Surgery of the Liver, Biliary Tract, Pancreas, and Spleen
LEARNING OBJECTIVES
After studying this chapter the reader will be able to:
• Identify the anatomy of the hepatobiliary system
• Correlate physiologic conditions of the hepatobiliary system with surgical interventions
• Discuss procedural considerations for select hepatobiliary surgical interventions
• Compare diagnostic methods for determining surgical interventions and/or approaches
• List five steps to protect patients with a latex allergy or sensitivity
• List the three main steps of Universal Protocol
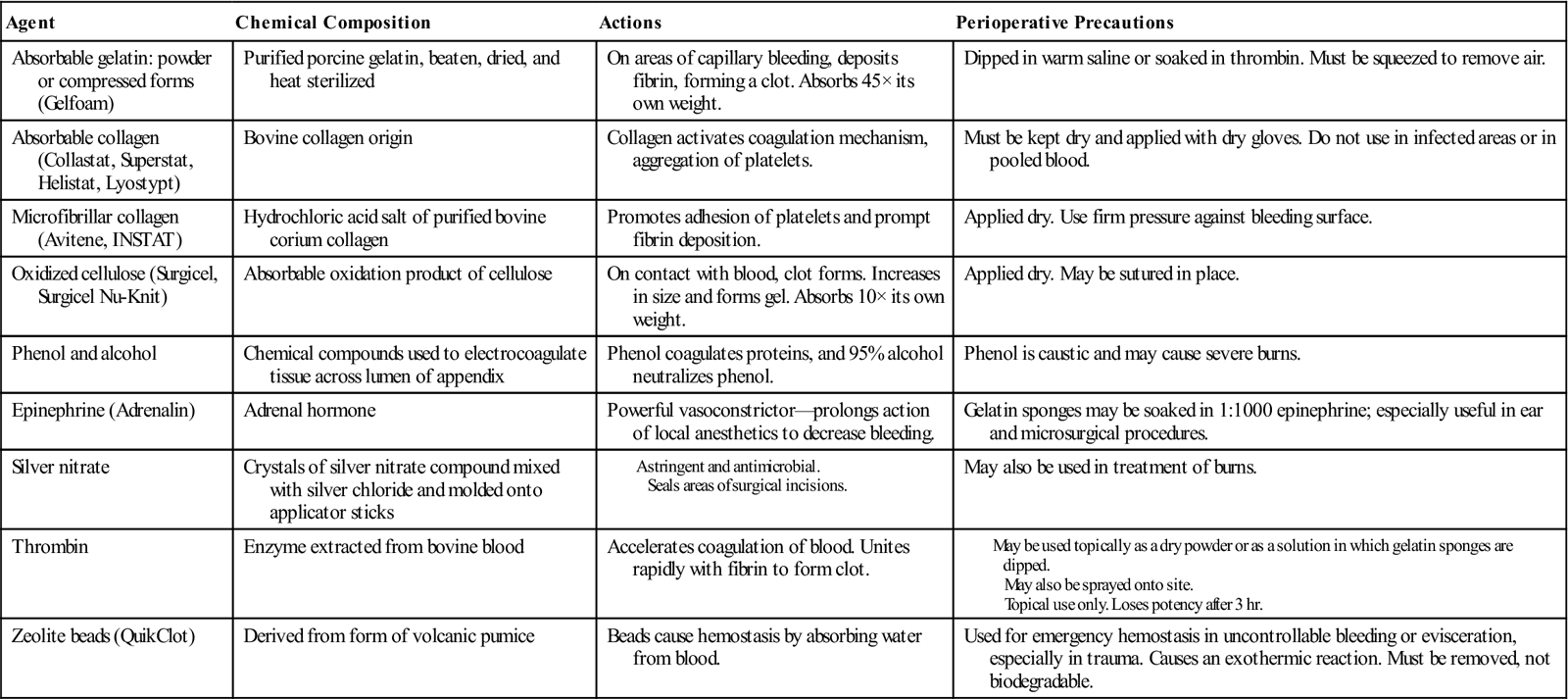
• List four pharmacologic/hemostatic agents utilized during biliary surgery and describe how they work
• Discuss types of hepatobiliary procedures
• List the two most common diseases of the biliary tree
• Contrast the advantages and disadvantages of laparoscopic and robotic-assisted surgery
• List the supplies needed for a cholangiogram and describe the procedural steps
• List the three types of pancreatic transplant
• List four common postoperative complications of liver transplants
Overview
A pathologic condition in the liver, biliary tract, pancreas, or spleen often requires surgical intervention. These organs are highly vascular and control many metabolic and immune functions of the body. Surgical intervention may be indicated for infection, cystic anomalies, congenital anomalies, metabolic diseases, trauma (see Chapter 18), or malignancy. Many new cases of malignancy of the pancreas, gallbladder, or extrahepatic biliary tract are diagnosed each year and the prognosis for these is often poor (Steer, 2008; Chari and Shah, 2008). Pancreatic cancer remains the fourth leading cause of death in the United States (McPhee et al, 2007). In the past decade, surgeries of the liver and biliary tract have become more advanced as research and new technology permit more complete diagnoses of pathologic conditions. A resection of the liver for carcinoma has achieved a recognized role for cure or substantial palliation with safety and low morbidity.
Cholecystectomy is the most common, nonemergency abdominal operation performed. In the United States more than 750,000 cholecystectomies are performed each year. It is one of the most frequently performed inpatient procedures in the United States (Afdhal and Vollmer, 2008; Chari and Shah, 2008). Laparoscopic cholecystectomy has become the gold standard surgical intervention for the treatment of cholecystitis. Since the early 1990s, laparoscopic cholecystectomy, as compared with open-incision cholecystectomy, has resulted in reduced trauma to tissues as well as shorter postoperative recoveries, both distinct advantages. About 94% of cholecystectomies are elective surgeries while the remainder are emergencies (Afdhal and Vollmer, 2008). Laparoscopic cholecystectomies were the precursor to numerous abdominal procedures now performed or assisted with the laparoscope. Current innovations in cholecystectomy are focusing on removal of the gallbladder through a natural orifice (referred to as NOTES, or natural-orifice transluminal endoscopic surgery) (Ramos et al, 2008).
New diagnostic technology and the intraoperative use of ultrasonography, biliary endoscopy, and radiography have enabled surgeons to better treat diseases of the biliary tract. Solid organ transplantation (History box), such as for the liver, pancreas, and kidneys, has become common as a means to treat primary hepatic tumors, end-stage liver disease, and insulin-deficient diabetes. Liver transplant procedures have advanced to include entire organ transplants as well as living-related organ donations.
This chapter explores the most common open and minimally invasive procedures performed on the liver, biliary tract, pancreas, and spleen.
Surgical Anatomy
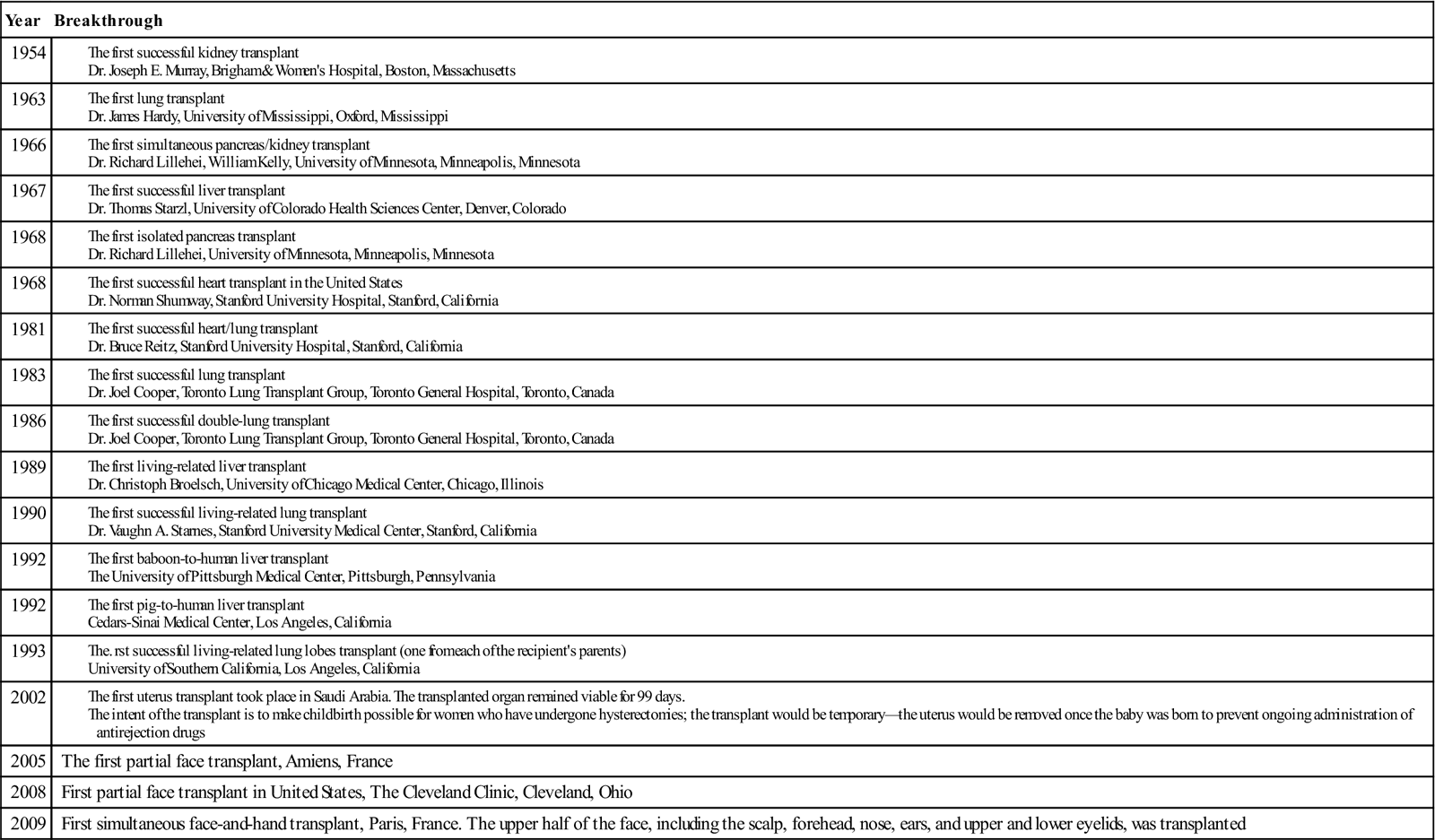
The liver is in the right upper quadrant of the abdominal cavity, beneath the dome of the diaphragm and directly above the stomach, duodenum, and hepatic flexure of the colon. The external covering, known as Glisson’s capsule, is composed of dense connective tissue. The visceral peritoneum extends over the entire surface of the liver, except at the point of posterior attachment to the diaphragm. This connective tissue branches at the porta hepatis into a network of septa that extends into an intrahepatic network of support for the more than 1 million hepatic lobules. The porta hepatis is located on the inferior surface of the liver and provides entry and exit for the major vessels, ducts, and nerves. The hepatic artery maintains the arterial blood supply, while venous blood from the stomach, intestines, spleen, and pancreas travels to the liver by the portal vein and its branches (Figure 3-1). The hepatic venous system returns blood to the heart via the inferior vena cava.
Lobules are the functional units of the liver. Each lobule contains a portal triad that consists of a hepatic duct, a hepatic portal vein branch, and a branch of the hepatic artery, nerves, and lymphatics. A central vein is located in the center of each lobule and provides venous drainage into the hepatic veins.
Lobules also contain hepatic cords, hepatic sinusoids, and bile canaliculi. The hepatic cords consist of numerous columns of hepatocytes—the functional cells of the liver. The hepatic sinusoids are the blood channels that communicate among the columns of hepatocytes. The sinusoids have a thin epithelial lining composed primarily of Kupffer cells—phagocytic cells that engulf bacteria and toxins. The sinusoids drain into the central vein.
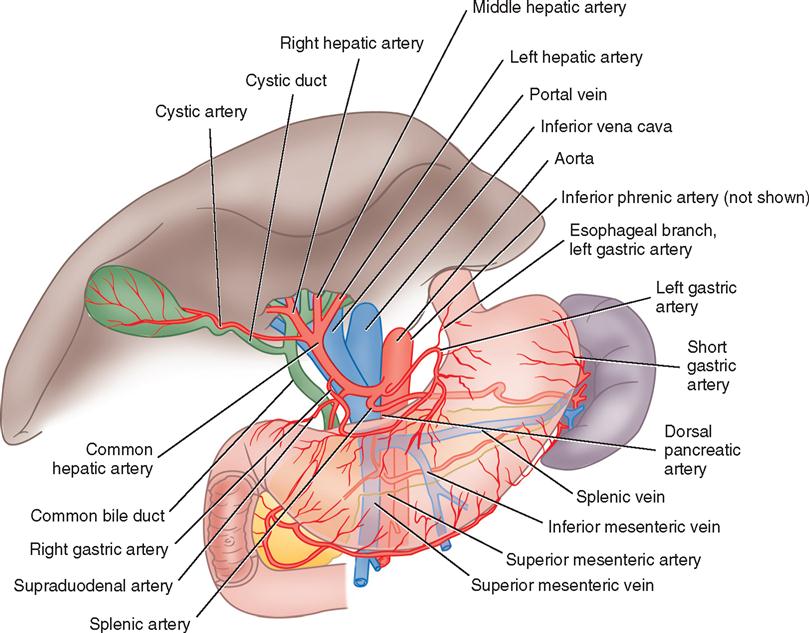
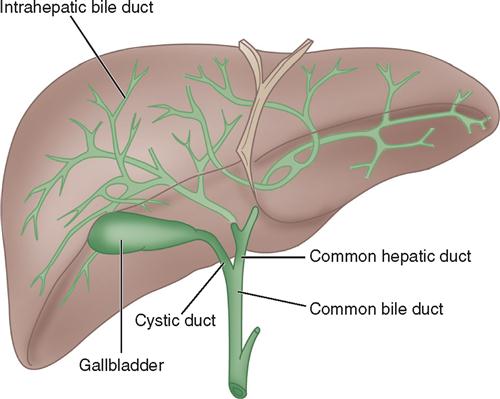
Bile is manufactured by the hepatocytes. The bile canaliculi are tiny bile capillary vessels that communicate among the columns of hepatocytes. The bile canaliculi collect and transport bile to the bile ducts in the portal triad of each lobule, from which bile then flows into the hepatic ducts at the porta hepatis. These ducts join immediately to form one common hepatic duct that merges with the cystic duct from the gallbladder to form the common bile duct (Figure 3-2). The common bile duct opens into the duodenum in an area called the ampulla, or papilla of Vater, located about 7.5 cm below the pyloric opening from the stomach.
Bile contains bile salts, which facilitate digestion and absorption, and various waste products. The liver is essential in the metabolism of carbohydrates, proteins, and fats. It metabolizes nutrients into stores of glycogen, used for regulation of blood glucose levels and as energy sources for the brain and body functions.
The liver plays several important roles in the blood-clotting mechanism. It is the organ that synthesizes plasma proteins, excluding gamma globulins but including prothrombin and fibrinogen. Vitamin K, a co-factor to the synthesis of prothrombin, is absorbed by the metabolism of fats in the intestinal tract as a result of bile formation by the liver. Patients with liver disease may have altered blood-coagulation abilities.
The liver also synthesizes lipoproteins and cholesterol. Cholesterol is an essential component of the blood plasma. It serves as a precursor for bile salts, steroid hormones, plasma membranes, and other specialized molecules. A diet high in cholesterol reduces the amount that must be synthesized by the liver. When the diet is deficient in cholesterol, the liver increases synthesis to maintain levels necessary for production of vital chemical molecules.
The liver also serves in the metabolic alteration of foreign molecules or biotransformation of chemicals. The microsomal enzyme system (MES) plays a major role in the body’s response to foreign chemicals, such as pollutants, drugs, and alcohol. Patients with liver disease may have an altered response to chemical substances. This consideration is important in the induction and management of general anesthesia for patients with liver disorders.
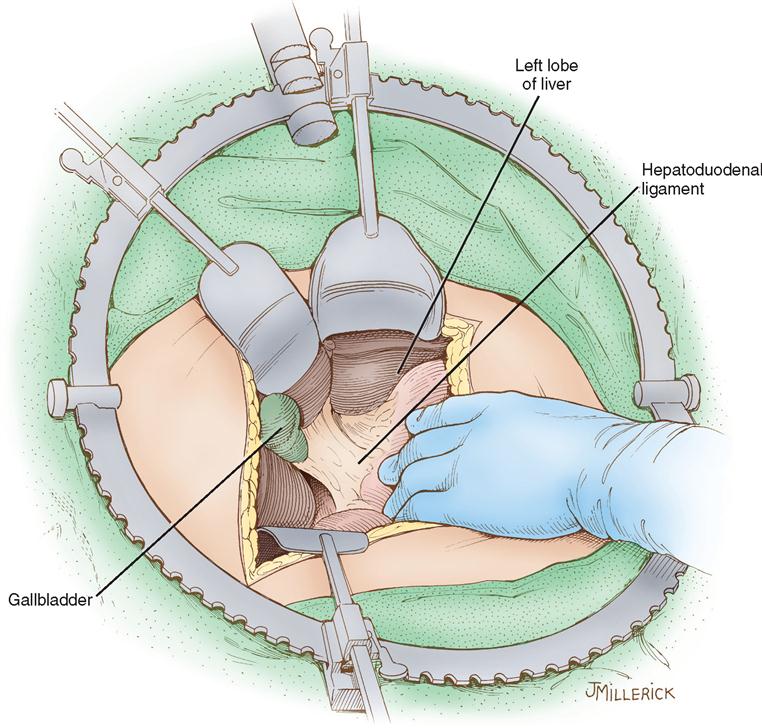
The gallbladder, which lies in a sulcus on the undersurface of the right lobe of the liver, terminates in the cystic duct (Figure 3-3). This ductal system provides a channel for the flow of bile to the gallbladder, where it becomes highly concentrated during storage. The liver produces about 600 to 1000 ml of bile each day. The gallbladder’s average storage capacity is 40 to 70 ml. As foods, especially fats, are ingested, the duodenal cells release cholecystokinin. As the musculature of the gallbladder contracts, bile is forced into the cystic duct and through the common duct. As the sphincter of Oddi in the ampulla of Vater relaxes, bile is released, flowing into the duodenum to aid in digestion by emulsification of fats. The gallbladder receives its blood supply from the cystic artery, a branch of the hepatic artery. The triangle of Calot contains the cystic artery (and possibly the right hepatic artery); it is an anatomic landmark in surgical removal of the gallbladder. Its boundaries may be remembered as “the 3 Cs”: Cystic duct, Common hepatic duct, and Cystic artery (Chari and Shah, 2008). Innervation for the gallbladder and biliary tree is controlled by the autonomic nervous system. Parasympathetic innervation stimulates contraction, whereas sympathetic innervation inhibits contraction.

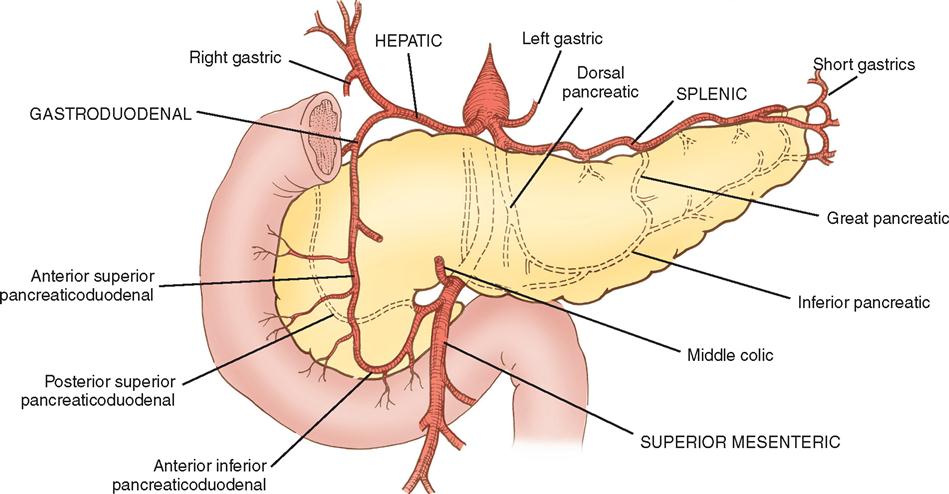
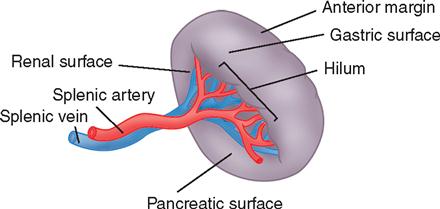
The pancreas (see Figure 3-3) is a fixed structure lying transversely behind the stomach in the upper abdomen. The head of the pancreas is fixed to the curve of the duodenum. Blood is supplied to the pancreas and the duodenum from the celiac axis and superior mesenteric artery (Figure 3-4). The body of the pancreas lies across the vertebrae and over the superior mesenteric artery and vein. The tail of the pancreas extends to the hilum of the spleen. In total, the pancreas extends about 25 cm. Pancreatic secretions containing digestive enzymes are collected in the pancreatic duct, or duct of Wirsung, which joins with the common bile duct to enter the duodenum about 7.5 cm below the pylorus. The dilated junction of the two ducts at the point of entry forms the ampulla of Vater.
The pancreas also contains groups of cells, called islets, or islands, of Langerhans, that secrete hormones into the blood capillaries instead of into the duct. These hormones are insulin and glucagon, and both are involved in carbohydrate metabolism.
Get Clinical Tree app for offline access