OUTLINE
Definitions and Nomenclature for Semisolids
Uses of Semisolid Dosage Forms
Principles of Compounding Ointments
I. DEFINITIONS AND NOMENCLATURE FOR SEMISOLIDS
A. As was described in the beginning section of Chapter 27, the nomenclature for pharmaceutical dosage forms is in transition between use of traditional terms and definitions and a more systematic approach that has been proposed to more accurately and consistently describe drug products and preparations. During the period when traditional terms are still used but new terms and definitions are being suggested, it is important that pharmacists and pharmacy technicians understand proposed dosage form taxonomy and nomenclature but also recognize the various traditional terms used to describe dosage forms. The definitions and descriptions in this section should help in this regard. The quoted definitions below that are printed in regular font are the traditional ones from USP 31/NF 26 Chapter 〈1151〉 Pharmaceutical Dosage Forms (1); the definitions in italic type are taken from the FDA CDER Data Standards Manual (2).
B. Definitions
1. Ointment
Ointments are semisolid preparations intended for external application to the skin or mucous membranes (1).
A semisolid dosage form, usually containing <20% water and volatiles and> 50% hydrocarbons, waxes, or polyols as the vehicle. This dosage form is generally for external application to the skin or mucous membranes (2).
The problem with the first definition is that, although the word ointment has this general meaning, which applies to any semisolid dosage form for external application (e.g., oleaginous ointments, creams, gels), pharmaceutical manufacturers use this term for a specific semisolid dosage form type, oleaginous ointments. When the same term is applied to more than one entity, it can lead to confusion. The CDER nomenclature, which was adopted in 2006, addresses this issue by using the general term semisolid [which the CDER Data Standards Manual defines as a system that is not pourable and does not flow under low shear stress at room temperature (2)] for the type of physical system and reserves the term ointment for oleaginous semisolids as just defined in italics. While the proposed nomenclature clarifies the terminology, you can easily see the difficulty in making this change because the word ointment in its more general sense is part of many traditional derived terms such as ointment jar, ointment slab, and ointment mill. Will these terms be changed to semisolid jar, semisolid slab, semisolid mill, etc.? Therefore, for the purposes of this chapter, the term semisolid is used interchangeably with the term ointment, which is used in its traditional general sense, encompassing all semisolid dosage forms intended for application to skin or mucous membranes.
2. Cream
Creams are semisolid dosage forms containing one or more drug substances dissolved or dispersed in a suitable base. The term has traditionally been applied to semisolids that possess a relatively fluid consistency formulated as either water-in-oil (e.g., Cold Cream) or oil-in-water (e.g., Fluocinolone Acetonide Cream) emulsions. However, more recently the term has been restricted to products consisting of oil-in-water emulsions or aqueous microcrystalline dispersions of long-chain fatty acids or alcohols that are water-washable and more cosmetically and aesthetically acceptable (1).
An emulsion, semisolid dosage form, usually containing >20% water and volatiles and/or <50% hydrocarbons, waxes, or polyols as the vehicle. This dosage form is generally for external application to the skin or mucous membranes (2).
Notice the new CDER definition applies to any semisolid emulsion, not just water-washable (o/w) types.
3. Gel
Gels (sometimes called Jellies) are semisolid systems consisting of either suspensions made up of small inorganic particles or large organic molecules interpenetrated by a liquid (1).
A semisolid dosage form that contains a gelling agent to provide stiffness to a solution or a colloidal dispersion. A gel may contain suspended particles (2).
4. Paste
Pastes are semisolid dosage forms that contain one or more drug substances intended for topical application. One class is made from a single-phase aqueous gel (e.g., Carboxymethylcellulose Sodium Paste). The other class, the fatty pastes (e.g., Zinc Oxide Paste), consists of thick, stiff ointments that do not ordinarily flow at body temperature, and therefore serve as protective coatings over the areas to which they are applied (1).
A semisolid dosage form, containing a large proportion (20–50%) of solids finely dispersed in a fatty vehicle. This dosage form is generally for external application to the skin or mucous membranes (2).
5. Collodion
Although there are collodion dosage forms in the USP, this term is not defined in Chapter 〈1151〉, and it is not in the CDER Data Standards Manual. Traditionally, collodions have been considered solutions but, because they are very viscous systems applied to the skin, in the proposed USP taxonomy (described in Chapter 27), collodions are under the topical semisolid classification. Basically, a collodion is a thick solution composed of pyroxylin (a breakdown product of cellulose) that is dissolved in a solvent mixture of alcohol and ether. An example is given in Sample Prescription 30.8.
C. In USP 31, four general classes of ointment (semisolid) bases are defined and briefly described: hydrocarbon, absorption, water-removable, and water-soluble. These base types, their composition, and their characteristics are discussed in Chapter 23, Ointment Bases.
II. USES OF SEMISOLID DOSAGE FORMS
A. To protect skin or mucous membrane from chemical or physical irritants in the environment and to permit rejuvenation of the tissue
B. To provide hydration of the skin or an emollient effect
C. To provide a vehicle for applying a medication either for local or systemic effect (e.g., local—a topical antibiotic; systemic—a nitroglycerin ointment for treating angina)
III. CHOICE OF A BASE
A. The choice of a base depends on the following factors:
1. The action or effect desired (see preceding section, II)
2. The nature of the incorporated medication
a. Bioavailability
b. Stability
c. Compatibility
3. The area of application
B. If a prescription order specifies a particular semisolid base and a change of base is necessary for compatibility or stability reasons, the prescriber should be consulted.
IV. PRINCIPLES OF COMPOUNDING OINTMENTS
A. Compounding equipment
1. Ointment slabs or pads
a. In the United States, ointments are commonly made using a spatula and an ointment slab or pad. In some other countries, mortars and pestles are the preferred compounding equipment.
b. Although ointment pads offer the advantage of easy cleanup, they do have some limitations. Ointment slabs are preferred when a liquid ingredient must be incorporated into the ointment, especially if the liquid is an aqueous solution. Liquids soak into the parchment paper of ointment pads, and excess weight can be lost. Very sticky or thick ointments are often more easily made on a slab. Loss is usually less when an ointment is compounded on an ointment slab rather than on a pad. Ointment slabs and pads are pictured and are described in greater detail in the compounding equipment section of Chapter 13.
2. Spatulas
a. Generally, the large metal spatulas are used for levigation, spatulation, and incorporation of solid and liquid ingredients. Smaller metal spatulas are useful for removing a preparation from the large spatula and for transferring a preparation from the ointment slab or pad to the ointment jar.
b. Black rubber or plastic spatulas are special-purpose spatulas that are used when an ingredient (e.g., iodine) reacts with a metal spatula. They are not for general use in compounding ointments because they do not have the proper combination of flexibility and strength to give efficient shear and mixing.
c. Various types of spatulas are pictured and are described in greater detail in the compounding equipment section of Chapter 13.
3. For pharmacies that compound a significant number of customized semisolid preparations, small-scale ointment mills and specialized electric ointment mixers are available. This sort of equipment can produce smooth, elegant preparations with minimal effort and have the added advantage of minimizing exposure of compounding personnel to the drugs and other components of preparations being compounded.
B. Amount of excess preparation to make to compensate for loss during compounding
1. The amount of excess preparation needed depends on factors such as compounding technique, number and type of ingredients, and difficulty of the compounding process. When using traditional equipment, such as an ointment slab and spatula, between 2 and 4 g of an ointment may be lost in the compounding process when preparing a moderate amount of ointment (e.g., 15 to 120 g).
2. Either a percentage of excess (e.g., 10%) or a given amount of excess preparation (e.g., 3 g) is usually made to compensate for this loss. The amount of excess made is based on the compounder’s own experience and professional judgment.
C. Incorporation of solid drugs and chemicals
1. General principles
a. Because a smooth, nongritty preparation is desired, any solids incorporated into an ointment base should be either solubilized or in the finest state of subdivision possible.
b. Auxiliary agents, such as levigating agents and solvents, are sometimes added during the formulation of an ointment to facilitate making a smooth, elegant preparation. Auxiliary agents that make a substantive change in the preparation’s properties should be avoided. For example, if a stiff, paste-like ointment is desired, avoid adding an auxiliary agent that would significantly decrease the viscosity of the formulation.
2. Choice of drug form
a. If possible, pick a form of the drug that is a fine powder (e.g., boric acid powder rather than boric acid crystals, or colloidal sulfur rather than precipitated sulfur).
b. If the prescription order specifies a certain form, that form should be used unless the prescriber is consulted.
c. If a drug or chemical is available in more than one form, the selected form should be specified on the face of the prescription document or in the compounding record.
3. Levigation

a. As described in Chapter 25, Powders, levigation is the process of reducing particle size of a solid by triturating it in a mortar or spatulating it on an ointment slab or pad with a small amount of a liquid or melted base in which the solid is not soluble. This process is illustrated on Color Plate 9 and is demonstrated on the CD that accompanies this book.
b. Optimally, the liquid, called a levigating agent, is somewhat viscous and has a low surface tension to improve ease of wetting the solid. When solids are added directly to a semisolid base, a certain amount of energy is required just to overcome the high resistance to flow of the semisolid. This resistance to flow makes it difficult to provide adequate shear to reduce the particle size of a solid when the manual techniques used in compounding are employed. Levigating agents act as lubricating agents. They make incorporating solids easier, and they usually give smoother preparations.
c. Often, the ointment formulation contains an ingredient that can be used as a levigating agent. This is the preferred situation. If the prescribed formula does not contain an ingredient that may be used for this purpose, it is generally acceptable to add an auxiliary agent, provided it is bland and nontoxic and does not make a substantive change in the preparation’s physical or therapeutic properties.
d. It is important to remember that auxiliary levigating agents are added to facilitate making a smooth, elegant preparation. A levigating agent is generally not added when
(1) The solid being incorporated has very fine particles.
(2) The quantity of solid to incorporate is small.
(3) The ointment base is soft.
(4) The final preparation is intended to be a stiff paste.
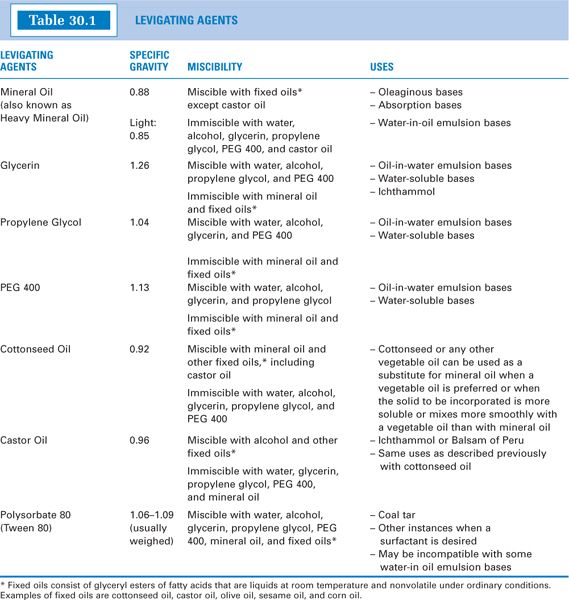
e. Types of levigating agents
(1) Commonly used levigating agents are listed in Table 30.1; their specific gravities, miscibilities, and general uses are given. More complete descriptions of the oils and solvents can be found in Chapter 15, Pharmaceutical Solvents and Solubilizing Agents, and the surfactant polysorbate 80 is described in Chapter 20, Surfactants and Emulsifying Agents.
(2) Melted ointment base may also be used.
(3) Special levigating agents are sometimes required for compatibility or stability reasons.
f. Choosing a levigating agent
(1) If the formulation already contains an ingredient that can function as a levigating agent, that liquid should be used.
(2) When adding a levigating agent, check for compatibility with the other formulation ingredients and with the ointment base. Fortunately, most levigating agents are fairly nonreactive and cause few compatibility problems.
(3) General Rule: Assuming that there are no compatibility problems with other ingredients in the formulation, levigating agents are usually chosen to be chemically similar to the ointment base. For example, mineral oil is the levigating agent of choice for oleaginous bases, such as hydrocarbon, absorption, and water-in-oil emulsion bases (see Sample Prescription 30.1). Glycerin, which is miscible with water, is usually used for water-removable and water-soluble bases.
(4) Some active ingredients require special levigating agents. Several of these are listed here.
(a) Polysorbate 80 (Tween 80) is used as the levigating agent for coal tar. Coal Tar Ointment USP contains an amount of polysorbate 80 equal to half the weight of coal tar (see Sample Prescription 30.3). Coal tar can be directly incorporated into some semi-solid bases, but polysorbate 80’s surfactant properties provide the advantage of facilitating removal of the preparation when this is desired. Coal tar will not mix with mineral oil or glycerin.
(b) Castor oil has been recommended for levigating Peruvian balsam. This stems from the fact that the resinous part of Peruvian balsam separates from semisolid preparations that also contain sulfur unless an equal quantity of castor oil is used as the levigating agent for the Peruvian balsam (3).
(c) Although ichthammol is a black, tarry substance, it is water-washable. Because it mixes with glycerin and fixed oils, these are suitable levigating agents. Some compounders think they get a better preparation by levigating ichthammol with an absorption base such as Hydrophilic Petrolatum USP, Aquabase, or Aquaphor. In this case, the amount of absorption base used is equal to the amount of ichthammol in the prescription order.
(5) Some levigating agents have compatibility problems with certain semisolid bases or additives. Some examples are listed here.
(a) Some nonionic surfactants are incompatible with certain emulsion bases if the surfactant favors the opposite emulsion type. For example, polysorbate 80, which forms o/w emulsions, can cause a phase inversion and breakdown when mixed with some w/o emulsion bases.
(b) Depending on the relative amounts, castor oil may be incompatible with a semisolid base or formulation that contains a significant amount of mineral oil. This is because castor oil is immiscible with mineral oil.
(6) Levigating agents should be nonsensitizing and nonallergenic. The prescriber and patient should be consulted before lanolin-type compounds are added to a compounded preparation, because some patients are allergic to these compounds (whereas a much smaller percentage are allergic to purified derivatives such as wool-wax alcohol present in Aquaphor and Aquabase).
g. Amount of levigating agent to use
(1) Factors that determine the amount of levigating agent needed include
(a) The quantity and properties of the solids to be incorporated.
(b) The levigating agent selected.
(c) The properties of the ointment base.
(d) The desired spreading consistency of the ointment.
(2) Amounts of levigating agents used in official preparations can serve as a guide, but these vary considerably. Several examples illustrate this point.
(a) Zinc Oxide Ointment USP calls for 15% mineral oil to be used for 20% zinc oxide in White Ointment USP. (White Ointment contains the stiffening agent white wax 5% in white petrolatum.)
(b) Zinc Oxide Paste USP uses no levigating agent for 50% solids (25% zinc oxide and 25% starch) in white petrolatum.
(c) Sulfur Ointment USP uses 10% mineral oil for 10% sulfur in white petrolatum.
(3) General rule: Unless a prescription order specifically calls for a given amount of levigating agent, the minimum amount of levigating agent necessary to lubricate the powders is generally recommended.
(a) To give you some idea of how much this is, 3 g of zinc oxide (10% zinc oxide for a 30-g semisolid preparation) requires approximately 1.5 to 2 mL of mineral oil or 1 to 1.25 mL of glycerin to get adequate lubrication of the powder for levigation.
(b) In both of these cases, the percent w/w concentration of levigating agent for a 30-g ointment is 4% to 6%, which is an average amount to use. You can easily see, however, that the amount really depends on the amount of powder to be levigated.
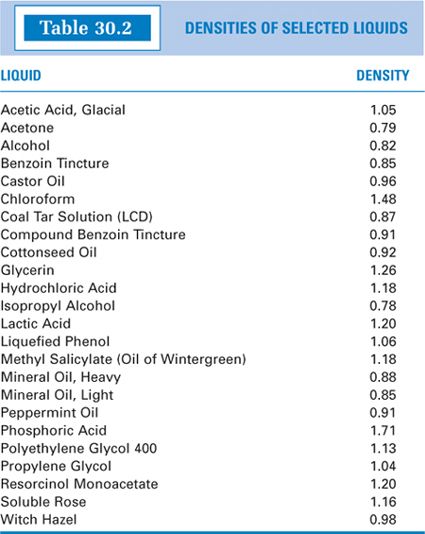
(4) If an auxiliary levigating agent is used, the weight must be determined, and there should be a corresponding decrease in the weight of the ointment base to bring the preparation to the desired final weight. When added by volume, the amount of levigating agent used should be measured in a syringe or graduate. The weight of the levigating agent that is used should then be calculated using the agent’s specific gravity and the volume incorporated. Specific gravities of various levigating agents are given in Table 30.1.
h. Documentation: The name of the levigating agent and the quantity used should be noted on the face of the prescription order or the compounding record.
4. Dissolution
a. Under certain circumstances, dissolving a solid ingredient in a solvent or oil and subsequent incorporation of the solution into the ointment base is the preferred treatment. Certain crystalline ingredients such as urea and camphor are difficult or impossible to levigate to a fine powder and should be dissolved in a suitable solvent before incorporation in the ointment base. Testosterone is an example of a compound that gives gritty ointments unless it is first dissolved in an appropriate vegetable oil. It is not soluble in mineral oil.
b. Types of solvents
(1) Water-miscible solvents include water, alcohol, isopropyl alcohol, glycerin, propylene glycol, and polyethylene glycol 400. Descriptions of these solvents appear in Chapter 15, Pharmaceutical Solvents and Solubilizing Agents.
(2) Lipophilic solvents include mineral oil and the various fixed oils, including castor oil, cottonseed oil, olive oil, and corn oil. Descriptions of these oils can also be found in Chapter 15.
c. Choosing a solvent
(1) Check a reference such as Remington: The Science and Practice of Pharmacy, The Merck Index, or Martindale—The Complete Drug Reference for solubility information on the ingredient(s) to be dissolved.
(2) If the formulation already contains an ingredient that would be a suitable solvent, that liquid should be used to dissolve the solid.
(3) Check for compatibility of the potential solvent with the semisolid base and the other formulation ingredients.
d. Capacity of various ointment bases to absorb solvents
Based on their makeup, ointment bases have varying capacities to absorb liquids.
(1) Hydrocarbon bases absorb no water and only very limited amounts of alcoholic solutions. Most oils mix easily with hydrocarbon bases, but they reduce the viscosity of the preparation.
(2) Anhydrous absorption bases can absorb large quantities of aqueous solutions and lesser amounts of alcoholic solutions.
(a) Hydrophilic Petrolatum USP and its branded commercial counterparts such as Aquabase and Aquaphor absorb an equal weight of water with relative ease and up to several times their weight of water with adequate spatulation and patience.
(b) These bases absorb less alcohol and isopropyl alcohol, possibly up to an equal weight, because these alcohols eventually dissolve the emulsifiers in these absorption bases and destroy their ability to emulsify extra hydroalcoholic liquid.
(c) As with hydrocarbon bases, absorption bases easily incorporate most oils, with a corresponding decrease in the viscosity of the system.
(3) Water-in-oil emulsion bases accept varied amounts of water and alcoholic solutions.
(a) Cold Cream and Rose Water Ointment USP are w/o emulsion bases that can absorb very little water.
(b) Two commercially available w/o emulsion bases, Hydrocream and Eucerin, absorb much more water than these but much less than their counterpart anhydrous absorption bases, Aquabase and Aquaphor.
(c) Although w/o emulsions bases easily accept most oils, as with hydrocarbon and absorption bases, their consistency may be decreased, depending on the amount of oil added.
(4) Water-removable oil-in-water emulsion bases accept water or water-miscible liquids in their external phase but eventually thin to a liquid emulsion with the addition of significant amounts of these liquids.
(a) The o/w emulsion base Hydrophilic Ointment USP and its commercial counterparts such as Dermabase and Aquaphilic Ointment take up about 30% of their weight of water without thinning. They will accept a somewhat lesser amount of alcohol and will eventually break down if too much alcohol is added.
(b) These ointment bases emulsify some amount of added oil because there is usually some excess emulsifying agent in the formulation. Larger quantities of oil may require the addition of a small amount of an o/w emulsifier, such as polysorbate 80 (Tween 80).
(5) As their name indicates, water-soluble bases are soluble in water. They are also soluble in alcohol.
(a) They accept a very limited amount of water or alcohol without loss of viscosity.
(b) Addition of an oil may require prior levigation with a liquid of intermediate chemical properties, such as glycerin or propylene glycol.
e. Strategies for adding solvents to nonabsorbing bases
If an aqueous or alcoholic solution must be added to a base that will not absorb it, the compounder may use one of the following strategies.
(1) Change the ointment base, in part or whole, to an absorption base or other base that will take up the liquid. If it is possible, stay within the same base class. For example, change from Cold Cream to Hydrocream, both w/o emulsion bases, rather than to Aquabase, an anhydrous absorption base. The prescriber should be consulted about base changes. This is illustrated with Sample Prescription 30.4.
(2) Add a nonionic auxiliary emulsifier or emulsifier combination.
(a) Fatty alcohols such as cetyl or stearyl alcohol can be added to hydrocarbon bases for this purpose. For example, when 5% cetyl alcohol is added to white petrolatum, the combination will absorb 40% to 50% of its weight in water (4). Because these fatty alcohols are waxy substances, the combination must be melted and then allowed to congeal so that a smooth base results.
(b) Stearyl or cetyl alcohol can also be added to water-soluble polyethylene glycol bases to improve their water or alcohol absorption properties. The Polyethylene Glycol Ointment monograph in the National Formulary notes that if 5% of the PEG 3350 is replaced with an equal quantity of stearyl alcohol, 6% to 25% of an aqueous solution can be incorporated in Polyethylene Glycol Ointment (5).
(c) Span nonionic surfactants are very useful for this purpose. For example, 2% to 5% Span 80, when directly incorporated into a hydrocarbon base, will enable the base to take up a significant amount of aqueous solution. This is illustrated with Sample Prescription 30.5.
(3) Spatulate the solution and ointment base until a sufficient amount of the solvent evaporates.
f. Be cautious when adding water to formulations containing drugs that are subject to hydrolysis. It may affect the stability of the preparation. Also, water-containing preparations must contain an antimicrobial ingredient or a preservative.
g. Amount of solvent to add
(1) General rule: Unless a prescription order specifically calls for a given amount of the solvent, use the minimum amount necessary to dissolve the solid ingredients.
(2) If a solvent is added to the formulation, the weight must be determined, and there should be a corresponding decrease in the weight of the ointment base to bring the preparation to the desired final weight. The volume of solvent used should be measured in a syringe or graduate; the weight of this volume can then be calculated using the solvent’s specific gravity and the volume incorporated. This is illustrated with Sample Prescriptions 30.4 and 30.5. Specific gravities of pharmaceutical solvents are given in the solvent descriptions in Chapter 15, and specific gravities for solvents and some liquids that are commonly added to compounded semisolids are given in Table 30.2.
h. Documentation: The name of the solvent and the quantity used should be noted on the face of the prescription order or in the compounding record.
D. Incorporation of liquids
1. Formulations that include liquids require semisolid bases that will absorb the liquid. If a liquid must be added to a base that will not absorb it, one of the strategies given earlier (in section C. 4.e.) for incorporating solutions may be used.
2. When adding a nonviscous liquid such as an aqueous or hydroalcoholic solution, good technique and care must be used during incorporation. Because we are working with semisolids, even those bases that can take up a significant amount of liquid do not just absorb the liquid; the liquid must be carefully spatulated or triturated into the base.
a. One method is to place the semisolid base on an ointment slab and create a depression in the base mass; then carefully pour the liquid that is to be incorporated into that depression to keep the liquid contained during the process. The liquid is then carefully spatulated in small portions into the base. This is illustrated in Color Plate 10.
b. A second method is to put the semisolid base in a mortar and gradually add the liquid in portions with trituration until all has been added.
3. Certain thick liquids are measured by weight rather than by volume. Examples include coal tar, Peru balsam, ichthammol, and the polysorbate-sorbitan type emulsifiers. For thick and sticky liquids, it works well to wipe the weighing paper first with the chosen levigating agent before taring out the weight of the paper and adding the sticky liquid. The thick liquid is then easier to transfer completely from the weighing paper onto the ointment slab because the thick liquid slides more readily off the slippery surface created by the levigating agent on the weighing paper.
4. Some liquids require special levigating agents. Several of these are listed previously in the section on levigating agents (see IV. C.3).
5. Some liquids, especially aqueous and alcoholic solutions, soak into the paper of ointment pads. Ointment slabs are the preferred equipment for these preparations.
V. COMPATIBILITY AND STABILITY
A. Physical stability
1. Ointments are semisolid dosage forms and are therefore naturally more physically stable than are liquid preparations such as solutions, suspensions, or emulsions; sedimentation, caking, creaming, and precipitation of solids are not problems we encounter with ointments. This allows more flexibility with assigning beyond-use dates.
2. The strategies for achieving uniform, smooth, nongritty ointments and for incorporating liquid ingredients in ointment bases have been discussed previously in this chapter.
3. “Bleeding” of liquid ingredients and phase separation can occur. Special levigating agents, as outlined previously, may be helpful.
4. Glycerin or propylene glycol may be added as humectants to ointment bases containing water, to retard evaporation of the water and prevent drying out of the ointment base.
B. Chemical stability
1. Ingredients
a. Check suitable references for information on chemical stability of the active ingredients and other formulation components. Issues of chemical stability are discussed in Chapter 37, and specific illustrations are given with sample prescriptions in this chapter and in the other dosage form chapters. The general topic of beyond-use dating is discussed in Chapter 4.
b. Professional judgment is needed in applying USP Chapter 〈795〉 beyond-use dating (BUD) recommendations to semisolids because they are not specifically mentioned. Obviously, when stability information is known for a specific formulation and storage conditions, that information can be applied in setting the BUD for the preparation. In the absence of such information, the following information from USP Chapter 〈795〉 can act as a guideline (6).
(1) If there is no water in the formulation, the standard for nonaqueous liquids and solid formulations could be applied to semisolids. This allows a 6-month BUD if the active ingredient(s) is a USP or NF substance, or 25% of the time remaining on the expiration date of a manufactured product used as an ingredient, whichever is less.
(2) If a formulation contains water, the standard for water-containing liquid preparations would be prudent in many circumstances. This allows a maximum BUD of 14 days when the preparation is stored in the refrigerator. Since most topical preparations are stored at room temperature, this variable should be considered in setting a BUD for the preparation.
(3) The third possibility in USP Chapter 〈795〉 uses the classification “all other formulations.”This limits the BUD to the length of time for the intended therapy or 30 days, whichever is earlier.
(4) Finally, when there are concerns for chemical stability of known labile active ingredient(s), this must be considered and may further limit the BUD.
2. Emulsifiers: For general compatibility information on emulsifiers, see Chapter 20, Surfactants and Emulsifying Agents. Soap-type emulsifiers cause most of the compatibility problems. Some specific ointment base examples include the following:
a. Oil-in-water emulsions: Traditional vanishing creams used stearate soap-type emulsifiers, which are sensitive to pH and multivalent ions. Some manufacturers have reformulated vanishing creams to use nonionic surfactants. The formula of a cream should be checked if compatibility problems are suspected. Some oil-in-water emulsions, such as Hydrophilic Ointment USP, do not have these compatibility problems because the emulsifying agent is the detergent sodium lauryl sulfate plus nonionic emulsifiers stearyl and cetyl alcohol.
b. Water-in-oil emulsions: Again, emulsion ointments that have soap-type emulsifiers may have problems. Two examples are Cold Cream and Rose Water Ointment. Both have emulsifiers formed by the reaction of sodium borate with the fatty acids in cetyl esters wax. This emulsion is sensitive to acid ingredients such as salicylic acid or phenolic compounds. Eucerin and Hydrocream are water-in-oil emulsion bases that contain nonionic emulsifiers. These bases are stable to acid ingredients.
C. Microbiologic stability
1. Ointments that contain water are subject to microbial growth. USP Chapter 〈1151〉 specifically states that all emulsions require an antimicrobial agent because the aqueous phase is favorable to the growth of microorganisms (1). This is especially true of ointments in which water is the external phase. For information on acceptable preservatives, see Chapter 16, Antimicrobial Preservatives.
2. If antimicrobial ingredients are present in the formulation, either for therapeutic purposes or as part of the preservative system of an ingredient, extra preservatives may not be needed. For example, Hydrophilic Ointment USP and manufactured emulsion ointment bases such as Dermabase, Acid Mantle Cream, Aquaphilic Ointment, Velvachol, and Dermovan contain preservatives.
3. It is important to remember that some preservatives, such as parabens and benzoic acid, are highly lipophilic; a significant portion of these may partition into the oil phase of the ointment and leave the water phase with an insufficient preservative concentration. Extra preservative may be needed.
The first two prescription orders given below are for USP formulations and are available as manufactured products from various sources. They would, therefore, not normally be compounded in the pharmacy. They are given here in an abbreviated format as examples of basic ointment preparations, one using a levigating agent for incorporation of insoluble powder and one using direct incorporation of a large quantity of powder to give a paste. The procedure for Sample Prescription 30.1 is also demonstrated on the CD that accompanies this book. Neither of these USP formulations have stability or compatibility issues. The manufactured products would have expiration dates of at least 2 years as assigned by the manufacturer following stability testing of the product. If such a preparation were compounded, a beyond-use date of 6 months for a nonaqueous preparation made with USP ingredients would be acceptable. Sample Prescription 30.3 is also for a USP preparation, but it is currently not available commercially and requires compounding. The other sample prescriptions in this section are custom formulations.

CALCULATIONS
Dose/Concentration: Zinc Oxide is often used as a protectant, which is defined as a substance that protects injured or exposed skin surfaces from harmful or annoying stimuli. The concentration range specified by the FDA for zinc oxide as a protectant is 1% to 25%. Using the USP formula given below, the concentration of zinc oxide in this preparation can be calculated as:

Ingredient Amounts
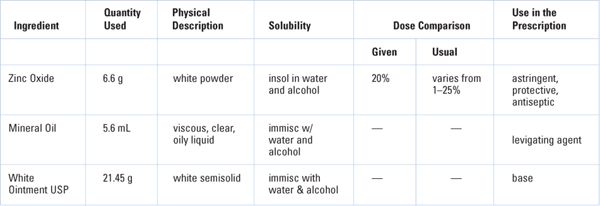
Zinc Oxide Ointment USP has the following formula (7):
Zinc oxide | 200 g |
Mineral oil | 150 g |
White Ointment | 650 g |
To make | 1,000 g |
Amounts of each ingredient (in g) to make 33 g of ointment (3 g excess):
Zinc oxide: 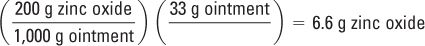
Mineral oil: 
White Ointment: 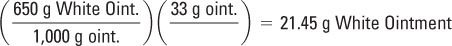
The quantity of mineral oil is given in grams but, in small-scale compounding, it is usually measured volumetrically. Using the specific gravity (s.g.) of mineral oil, the number of milliliters needed for this prescription is calculated:

White Ointment is an official USP preparation. Its formula and method of preparation is given in Table 23.2 in Chapter 23, Ointment Bases, and is demonstrated with this sample prescription on the CD that accompanies this book.
COMPOUNDING PROCEDURE: On a class III torsion or electronic balance, weigh 6.6 g of zinc oxide. With a 10-mL syringe or graduated cylinder, measure 5.6 mL of mineral oil. Place the zinc oxide on an ointment pad or slab. Add the mineral oil stepwise in small portions with levigation to the zinc oxide to form a smooth paste. Weigh 21.45 g of White Ointment, and incorporate the zinc oxide–mineral oil paste into the White Ointment. Do this by adding the White Ointment stepwise in portions with spatulation to the paste. Spatulate the ointment well to achieve a smooth, uniform preparation.
DESCRIPTION OF FINISHED PREPARATION: The preparation should be a smooth, white opaque ointment of moderate consistency and with a slight sheen. The actual weight should be within the range of 30 g ± 10%.
LABELING

Auxiliary Labels: For External Use Only. This medicine was compounded in our pharmacy for you at the direction of your prescriber.
INGREDIENTS USED: (For 10% excess, 66 g)
CALCULATIONS
Dose/Concentration
This formulation is actually Zinc Oxide Paste USP. All concentrations are okay for the intended use.
Percent concentrations of zinc oxide and starch:
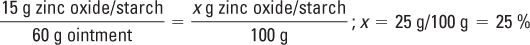
Ingredient Amounts
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree