drug Facts and Comparisons, the PDR for Ophthalmology, and the product prescribing information (product package insert) list the ingredients of artificial tears products.
e. Amount of buffer solution to use
(1) The buffer solution is often used as the tonicity adjustor for the ophthalmic solution. Under these circumstances, isotonicity calculations determine the amount of buffer solution to use.
(2) A minimum amount of buffer is needed to provide a buffering effect. One general rule states that the concentration of the buffer should be ten times that of the drug, the concentrations of both expressed in molar quantities (6). Another recommendation states that a concentration of 0.05 to 0.5 M of buffer gives sufficient buffering capacity (7). Both of these general rules are somewhat arbitrary; they are based on buffer solutions that are made from conjugate pairs with a p Ka within one unit of the desired pH. Although Boric Acid Vehicle has a higher molar concentration (approximately 0.3 M) than has Sorenson’s Modified Phosphate Buffer (0.067 M), Sorensen’s buffer has significantly greater buffering capacity because it consists of a conjugate pair, whereas Boric Acid Vehicle is merely a solution of a mild acid.
(3) Because the aforementioned general rules require a bit of calculation, a more simplified recommendation, which gives a “ballpark” figure, is for the volume of the buffer solution to be one-third of the volume of the finished product. If isotonicity calculations show that less than one-third of the final volume should be buffer solution, a compromise between isotonicity and buffering is needed. The one-third of the volume is an oversimplified but convenient figure (6).
4. Tonicity adjustor
a. As indicated earlier, the buffer solution is convenient to use as the tonicity adjustor.
b. In circumstances when a buffer is not needed, any compatible salt or nonelectrolyte that is approved for ophthalmic preparations may be used. Sodium chloride, sodium nitrate, sodium sulfate, and dextrose are common neutral tonicity adjustors.
5. Antimicrobial preservatives
a. USP Chapter 〈1151〉 states the following concerning the use of preservatives in ophthalmic solutions:
Each solution must contain a suitable substance or mixture of substances to prevent the growth of, or to destroy, microorganisms accidentally introduced when the container is opened during use. Where intended for use in surgical procedures, ophthalmic solutions, although they must be sterile, should not contain antibacterial agents, since they may be irritating to the ocular tissues (1).
b. The authors of Extemporaneous Ophthalmic Preparations give the following advice on the use of antimicrobial preservatives in ophthalmic solutions (8):
(1) Because of preservative toxicity, especially following ocular surgery, avoid preservatives, if possible, and use either unpreserved Sterile Water for Injection or Sodium Chloride Injection 0.9% as vehicles for ophthalmic drugs. This means that the solution should be discarded after 24 hours because of the danger of contamination by microorganisms. This practice is practical only in a hospital or institutional setting where fresh solution can be furnished every day, so it is useful primarily for inpatients.
(2) For ambulatory patients and when hospitalized patients are discharged, it can be assumed that the eye has sufficiently healed so that it is less vulnerable to irritation and toxicity due to preservatives. At this time, the solution vehicle can be changed to Bacteriostatic Water for Injection or Bacteriostatic Sodium Chloride Injection. The beyond-use date is then based on the chemical stability of the active ingredient(s). (Note: A recent revision to USP Chapter 〈797〉 puts additional time limits on the beyond-use dates of these compounded sterile preparations; see the section later in this chapter on beyond-use dates.)
(3) Manufactured multidose artificial tears products contain one or more preservatives; they provide another alternative ophthalmic vehicle for ambulatory patients. When using these products for vehicles, consideration must be given to the volume of any added solution so that the preservative in the product is not diluted beyond its effective concentration.
c. Agents: Although more than a dozen preservatives are approved for ophthalmic solutions (see Table 16.2 in Chapter 16, Antimicrobial Preservatives), there is no ideal ophthalmic preservative.
(1) Benzalkonium chloride (BAC), phenylmercuric acetate (PMA) or phenylmercuric nitrate (PMN), thimerosal, and chlorobutanol are the most commonly used ophthalmic preservatives. Information on each of these agents, including official articles, solubilities, effective concentrations, and information on incompatibilities, can be found in Chapter 16.
(2) Benzyl alcohol and the parabens are not often used in manufactured ophthalmic products, but they are approved for ophthalmic use (9). They are the preservatives most commonly found in Bacteriostatic Water for Injection and Bacteriostatic Sodium Chloride Injection.
d. Before adding a preservative, always check on patient sensitivity, on compatibility of the preservative with all other ingredients in the formulation, and on recommended preservative concentration. Martindale:The Complete Drug Reference and Handbook of Pharmaceutical Excipients are two excellent resources for compatibility information.
e. Table 33.1 gives the maximum concentrations of some common preservatives approved for use in nonprescription ophthalmic products (10).
6. Antioxidants
a. Check references and use your general knowledge of chemistry to decide whether the active ingredient or ingredients are subject to oxidation. If oxidation is a problem, an antioxidant may be necessary or recommended. If an antioxidant is recommended, check references for compatibility information. Maximum concentrations of antioxidants approved for use in nonprescription ophthalmic products are given in Table 33.2 (10).
b. Agents:Although several antioxidants are approved for ophthalmic solutions, as with preservatives, they all have some disadvantages. Sodium bisulfite, sodium metabisulfite, and disodium edetate are commonly used antioxidants for ophthalmic products. Information on each of these agents, including descriptions of official articles, solubilities, and effective concentrations, can be found in Chapter 17, Antioxidants.
(1) As reported in Chapter 17, sulfites must be used with caution because of reported incidents of allergic reactions to these compounds. Even though they have been used as antioxidants in solutions of epinephrine, they are reported to inactivate this compound. The reaction is pH dependent, and boric acid has a stabilizing effect (11).
(2) As discussed in Chapter 17, disodium edetate is technically not an antioxidant but a chelator of heavy metal ions. It serves as an antioxidant for drugs that have their oxidation catalyzed by heavy metals. Edetate is reported to have a synergistic effect on the preservative effectiveness of BAC (12); this may be a reason for its presence in many commercial ophthalmic products preserved with BAC. Although edetic acid is also an effective antioxidant, it is not often used in compounding ophthalmic solutions because of its poor water solubility; disodium edetate is the preferred form because it is water-soluble.
7. Other agents:When needed, wetting, clarifying, and viscosity-inducing agents may be added to ophthalmic solutions. Maximum concentrations of these components approved for use in non-prescription ophthalmic products are given in Table 33.2.
II. NASAL SOLUTIONS
A. Definition: These solutions are sprayed or instilled into the nasal cavity. Although they are not specifically mentioned as a class in USP Chapter 〈1151〉, there are official USP nasal solutions, such as Naphazoline Hydrochloride Nasal Solution. Nasal solutions are most often used for local action, but they may also be used for systemic effect.
1. While not quite as critical as for ophthalmic solutions, nasal solutions should be sterile, and compounders should make this dosage form only when they have the knowledge and necessary equipment and when there is no alternative commercial product.
2. At one time, nasal solutions were used almost exclusively for local action but, in recent years, this route of administration has also been used for systemic effects. Compounding and dispensing nasal solutions for systemic administration should be done with the greatest caution. Although procedures have been described in the literature for calibration of droppers and nasal sprays, results in practice have not been encouraging. In one study of nasal droppers, ten physicians acted as the test subjects; all ten overused the drug by a range of 41% to 338% (13). Similar results were found during an informal study conducted by pharmacy students using a procedure recommended for calibration of nasal spray bottles in which nasal solution was sprayed into a tared plastic bag and the bag then reweighed to determine quantity per spray. For systemic nasal drug administration, metered-dose drug delivery systems are recommended, especially when potent drugs are involved. Nasal actuators that deliver 0.1 mL per pump are available from some vendors of compounding supplies.
C. Desired properties and components
1. The FDA Center for Drug Evaluation and Research (CDER) has issued Guidance for Industry: Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—Chemistry, Manufacturing, and Controls Documentation (available on the FDA Internet site at http://www.fda.gov/cder/guidance/4234fnl.htm, accessed February 2008) (14). This guidance was written for the pharmaceutical industry, and while the information and recommendations do not impose mandatory requirements either on the industry or on pharmacies that compound these preparations, the information provided is very useful.
2. Nasal solutions should be sterile when dispensed. The section on nasal solutions in the FDA Guidance for Industry states that microbial quality should be controlled by tests and criteria as given in USP Chapter 〈61〉, Microbial Limit Tests (14). Though there is no absolute requirement for sterility of nasal solutions in the USP (15) or the FDA Guidance for Industry (14), sterility is considered to be an important safety issue, so it is recommended that pharmacists who compound nasal solutions render them sterile at the time of dispensing (16).
3. Multidose containers of nasal solutions should contain an antimicrobial preservative or other agent(s) that will prevent the growth of microorganisms, which may be introduced in the preparation during use.
4. Normal nasal secretions have a pH in the range of 5.5 to 6.5. Because nasal secretions lack significant natural buffer capacity, highly buffered solutions, especially outside the normal pH range, should be avoided (17).
5. The cilia in nasal passages are sensitive to osmotic pressure, so nasal solutions should be as close to isotonic as is possible. Nasal solutions with osmolarity comparable to aqueous 0.5% to 2% sodium chloride solutions are relatively comfortable and should not harm nasal cilia.
D. Active ingredients and components
1. As with ophthalmic solutions, active ingredients for nasal solutions may be bulk powders or liquids or sterile manufactured products.
2. The vehicle for nasal solutions is usually water, but it may be a cosolvent system, provided the other solvent or solvents are approved for internal use.
3. Buffers
a. If a buffer is desired with a pH in the neutral range, a dilute phosphate buffer at pH 6.5 is recommended (16). The isotonic Sorenson’s Modified Phosphate Buffer Solution at pH 6.5, as given in Table 18.3 in Chapter 18, would be a good choice.
b. If a buffer is needed for purposes of drug stability or solubility with a pH that is outside the normal range, a buffer or pH-adjusting agent that has low buffer capacity should be selected. See Chapter 18 for more information on this subject.
4. Antimicrobial preservatives
a. Antimicrobial preservatives that are approved for internal use would be suitable for preserving nasal solutions. These are listed and described in Chapter 16.
b. When selecting a preservative, check for compatibility with active ingredients and other necessary added excipients. Also check to be sure that the preservative is active at the pH of the finished nasal solution.
5. Tonicity adjustors: Sodium chloride and dextrose are recommended tonicity adjustors for nasal solutions (17).
6. Other components
a. In addition to preservatives, buffers, and tonicity adjusting agents, nasal solutions may also contain antioxidants, surfactants, and viscosity-inducing agents.
b. The effect of tonicity and the specific effects of various drugs, salts, surfactants, cosolvents, oils, and preservatives was investigated in the late 1940s and early 1950s by Proetz (18). Sample Prescription 33.5 uses a formula developed by Proetz for a nasal preparation. A summary of this work can be found in the seventh edition of Dispensing of Medication, pages 913–915. Some findings helpful in formulating compounded nasal solutions are given here (19).
(1) Although nasal passages can tolerate a relatively wide range of tonicity without pain, isotonicity is important. Highly hypertonic solutions (4% to 4.5% sodium chloride solutions) and hypotonic solutions (0.3% or less sodium chloride solutions) were found to cause damage to nasal cilia.
(2) Alcohol in concentrations up to 10%, when in an isotonic solution, caused no problems.
(3) BAC in concentrations up to 0.1%, if incorporated into an isotonic saline solution, showed no damage to cilia.
(4) Anionic surfactants such as sodium lauryl sulfate and docusate sodium could be used in concentrations of 0.01% without pain or a burning sensation, but concentrations of 0.05% caused some discomfort. Nonionic surfactants were acceptable at much higher concentrations.
III. INHALATION SOLUTIONS (1,20)
A. Definition: Inhalations are dosage forms “designed to be dispersed in a current of air and drawn into the airways when the patient breathes in” (20). They are administered either by the nasal or oral route, with the respiratory tract as the intended site for local effect or for systemic absorption of an active ingredient. They may be drugs, solutions, or suspensions (1).
B. Cautions: As with ophthalmic and nasal dosage forms, inhalations should be compounded with great care and only when there is no alternative commercial product available.
C. Desired properties and components
1. Inhalation solutions are required to be sterile: Section III. F.2.f of the FDA Guidance for Industry states, “All aqueous-based oral inhalation solutions, suspensions, and spray drug products must be sterile” (14). Although this guidance was written for the pharmaceutical industry, compounded inhalation solutions must also be sterile.
2. The usual vehicles for inhalation solutions are Sterile Water for Inhalation and Sodium Chloride Inhalation Solution 0.9%. Both meet the sterility standards in USP Chapter 〈71〉, Sterility Tests. Neither contains an antimicrobial preservative, so if either of these is used as the vehicle for a compounded inhalation solution, it must be packaged and labeled for single use (15), or a preservative must be added. Sodium Chloride Injection 0.9% and Sterile Water for Injection are also acceptable vehicles. Small quantities of cosolvents such as alcohol or glycerin may be added when needed (20).
3. As with ophthalmic and nasal solutions, inhalation solutions should be as close to isotonic as is possible. Sodium chloride is the most common tonicity–adjusting agent.
4. An antimicrobial preservative is required for any inhalation solution dispensed in a multidose container (21). Preservatives that are approved for internal use would be suitable for preserving inhalation solutions; these are listed and described in Chapter 16. When selecting a preservative, check for compatibility with active ingredients and other necessary added excipients. Also check to be sure that the preservative is active at the pH of the finished inhalation solution.
5. Inhalation solutions may contain additives similar to those used in nasal solutions, but agents such as preservatives, antioxidants, buffers, and surfactants should be used only as necessary, and the concentration of these additives should be as low as possible. These solutions are delivered to very sensitive tissues in the lungs, and this should always be considered in formulating a solution for inhalation.
6. For proper delivery of solution to the respiratory tract, inhalation solutions must first be nebulized to form very small, uniform droplets that will pass through the mouth or nose, throat, and bronchial tree to the bronchioles and alveoli of the lungs. Hand-held nebulizers and intermittent positive-pressure breathing (IPPB) machines are available for this purpose (1).
NEBULIZER
IV. IRRIGATION SOLUTIONS
A. Definition: These are sterile solutions used to soak, flush, or irrigate wounds or body cavities, such as the bladder (1,20).
B. Cautions: As with the other sterile solutions discussed in this chapter, irrigation solutions should be compounded with great care and only when there is no alternative commercial product available. In 1990, the FDA issued an “alert letter” to pharmacists after the death of two hospital patients; the incident involved incorrectly prepared irrigation solutions for surgical procedures (2). Such solutions are administered to vulnerable body tissues and must be sterile; they should be handled in the same fashion as parenteral products.
C. Desired properties and components
1. Irrigation solutions must be sterile.
2. They are not for parenteral use and should be labeled “not for injection” and “for irrigation only.”
3. The usual vehicle for irrigating solutions is water.
4. Because irrigating solutions come in contact with open wounds and delicate body tissues and membranes, special consideration must be given to isotonicity and pH of the solution. Additives may be necessary to achieve these objectives, but they must be chosen with great care.
V. COMPOUNDING PROCEDURES
A. Follow the usual compounding procedures with respect to checking doses and concentrations, general stability and compatibility of the formulation ingredients, selection of ingredients and equipment, and so on as outlined in Chapter 12, General Guidelines for Preparing Compounded Drug Preparations, and as illustrated in the sample prescriptions that follow. For ophthalmic solutions, the ASHP Technical Assistance Bulletin on Pharmacy-Prepared Ophthalmic Products gives good general instructions on preparing these solutions (22). (Available on the American Society of Health-System Pharmacists’ Internet site at http://www.ashp.org/, Accessed December 2007).
B. Because these are sterile dosage forms, their preparation and packaging should be done in a sterile environment such as a laminar airflow workbench or a barrier isolator. A sterile, particle-free solution can be achieved by one of the following methods.
1. Using sterile parenteral drug products as the solution ingredients, prepare the solution as you would a parenteral preparation, using aseptic technique and packaging the solution in a clean, particle-free, sterile container. See Chapter 32, General Principles of Sterile Dosage Form Preparation, and Chapter 34, Parenteral Preparations, for a discussion of the principles and sample prescriptions using techniques for this type of aseptic processing. Sample Prescriptions 33.3 and 33.4 in this chapter illustrate the use of sterile manufactured products for making compounded ophthalmic solutions.
2. Prepare the solution using nonsterile but high-quality ingredients, and filter the solution using a 0.2- or 0.22-μm bacterial filter into a dispensing container that is clean, particle-free, and sterile. For solutions sterilized by bacterial filtration, a small excess is often prepared to allow for loss during the filtration process. Compatibility and stability of the preparation ingredients should be considered, because some bacterial filters adsorb certain types of drug molecules; large molecules such as peptides or proteins are particularly vulnerable. Sample Prescriptions 33.1, 33.2, and 33.5 illustrate the use of a bacterial membrane filter unit.
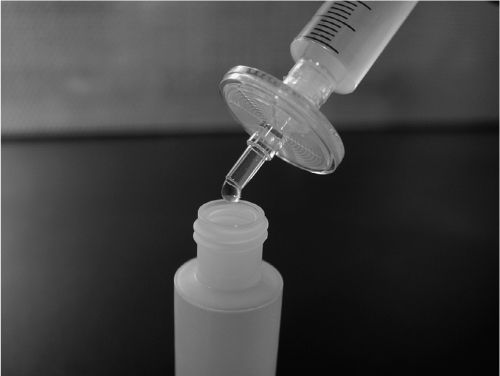
STERILIZATION OF A COMPOUNDED SOLUTION USING A BACTERIAL MEMBRANE FILTER
3. If an autoclave is available, terminal steam sterilization can be used. In this case, the solution may be prepared using nonsterile but high-quality ingredients and packaged in an appropriate clean, particle-free container that is stable to the elevated temperature and pressure needed for steam sterilization. The preparation is then autoclaved in the dispensing container.
a. Usual quality control procedures for steam sterilization must be used. Monitoring devices that track and record time, temperature, and pressure are used, as is verification of the autoclave cycle through use of biologic and other indicators. For pharmacies that have access to autoclaves in institutional settings such as hospitals, the methods are well controlled and documented as required by accreditation standards. Pharmacists using their own equipment should employ standard operating procedures that assure that the equipment and methods give preparations that are sterile. The accidents with nonsterile ophthalmic solutions, which were discussed at the beginning of this chapter, involved steam sterilization procedures that were inadequate (2).
STERILE DROPPER BOTTLES
NASAL SPRAY BOTTLES

NASAL ACTUATOR
b. Because steam sterilization uses elevated temperature and pressure, consider drug and container stability under these conditions before using this method.
4. Packaging must be in a sterile container.
a. For small volumes of solution, presterilized dropper containers, in sizes 3 mL, 7 mL, and l5 mL, are available for purchase from vendors of compounding supplies.
b. An alternative for an ophthalmic or nasal preparation is to empty the contents from the container of a manufactured sterile product (such as artificial tears for an ophthalmic solution or sterile saline nasal mist for a nasal solution), rinse the sterile container with sterile water, and use this container for the compounded preparation.
c. Dropper bottles, nasal spray bottles, and nasal actuators (with controlled volume per spray) are available from compounding suppliers. If these are not sterile as purchased, they should be sterilized before use.
d. Most often, sterile irrigation solutions are prepared by adding ingredients to a bottle of Sterile Water for Irrigation or Sodium Chloride Irrigation. This then also provides the sterile container.
e. Nonsterile containers can be sterilized using either an autoclave or an ethylene oxide sterilizer. Before using one of these methods, check to be sure that the container and closure materials will withstand the required sterilization conditions.
VI. BEYOND-USE DATING
A. Manufactured products
1. Even for multidose manufactured products that contain antimicrobial preservatives, shorter beyond-use dates (BUDs) are recommended for these products than for nonsterile dosage forms because of the danger of contamination during use and the serious consequences of using a nonsterile product. Research has been done and papers have been published on loss of sterility of ophthalmic solutions under conditions of use (23). As a result, some hospitals and nursing homes use a policy of discarding ophthalmic products 30 days after unsealing. The nurse or caregiver dates the sealed ophthalmic container when the seal is broken and the bottle is entered for the first time, and any unused portion is discarded after 30 days. For sterile multi-dose products, USP Chapter 〈797〉 recommends a 28-day BUD after first entry; this recommendation is for sterile, manufactured, preserved solutions handled by health care professionals (24). The pharmacist should consider the expected storage and handling conditions for dispensed sterile products when assigning a BUD.
2. For unpreserved sterile manufactured products that are available in single-dose or single-use quantities, manufacturers recommend immediate use on unsealing the container and discarding any unused solution. USP Chapter 〈797〉 recommends a 1-hour limit if the container is opened outside a controlled ISO Class 5 environment and a 6-hour limit if the container is opened and maintained within a Class 5 or cleaner air space (24). (See Chapter 32 for a discussion of ISO classes.)
B. Compounded solutions
Compounded sterile solutions are discussed in both USP Chapter 〈795〉 and USP Chapter 〈797〉; using these two chapters as guides, the most conservative recommended BUD should be used.
1. USP Chapter 〈795〉
Chapter 〈795〉 on pharmacy compounding of nonsterile preparations recommends a maximum 14-day dating for all water-containing liquid preparations made with ingredients in solid form when the stability of the ingredients in the formulations is not known (25). Obviously, for drugs that are labile to chemical degradation, a shorter dating must be used.
2. USP Chapter 〈797〉
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree