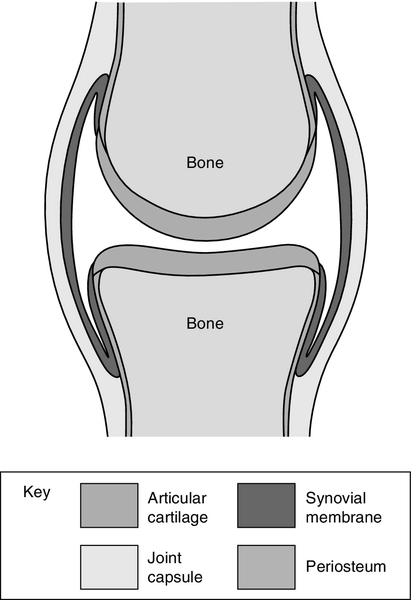
CHAPTER 32 CHAPTER OUTLINE DISORDERS OF THE ARTICULAR SYSTEM The connective tissue diseases ARTICULAR INVOLVEMENT IN ENDOCRINE AND METABOLIC DISEASES LABORATORY TESTING IN ARTICULAR DISEASE Anaemia in rheumatoid arthritis While biochemical abnormalities themselves are responsible for few articular disorders, clinical biochemistry plays an important part in the diagnosis, treatment and monitoring of many of these conditions. This chapter starts with a description of the articular system and its diseases, which will put the role of biochemistry in these conditions into perspective. Crystal synovitis, as seen in gout and pseudogout, is the only common articular condition caused by an underlying biochemical abnormality. This is described in some detail along with examination of synovial fluid, the one biochemical investigation that is primarily the province of the rheumatologist. Some diseases with a primary biochemical abnormality like diabetes, alkaptonuria, endocrine conditions and haemochromatosis have articular manifestations and also warrant discussion. Immunological abnormalities are at the root of an important group of rheumatic diseases, the connective tissue diseases. Autoantibody formation is a major disease manifestation and, in some hospitals, tests to identify and measure autoantibodies are performed in the biochemistry laboratory. These tests are described briefly. The joint is the pivotal structure of the articular system. Joints can be considered as discontinuities in the skeleton that permit controlled mobility. They have different structures depending on their functional requirements. When no movement is needed, the joint is bound together by tough fibrous tissue (e.g. the skull ‘sutures’). In cartilaginous joints, the bone ends are joined by compressible fibrocartilage and reinforced by a surrounding tough fibrous tissue (as in the symphysis pubis and the manubriosternal joint) and permit only a limited amount of movement. This type of joint tends to be located centrally. If a moderate or wide range of movement is required, a space must exist between the bone ends, forming a discontinuous ‘synovial’ joint. Most of the peripheral joints fall into this category. The most important joints from a clinician’s point of view are the peripheral ones, which enable our arms and legs to move through such a wide range, our hands to be endowed with such dexterity and strength and our feet to carry us so uncomplainingly when we stand, walk, run and jump. While synovial joints have the same basic structure and physiology, individual joints have evolved differently, depending on their situation and what is expected of them. For example, the knee, ankle and finger joints, which move mainly in one plane, are ‘hinge’ joints, while the hip and shoulder, which move in all directions, are ball and socket joints. Of course, to carry out even the simplest of tasks, like opening a door or walking up a step, we have to coordinate the movement of several joints, not to mention contracting and relaxing the muscles on either side of the joints. The archetypical synovial joint is an enclosed space with a negative pressure (Fig. 32.1). The bone ends that move against each other (the articular surfaces) are covered with articular cartilage. This is made up of proteoglycans and collagen, combined in such a way as to allow it to absorb huge forces of pressure like a shock absorber, while providing a shiny surface for smooth, low-friction movement. A healthy joint is lubricated by a small amount of synovial fluid (0–4 mL). This is an ultrafiltrate of plasma with additional components secreted by the synovium. The most important of these is hyaluronate, a linear repeating disaccharide with a molecular weight of some 107 Da. This provides the fluid with its viscoelastic properties without which the cartilage would fail and smooth movement would be impossible. Cartilage and synovial fluid together maintain coefficients of friction of < 0.02. The bone ends are enclosed by the tough joint capsule which is lined by the synovial membrane, a structure only a cell or two thick (Fig. 32.1). Synovial cells phagocytose intra-articular debris, secrete many components of synovial fluid and possess immunological functions. While we talk of ‘articular disorders’, it is not really possible to consider the joint in isolation. Each joint depends on other structures like ligaments, tendons, muscles and bone for its function and stability. Bone and muscle disease are dealt with in separate chapters. There are over 100 rheumatic disorders with different underlying causes, different treatments and different outcomes. These conditions can be broadly divided into non-inflammatory diseases, of which osteoarthritis is the most important, and inflammatory conditions such as rheumatoid arthritis (RA), ankylosing spondylitis and systemic lupus erythematosus (SLE or lupus). The most common form of articular disease is osteoarthritis, in which the articular cartilage becomes fissured and gradually wears away. The joint tries to heal itself by forming bony out-growths on the sides of the joints (osteophytes). Trauma to a joint predisposes to OA, as do some rare biochemical disorders like alkaptonuria. However, in most cases, apart from an inherited tendency and increasing frequency with age, the cause cannot be identified. For many years, OA was thought to be due to degeneration or ‘wear and tear’. Recently, we have appreciated that a degree of inflammation is often present if sought and that calcium pyrophosphate and basic calcium phosphate crystals are frequently found in osteoarthritic joints (see below). At present, we have no treatment to influence this process. The knee and hip are often affected and, if the pain and limitation to mobility are severe enough, the orthopaedic surgeon will remove the joint and replace it with an artificial one, usually with very gratifying results. The other major class of articular disease is inflammatory arthritis. This consists of a group of systemic immunological diseases that focus their attention on the synovium of the joint. Rheumatoid arthritis (RA) is the archetypical form of inflammatory arthritis. In RA, there is an imbalance between the proinflammatory and anti-inflammatory cytokines that favours the induction of autoimmunity (an immune reaction against one’s own tissues), chronic inflammation and joint damage. The synovium becomes inflamed, resulting in pain, tenderness, heat and stiffness in the joint as well as a systemic reaction consisting of malaise and fatigue. An acute phase reaction ensues, which can be detected and monitored by measurement of plasma C-reactive protein (CRP) concentration or the erythrocyte sedimentation rate (ESR). In what may well be a separate process, in time the synovial cells increase in size and number, the synovium becomes thickened and develops the changes of chronic inflammation with infiltration by macrophages and lymphocytes. There are also alterations in the small blood vessels. In addition, the amount of synovial fluid in the joint increases in quantity, which adds to the swelling and dysfunction of the affected joint. A number of different cytokines associated with the rheumatoid process can be found in the synovial tissue and fluid; these include tumour necrosis factor alpha (TNFα), interleukins one (IL-1) and six (IL-6). There are also abnormalities of the T and B cell lymphocytes. If left to its own devices, the thickened synovium will spread over the cartilage and erode it and the neighbouring bone. This results in joint damage, deformity and disability. Non-steroidal anti-inflammatory drugs, e.g. diclofenac, ibuprofen and naproxen are used less frequently than they once were. Although they often provide symptomatic relief, they increase the risk of cardiovascular events, can cause chronic kidney disease and have no influence on the long-term outcome of the disease. Of more concern in the long term, is the chronic inflammation that results in joint damage and disability. Until about 20 years ago, there was little that we could do to slow or even stop the inexorable progress of this destructive process, meaning that many people with RA became very disabled with joint deformity and pain. However, there have been dramatic improvements in our drug treatment of this most unpleasant condition. We have learnt that we need to diagnose the condition as early as possible. This enables treatment with one or more disease modifying anti-rheumatic drugs (DMARDs) (e.g. methotrexate, leflunomide, sulfasalazine), to be started as soon as possible. As a rule, these drugs rapidly bring the arthritis under control, but different drugs suit different people so sometimes a degree of trial and error is required. DMARDs are taken for long periods and can cause side-effects such as liver damage or bone marrow suppression, and regular blood tests (full blood count, ESR, CRP and liver function tests) are used to monitor their safety as well as the activity of the disease. More recently, the treatment of RA has been transformed by the introduction of so-called ‘biologics’ (monoclonal antibodies designed to counteract the effects of cytokines or alter the function of T or B cell lymphocytes). They are expensive and need to be taken long term, so at present they are used in the cases where DMARDs have been unsuccessful. However, in time, they may become the first-line in treatment of RA. Biologics in common use include agents against TNFα (e.g. etanercept) and anti IL-6 (tocilizumab), as well as B cell depleters (rituximab) and T cell blockers (abatacept). This is a field of active research with the potential to transform the treatment not only of RA but of many other immunological diseases. Other examples of chronic inflammatory arthritis include ankylosing spondylitis, which mainly affects the small joints of the spine, the arthritis associated with psoriasis and the ‘reactive’ arthritis one sees in response to certain intestinal or genitourinary infections. The connective tissue diseases (CTDs) are a fascinating group of conditions in which, for reasons we do not understand, the normal fine balance of the immune system gets disturbed, causing the body to react against itself. In health, the immune system consists of series of counterbalancing mechanisms that marshal defences against alien material like bacteria, viruses or foreign tissue, while not reacting in this way against itself. The identification of foreign tissue (the antigen) is the first step in the process and is followed by a series of defensive reactions including manufacturing antibodies. In CTDs, the person’s ability to recognize their own tissue goes awry and they form antibodies against their own tissue (autoantibodies). In some cases, these are responsible for the clinical manifestations of the disease; in others they are a peripheral effect of the underlying disease process. Theories of the pathogenesis of autoimmune diseases are discussed in Chapter 30. Systemic lupus erythematosus (SLE) mainly affects younger women and can involve one or more of the bodily systems. It is characterized by the formation of autoantibodies against the cell’s nucleus, or part of the nucleus, which are believed to be responsible for many of the manifestations of the disease. Joint pain is common, although it is unusual to see joint damage such as occurs in RA. SLE can result in a variety of manifestations in other systems, for example various rashes, haemolytic anaemia and glomerulonephritis leading to renal impairment, as well as cerebral, lung and heart problems. Some of these can be life threatening. Autoantibodies against cell nuclei (antinuclear factor, ANF) are present in the plasma in almost every patient with SLE. However, they are also frequently present in other CTDs and in a proportion of healthy people. Antibodies to specific components of the nucleus are more specific. For example, antibodies against double-stranded DNA are strongly suggestive of SLE. Management depends on the systems affected. The joint and skin manifestations can often be controlled with hydrochloroquine and/or low doses of prednisolone. More severe cases require the use of high doses of corticosteroids with immunosuppression using cyclophosphamide, azathioprine or mycophenolate mofetil. Other CTDs are associated with autoantibody formation, although in these cases the disease is not believed to be caused by specific antibody-antigen complexes as in SLE. Examples of these conditions include Sjögren syndrome, polymyositis, scleroderma and primary biliary cirrhosis. For details and associated immunological abnormalities, see Table 32.1. Table 32.1 is, of necessity, an oversimplified guide to some of the more important signs and symptoms seen in the CTDs, with their associated antibodies. There is often a crossover of antibodies in the various diseases and the antibodies are by no means seen in every case. TABLE 32.1 The connective tissue diseases, their common clinical features and frequently associated autoantibodies cANCA, cytoplasmic anti-neutrophil cytoplasmic antibody; pANCA, perinuclear anti-neutrophil cytoplasmic antibody; SLE, systemic lupus erythematosus. If we move from the laboratory to the clinic or surgery, ‘articular disorders’ take on another perspective. About one-fifth of the patients in a GP’s surgery will complain of musculoskeletal symptoms, such as aches, pain, stiffness or disability. Only a proportion of these patients will have a demonstrable disease or significant anatomical abnormality. Some will have abnormalities associated with the way they move their musculoskeletal system (‘mechanical’ pain), while in other cases, no abnormality can be identified. This does not mean the symptoms are imagined, but in the absence of an abnormality, attempts to diagnose and ‘cure’ a disease will, of course, be futile. Doctors are trained to diagnose diseases and then treat them. Too many doctors are uncomfortable when they are not able to make a diagnosis. This may provoke them to order unnecessary investigations in these often anxious patients, thus exposing both parties to the dangers of false positives, laboratory or clerical error and misinterpretation of the results.
Biochemistry of articular disorders
INTRODUCTION
THE ARTICULAR SYSTEM
DISORDERS OF THE ARTICULAR SYSTEM
Osteoarthritis (OA)
Inflammatory arthritis
The connective tissue diseases
Disease
Clinical features
Autoantibodies
SLE
Rashes, joint pains, glomerulonephritis, haemolytic anaemia, leukopenia, neuropsychiatric lupus, pericarditis, pleurisy, vasculitis, fatigue
Anti-nuclear factor
Anti-double-stranded DNA
Anti-Sm
Anti-Ro
Anti-La
Scleroderma
Thickened skin, Raynaud phenomenon, pulmonary hypertension, calcinosis, pulmonary fibrosis
Anti-nuclear factor
Anti-centromere
Sjögren syndrome
Dry eyes, dry mouth, joint pains, fatigue, Raynaud phenomenon
Anti-nuclear factor
Anti-Ro
Anti-La
Rheumatoid factor
Polymyositis
Muscle weakness and wasting, joint pains, pulmonary fibrosis, fatigue
Anti-nuclear factor
Anti-Jo-1
Anti-synthetase
Dermatomyositis
Proximal muscle weakness and wasting, rashes, joint pains, pulmonary fibrosis
Anti-nuclear factor
Anti-Mi2
Wegener granulomatosis
Vasculitis, sinusitis, pulmonary infiltration, glomerulonephritis, rashes
cANCA
Microscopic polyangiitis
Glomerulonephritis, joint/muscle pains, rashes, lung infiltration
pANCA
Primary biliary cirrhosis
Jaundice, itching, joint pains, Raynaud phenomenon
Antimitochondrial
Aches and pains
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree