CHEST PAIN
CHEST PAIN: ACUTE CORONARY SYNDROMES
 Definition
Definition
 Chest pain accounts for over 6 million annual emergency department visits and 3 million hospital admissions in the United States. The differential diagnosis for chest pain is broad and ranges from benign musculoskeletal conditions to life-threatening emergencies.
Chest pain accounts for over 6 million annual emergency department visits and 3 million hospital admissions in the United States. The differential diagnosis for chest pain is broad and ranges from benign musculoskeletal conditions to life-threatening emergencies.
 The prevalence of chest pain etiology varies greatly by location of the patient interaction. Acute coronary syndromes account for <2% of outpatient chest pain visits as opposed to 15% of emergency room visits. Of central importance in the evaluation of the patient with chest pain is a thorough history and physical supported by ancillary testing to determine if emergent treatment is required.
The prevalence of chest pain etiology varies greatly by location of the patient interaction. Acute coronary syndromes account for <2% of outpatient chest pain visits as opposed to 15% of emergency room visits. Of central importance in the evaluation of the patient with chest pain is a thorough history and physical supported by ancillary testing to determine if emergent treatment is required.
 Initial clinical assessment is focused on immediate threats to life: acute coronary syndrome, aortic dissection, pulmonary embolism, tension pneumothorax, pericardial tamponade, and mediastinitis (esophageal rupture).
Initial clinical assessment is focused on immediate threats to life: acute coronary syndrome, aortic dissection, pulmonary embolism, tension pneumothorax, pericardial tamponade, and mediastinitis (esophageal rupture).
 Evaluation of the patient with chest pain should differentiate non-cardiac from cardiac etiologies. Acute coronary syndrome (ACS) is a unifying term representing the potentially life-threatening syndrome of myocardial ischemia that results from a disparity between coronary blood flow and myocardial oxygen demand most often due to atherosclerosis, vasoconstriction, or thrombus with superimposed myonecrosis. ACS syndromes present as unstable angina (UA), or myocardial infarction either with ST-segment elevation on ECG (STEMI) or without (NSTEMI).
Evaluation of the patient with chest pain should differentiate non-cardiac from cardiac etiologies. Acute coronary syndrome (ACS) is a unifying term representing the potentially life-threatening syndrome of myocardial ischemia that results from a disparity between coronary blood flow and myocardial oxygen demand most often due to atherosclerosis, vasoconstriction, or thrombus with superimposed myonecrosis. ACS syndromes present as unstable angina (UA), or myocardial infarction either with ST-segment elevation on ECG (STEMI) or without (NSTEMI).
 NSTEMI and unstable angina comprise two thirds of ACS.
NSTEMI and unstable angina comprise two thirds of ACS.
 Immediate recognition of ACS in patients presenting with chest pain is important as the diagnosis triggers both triage and treatment decisions.
Immediate recognition of ACS in patients presenting with chest pain is important as the diagnosis triggers both triage and treatment decisions.
 It is prudent to have a low threshold for the diagnosis of ACS, which is made from the clinical characteristics of the presenting symptoms, ECG findings and the presence of myonecrosis as reflected by an elevation of cardiac biomarkers. These diagnostic tools also provide risk assessment, which may dictate therapy, management strategy and placement.
It is prudent to have a low threshold for the diagnosis of ACS, which is made from the clinical characteristics of the presenting symptoms, ECG findings and the presence of myonecrosis as reflected by an elevation of cardiac biomarkers. These diagnostic tools also provide risk assessment, which may dictate therapy, management strategy and placement.
 NSTEMI patients have an intermediate risk of acute complications when compared to unstable angina (lower) and STEMI patients (higher), approaching a 5% 30-day mortality rate.
NSTEMI patients have an intermediate risk of acute complications when compared to unstable angina (lower) and STEMI patients (higher), approaching a 5% 30-day mortality rate.
 Etiology
Etiology
 Cardiac sources of chest pain:
Cardiac sources of chest pain:
1.Ischemic/coronary heart disease—acute coronary syndromes (STEMI, NSTEMI, UA), stable angina pectoris
2.Ischemic/nonatherosclerotic—aortic stenosis, hypertrophic cardiomyopathy, severe systemic hypertension, right ventricular hypertension, aortic regurgitation, severe anemia, coronary vasospasm, anatomical abnormalities
3.Inflammatory—pericarditis, infectious and autoimmune vasculitis
4.Hyperadrenergic states—stress CM, severe hypertension, pheochromocytoma
5.Chest pain syndromes—mitral valve prolapse, psychosomatic
 Noncardiac sources: GI (GERD, esophageal rupture, esophagitis, esophageal motility/achalasia, referred pain—biliary colic, appendicitis), pulmonary (pneumonia, pulmonary embolism, pulmonary hypertension, sarcoidosis, effusion, pneumothorax, pleuritis, serositis), aortic syndromes, musculoskeletal, psychosomatic
Noncardiac sources: GI (GERD, esophageal rupture, esophagitis, esophageal motility/achalasia, referred pain—biliary colic, appendicitis), pulmonary (pneumonia, pulmonary embolism, pulmonary hypertension, sarcoidosis, effusion, pneumothorax, pleuritis, serositis), aortic syndromes, musculoskeletal, psychosomatic
 Who Should Be Suspected of ACS?
Who Should Be Suspected of ACS?
 Consideration of the pretest probability of coexisting coronary artery disease should influence the diagnosis of ACS.
Consideration of the pretest probability of coexisting coronary artery disease should influence the diagnosis of ACS.
 ACS should be considered a working diagnosis subject to reevaluation.
ACS should be considered a working diagnosis subject to reevaluation.
 Unstable angina (UA) and NSTEMI are closely related clinical conditions with similar pathophysiology but differing severity. NSTEMI is usually characterized by ischemic chest discomfort at rest, absence of ST-segment elevation on a 12-lead ECG, and positive necrosis biomarkers.
Unstable angina (UA) and NSTEMI are closely related clinical conditions with similar pathophysiology but differing severity. NSTEMI is usually characterized by ischemic chest discomfort at rest, absence of ST-segment elevation on a 12-lead ECG, and positive necrosis biomarkers.
 As an elevation of cardiac biomarkers may not be detectable for 12 hours, UA and NSTEMI may be initially indistinguishable. However, the distinction is important to make for early acute management. Increasing evidence supports early aggressive anticoagulation and mechanical revascularization as superior to conservative medical therapy for NSTEMI patients.
As an elevation of cardiac biomarkers may not be detectable for 12 hours, UA and NSTEMI may be initially indistinguishable. However, the distinction is important to make for early acute management. Increasing evidence supports early aggressive anticoagulation and mechanical revascularization as superior to conservative medical therapy for NSTEMI patients.
 Unstable angina presents as three scenarios: ischemic discomfort at rest (approximately 20 minutes’ duration), new-onset discomfort at mild exercise threshold in the last 6 weeks, or progression of previously stable angina to easily evoked with ordinary physical activity or increased in severity (Canadian Cardiovascular Society Class III).
Unstable angina presents as three scenarios: ischemic discomfort at rest (approximately 20 minutes’ duration), new-onset discomfort at mild exercise threshold in the last 6 weeks, or progression of previously stable angina to easily evoked with ordinary physical activity or increased in severity (Canadian Cardiovascular Society Class III).
 UA should be differentiated from stable angina pectoris. While similar in character, stable angina pectoris represents short-lived chest discomfort elicited from states of higher cardiac demand that is relieved with rest and is without a crescendo pattern as described above.
UA should be differentiated from stable angina pectoris. While similar in character, stable angina pectoris represents short-lived chest discomfort elicited from states of higher cardiac demand that is relieved with rest and is without a crescendo pattern as described above.
 Because of a lack of objective data for diagnosis, unstable angina is the most subjective of the ACS diagnoses. Nevertheless, history/exam, ECG, and cardiac biomarkers on presentation can be utilized to formulate a likelihood of an ACS diagnosis.
Because of a lack of objective data for diagnosis, unstable angina is the most subjective of the ACS diagnoses. Nevertheless, history/exam, ECG, and cardiac biomarkers on presentation can be utilized to formulate a likelihood of an ACS diagnosis.
 Elderly, diabetic, and female patients are more likely to present with ACS symptoms that do not include chest pain. Rather, symptoms may include dyspnea, diaphoresis, emesis, or hypotension.
Elderly, diabetic, and female patients are more likely to present with ACS symptoms that do not include chest pain. Rather, symptoms may include dyspnea, diaphoresis, emesis, or hypotension.
 While ACS syndromes are mediated through platelet activation, at present, use of platelet aggregation assays (P2Y12, etc.) and measurement of immature platelet forms are not recommended for the diagnosis of ACS.
While ACS syndromes are mediated through platelet activation, at present, use of platelet aggregation assays (P2Y12, etc.) and measurement of immature platelet forms are not recommended for the diagnosis of ACS.
 The inflammatory markers/mediators of CRP, serum amyloid A, and IL-6 have been shown to risk stratify UA and NSTEMI patients but cannot be recommended for routine use in clinical diagnosis or guiding therapy for ACS patients at this point in time.
The inflammatory markers/mediators of CRP, serum amyloid A, and IL-6 have been shown to risk stratify UA and NSTEMI patients but cannot be recommended for routine use in clinical diagnosis or guiding therapy for ACS patients at this point in time.
 Who Should Be Suspected of an MI?
Who Should Be Suspected of an MI?
 A diagnosis of NSTEMI implies ischemia severe enough to cause myocardial damage as evidenced by a release of cardiac biomarkers of necrosis. While the presence of objective markers of injury makes a diagnosis of MI less prone to error than UA, abnormal values must be interpreted in a clinical context to avoid false-positive interpretation.
A diagnosis of NSTEMI implies ischemia severe enough to cause myocardial damage as evidenced by a release of cardiac biomarkers of necrosis. While the presence of objective markers of injury makes a diagnosis of MI less prone to error than UA, abnormal values must be interpreted in a clinical context to avoid false-positive interpretation.
 Due to the increasing sensitivity of newer-generation biomarkers of myonecrosis, a universal definition of myocardial infarction was adopted in 2007 and specifies that one of the following criteria be met in a clinical setting consistent with myocardial ischemia:
Due to the increasing sensitivity of newer-generation biomarkers of myonecrosis, a universal definition of myocardial infarction was adopted in 2007 and specifies that one of the following criteria be met in a clinical setting consistent with myocardial ischemia:
1.Detection of rise and/or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit (URL), together with evidence of myocardial ischemia with at least one of the following:
 Symptoms of ischemia
Symptoms of ischemia
 ECG changes indicative of new ischemia (new ST-T changes or left bundle branch block, LBBB)
ECG changes indicative of new ischemia (new ST-T changes or left bundle branch block, LBBB)
 Development of pathologic Q waves on ECG
Development of pathologic Q waves on ECG
 Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
2.Sudden or unexpected cardiac death, often with symptoms suggestive of myocardial ischemia, and accompanied by presumably new ST elevation, or new LBBB, and/or evidence of fresh thrombus by coronary angiography and/ or at autopsy, but death occurring before blood samples could be obtained or at a time before the appearance of cardiac biomarkers in the blood.
3.For percutaneous coronary intervention (PCI), patients with normal baseline troponin, an elevation >3× 99th percentile URL is defined as a PCI-related MI. It is important to note the threshold for characterizing NSTEMI post-PCI is higher than in spontaneously presenting NSTEMI (3× 99th percentile). This is due to the fact the 99th percentile is reached in up to 50% of PCI patients but does not carry the same prognosis as chest pain patients. In fact, only 5–8× 99th percentile elevations of CK-MB carry negative long-term prognosis.
4.For coronary bypass patients with normal troponin, an increase of biomarkers >5× 99th percentile URL with pathologic Q waves, new LBBB, or angiographically documented new graft or native coronary artery occlusion or loss of a viable myocardium by imaging has been designated bypass-related MI.
5.Pathologic findings of acute myocardial infarction.
 Once the criteria for MI are met, a clinical classification of MI has been established to recognize different etiologies of myocardial necrosis. Each etiology differs in both short- and long-term mortality rates. The classifications are
Once the criteria for MI are met, a clinical classification of MI has been established to recognize different etiologies of myocardial necrosis. Each etiology differs in both short- and long-term mortality rates. The classifications are
1.Type 1 MI: spontaneous MI related to ischemia from a primary coronary event such as atherosclerotic plaque erosion, rupture fissure, or dissection with accompanying thrombus
2.Type 2 MI: ischemia/necrosis related to increased oxygen demand or decreased supply such as in coronary spasm, embolism, anemia, arrhythmia, hypertension, and hypotension
3.Type 3 MI: sudden cardiac death meeting criterion as listed above
4.Type 4 MI: PCI-related MI with further classification as type 4a or type 4b
 Type 4a MI: MI directly related to procedure
Type 4a MI: MI directly related to procedure
 Type 4b MI: MI due stent thrombosis as documented by angiography or autopsy
Type 4b MI: MI due stent thrombosis as documented by angiography or autopsy
5.Type 5 MI: coronary bypass related
 Type 1, 3, and 4b MIs have the highest short- and long-term mortalities and must be triaged and treated aggressively upon presentation. Prognosis for type 2, 4a, and 5 MIs is generally more favorable.
Type 1, 3, and 4b MIs have the highest short- and long-term mortalities and must be triaged and treated aggressively upon presentation. Prognosis for type 2, 4a, and 5 MIs is generally more favorable.
 MI classification is almost always based entirely on clinical context with supporting imaging/autopsy findings if needed. A notable exception may be the use of point of care platelet assays (see Chapter 16: Platelet Function Assay). High on-treatment platelet reactivity in a recently stented patient may suggest sub-optimal antiplatelet therapy or genetically determined resistance to treatment, greatly increasing the risk of stent thrombosis (type 4b MI).
MI classification is almost always based entirely on clinical context with supporting imaging/autopsy findings if needed. A notable exception may be the use of point of care platelet assays (see Chapter 16: Platelet Function Assay). High on-treatment platelet reactivity in a recently stented patient may suggest sub-optimal antiplatelet therapy or genetically determined resistance to treatment, greatly increasing the risk of stent thrombosis (type 4b MI).
 Any type of MI classification can present as either STEMI or NSTEMI depending upon the severity of ischemic insult.
Any type of MI classification can present as either STEMI or NSTEMI depending upon the severity of ischemic insult.
 Diagnosis
Diagnosis
The diagnosis of ACS depends on the likelihood of coronary atherosclerosis, characteristics of the chest pain, abnormalities on ECG, and levels of serum markers of cardiac injury. A rapid assessment of chest pain patients (Figure 3-1) is required to initiate appropriate and potentially lifesaving treatment and may need to be revisited until a final diagnosis is confirmed.
 The physical examination of patients with uncomplicated ACS is usually normal but has the goal of evaluating for precipitating factors (uncontrolled hypertension, anemia, thyrotoxicosis, sepsis), assessing hemodynamic consequences of ACS (CHF, third heart sound, new mitral regurgitant murmur, shock), revealing comorbid conditions that impact treatment decisions (malignancy), and ruling out other chest pain etiologies. A targeted initial exam should evaluate for unequal extremity pulses and aortic regurgitation (aortic dissection), a pericardial rub (pericarditis), pulsus paradoxus (tamponade), or reproducible chest pain with palpation (musculoskeletal).
The physical examination of patients with uncomplicated ACS is usually normal but has the goal of evaluating for precipitating factors (uncontrolled hypertension, anemia, thyrotoxicosis, sepsis), assessing hemodynamic consequences of ACS (CHF, third heart sound, new mitral regurgitant murmur, shock), revealing comorbid conditions that impact treatment decisions (malignancy), and ruling out other chest pain etiologies. A targeted initial exam should evaluate for unequal extremity pulses and aortic regurgitation (aortic dissection), a pericardial rub (pericarditis), pulsus paradoxus (tamponade), or reproducible chest pain with palpation (musculoskeletal).
 The ECG should be performed first and within 10 minutes of first medical contact and reviewed for ischemic findings as ECG changes have both diagnostic and prognostic implications.
The ECG should be performed first and within 10 minutes of first medical contact and reviewed for ischemic findings as ECG changes have both diagnostic and prognostic implications.
 ST-segment deviation (depression or elevation) is the most specific sign of ischemia.
ST-segment deviation (depression or elevation) is the most specific sign of ischemia.
 T-wave changes are the most sensitive.
T-wave changes are the most sensitive.
 ST-segment elevation of >1 mm in two contiguous precordial or two adjacent limb leads that is persistent and accompanied by symptoms consistent with ACS (>30 minutes) should be considered for immediate mechanical or pharmacologic reperfusion due to the poor short-term prognosis of STEMI. This category also includes ECG changes of hyperacute T waves, new LBBB, or posterior MI (may require posterior leads for diagnosis).
ST-segment elevation of >1 mm in two contiguous precordial or two adjacent limb leads that is persistent and accompanied by symptoms consistent with ACS (>30 minutes) should be considered for immediate mechanical or pharmacologic reperfusion due to the poor short-term prognosis of STEMI. This category also includes ECG changes of hyperacute T waves, new LBBB, or posterior MI (may require posterior leads for diagnosis).
 If STEMI (or equivalent) is excluded, the presence of ST-segment depressions and T-wave abnormalities should be assessed.
If STEMI (or equivalent) is excluded, the presence of ST-segment depressions and T-wave abnormalities should be assessed.
 Horizontal or downsloping depressions of ≥0.05 mV are important indicators of ongoing ischemia.
Horizontal or downsloping depressions of ≥0.05 mV are important indicators of ongoing ischemia.
 T-wave inversions or “pseudonormalizations” may aid diagnosis, particularly with symptoms, but are less sensitive for ischemia.
T-wave inversions or “pseudonormalizations” may aid diagnosis, particularly with symptoms, but are less sensitive for ischemia.
 As ACS is highly dynamic, serial ECGs (every 20–30 minutes) and clinical reassessment should be performed if the initial ECG is nondiagnostic and the patient remains symptomatic.
As ACS is highly dynamic, serial ECGs (every 20–30 minutes) and clinical reassessment should be performed if the initial ECG is nondiagnostic and the patient remains symptomatic.
 Continuous ECG monitoring should be performed in all UA/NSTEMI patients admitted to the hospital for surveillance of arrhythmias and ongoing ischemia.
Continuous ECG monitoring should be performed in all UA/NSTEMI patients admitted to the hospital for surveillance of arrhythmias and ongoing ischemia.
 Cardiac biomarkers, along with the ECG, remain a cornerstone for the diagnosis of MI. Cardiac troponin T and I are preferred markers given the myocardial specificity. CK-MB is the next favored biomarker and is released more rapidly with ischemia than troponin, although it lacks the former’s absolute tissue specificity (see Chapter 16, Troponin limitations).
Cardiac biomarkers, along with the ECG, remain a cornerstone for the diagnosis of MI. Cardiac troponin T and I are preferred markers given the myocardial specificity. CK-MB is the next favored biomarker and is released more rapidly with ischemia than troponin, although it lacks the former’s absolute tissue specificity (see Chapter 16, Troponin limitations).
 Most NSTEMI patients have troponin elevation within 4–6 hours after symptom onset. Initially negative biomarkers should be remeasured within 8–12 hours after symptom onset.
Most NSTEMI patients have troponin elevation within 4–6 hours after symptom onset. Initially negative biomarkers should be remeasured within 8–12 hours after symptom onset.
 New “high-sensitivity” troponin assays increase sensitivity with an associated loss of specificity, particularly in low-risk patients and must be interpreted in the clinical context.
New “high-sensitivity” troponin assays increase sensitivity with an associated loss of specificity, particularly in low-risk patients and must be interpreted in the clinical context.
 Even without ACS as an etiology, however, an elevation in troponin >99th percentile portends a worse prognosis when compared to patients without elevation.
Even without ACS as an etiology, however, an elevation in troponin >99th percentile portends a worse prognosis when compared to patients without elevation.
 Cardiac imaging is emphasized in the definition of acute MI and can aid in clinically indeterminate cases. Because of its widespread availability and mobility, echocardiography is often used to differentiate myocardial ischemia from nonischemic etiologies of chest pain. Regional wall motion abnormalities can help distinguish ischemia from perimyocarditis, valvular heart disease, cardiomyopathy, pulmonary embolism, or ascending aortic dissection. Wall thickness (or lack thereof) may aid in determining if MI is acute or subacute/old. While MRI is validated for these purposes as well, its availability, cost, and time make it less efficient for acute chest pain evaluation.
Cardiac imaging is emphasized in the definition of acute MI and can aid in clinically indeterminate cases. Because of its widespread availability and mobility, echocardiography is often used to differentiate myocardial ischemia from nonischemic etiologies of chest pain. Regional wall motion abnormalities can help distinguish ischemia from perimyocarditis, valvular heart disease, cardiomyopathy, pulmonary embolism, or ascending aortic dissection. Wall thickness (or lack thereof) may aid in determining if MI is acute or subacute/old. While MRI is validated for these purposes as well, its availability, cost, and time make it less efficient for acute chest pain evaluation.
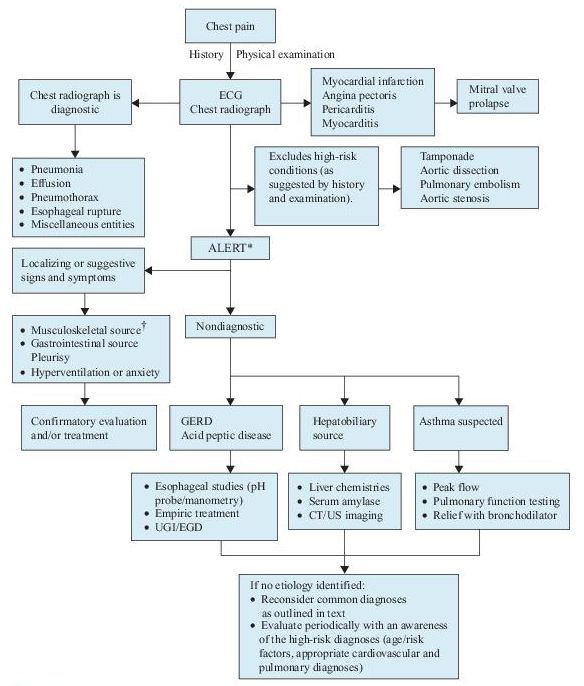
Figure 3–1 Algorithm for the diagnosis of chest pain.
*This algorithm is intended to direct the workup in patients with chest pain of unclear etiology.
†Many of these patients will be discovered to have musculoskeletal syndromes that are diagnosed through a detailed history and physical examination. Musculoskeletal diagnoses to specifically consider include overuse syndromes, costochondritis, pectoral girdle syndrome, and xiphodynia. CT, computed tomography; ECG, electrocardiogram; EGD, esophagogastroduodenoscopy; GERD, gastroesophageal reflux disease; UGI, upper gastrointestinal series; US, ultrasound.
 Laboratory and Additional Testing
Laboratory and Additional Testing
While the diagnosis of MI is in part dependent upon laboratory testing, cardiac biomarkers and supplemental imaging may also be utilized for risk stratification and delivery of cost-effective care based on patient risk.
 The diagnosis of STEMI is made by clinical history, ECG findings, and, if needed, cardiac imaging. It should not be dependent upon the results of cardiac biomarker assays given the time-dependent nature of reperfusion therapy efficacy in this high-risk population. Stat renal function and CBC to assess for anemia and baseline platelet levels are recommended in patients presenting with STEMI. Cocaine history/tox screen should be considered.
The diagnosis of STEMI is made by clinical history, ECG findings, and, if needed, cardiac imaging. It should not be dependent upon the results of cardiac biomarker assays given the time-dependent nature of reperfusion therapy efficacy in this high-risk population. Stat renal function and CBC to assess for anemia and baseline platelet levels are recommended in patients presenting with STEMI. Cocaine history/tox screen should be considered.
Up to 25% of hospital admissions are due to symptoms consistent with ACS, yet up to 85% of these patients do not have ACS as a final diagnosis. Serial biomarkers with stress testing may help identify low- and intermediate-risk patients who may be safely discharged home to continue a cardiovascular evaluation as an outpatient. Based on history, exam, ECG, and laboratory testing, risk assessment may be performed. The presence of ischemic symptoms, hypotension, dynamic ECG changes, heart failure, or advanced age indicates high-risk ACS, and these patients admitted as either NSTEMI (positive biomarkers) or high-risk UA (negative markers).
 The distinction between NSTEMI and UA is determined by the presence or absence of detectable biomarkers of necrosis. Troponin is widely accepted as the “gold standard” for cardiac myonecrosis and appears in serum 4 hours after onset of ischemia and peaks in 8–12 hours. Patients with negative biomarkers within 6 hours of chest symptoms should have a second set obtained 8–12 hours after symptom onset.
The distinction between NSTEMI and UA is determined by the presence or absence of detectable biomarkers of necrosis. Troponin is widely accepted as the “gold standard” for cardiac myonecrosis and appears in serum 4 hours after onset of ischemia and peaks in 8–12 hours. Patients with negative biomarkers within 6 hours of chest symptoms should have a second set obtained 8–12 hours after symptom onset.
 At conclusion of two biomarker evaluations (observed for 8–24 hours postsymptoms), the decision for admission (positive biomarkers) or noninvasive provocative testing (normal biomarkers without high-risk clinical features) may be made (Figure 3-2).
At conclusion of two biomarker evaluations (observed for 8–24 hours postsymptoms), the decision for admission (positive biomarkers) or noninvasive provocative testing (normal biomarkers without high-risk clinical features) may be made (Figure 3-2).
 There are a number of abbreviated biomarker strategies (<6 hours) to potentially discharge low-risk patients home earlier than current practice. Due to superior release kinetics, initially CK-MB was used for these protocols. CK-MB has fallen out of favor due to superior troponin sensitivity for MI. Troponin-based protocols incorporate assays performed 2 hours apart combined with either risk model assessment (TIMI score) or potentially imag-ing modalities (CT). While these strategies show promise, due to the use of point-of-care testing, they require institution-specific customization of cutoff thresholds of troponin as point-of-care testing has lower sensitivity than cen-tral laboratory troponin assays (which result more slowly).
There are a number of abbreviated biomarker strategies (<6 hours) to potentially discharge low-risk patients home earlier than current practice. Due to superior release kinetics, initially CK-MB was used for these protocols. CK-MB has fallen out of favor due to superior troponin sensitivity for MI. Troponin-based protocols incorporate assays performed 2 hours apart combined with either risk model assessment (TIMI score) or potentially imag-ing modalities (CT). While these strategies show promise, due to the use of point-of-care testing, they require institution-specific customization of cutoff thresholds of troponin as point-of-care testing has lower sensitivity than cen-tral laboratory troponin assays (which result more slowly).
 The role of very high-sensitivity troponin for rapid assessment (not approved yet in United States) shows promise due to superior sensitivity and earlier detection (hs-TnT 100% sensitivity for MI within 4–6 hours after symptoms or 0–2 hours after ED presentation).
The role of very high-sensitivity troponin for rapid assessment (not approved yet in United States) shows promise due to superior sensitivity and earlier detection (hs-TnT 100% sensitivity for MI within 4–6 hours after symptoms or 0–2 hours after ED presentation).
 Assessment of platelet reactivity at this point in time cannot be recommended for the diagnosis of ACS.
Assessment of platelet reactivity at this point in time cannot be recommended for the diagnosis of ACS.
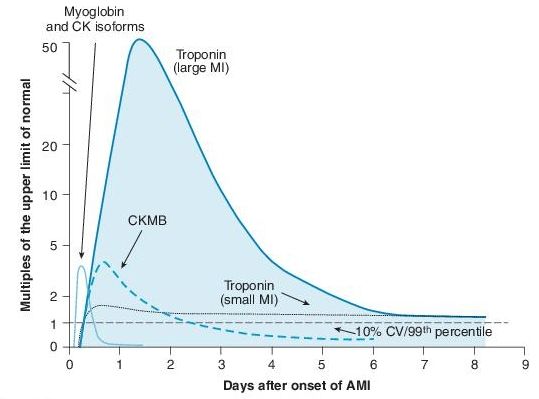
Figure 3–2 Graph of temporal expression of cardiac biomarkers. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
Without positive biomarkers, low-risk patients (age < 70, no rest pain, pain <2 weeks without prolonged episodes, normal ECG, no prior CAD or diabetes mellitus) may be discharged home and additional evaluation performed as an outpatient. Appointment should be made within 72 hours for evaluation. Intermediaterisk patients without high-risk features require in-hospital triage with provocative noninvasive imaging. The sensitivity and specificity of stress testing can be combined with pretest risk to give a prognosis of coronary heart disease.
 Exercise treadmill testing should be considered first, particularly in lowrisk groups; low sensitivity, predictive value, and inability to identify and quantify ischemic areas when compared with imaging modalities; cannot interpret ECG if LBBB, ventricular paced rhythm, LVH hypertrophy, and conduction abnormalities. Duke prognostic treadmill score establishes the risk of death from CAD; combined with imaging (SPECT, MRI, or echo) to improve sensitivity and specificity in women and those with confounding baseline ECGs.
Exercise treadmill testing should be considered first, particularly in lowrisk groups; low sensitivity, predictive value, and inability to identify and quantify ischemic areas when compared with imaging modalities; cannot interpret ECG if LBBB, ventricular paced rhythm, LVH hypertrophy, and conduction abnormalities. Duke prognostic treadmill score establishes the risk of death from CAD; combined with imaging (SPECT, MRI, or echo) to improve sensitivity and specificity in women and those with confounding baseline ECGs.
 The advantage of echocardiography over SPECT (single-photon emission computed tomography) is lack of radiation exposure but carries higher false-negative results at submaximal heart rates. SPECT has higher positive-negative predictive values over treadmill testing alone.
The advantage of echocardiography over SPECT (single-photon emission computed tomography) is lack of radiation exposure but carries higher false-negative results at submaximal heart rates. SPECT has higher positive-negative predictive values over treadmill testing alone.
 Cardiac MRI has excellent spatial resolution without radiation; similar to SPECT, may assess myocardial viability (unlike SPECT can differentiate pericarditis). Stress MRI can be performed with dobutamine or adenosine; difficulty imaging irregular heart rhythms and patients with metal implants. No large comparative studies published yet.
Cardiac MRI has excellent spatial resolution without radiation; similar to SPECT, may assess myocardial viability (unlike SPECT can differentiate pericarditis). Stress MRI can be performed with dobutamine or adenosine; difficulty imaging irregular heart rhythms and patients with metal implants. No large comparative studies published yet.
 Cardiac CT (64 slice) has excellent negative predictive value (>90%), slightly diminished positive predictive value (80%). Rapid acquisition but requires lower heart rates for image analysis with a tendency to overestimate disease severity; provides only anatomic, not functional information (i.e., culprit lesion). At present, no consensus for use of CT as a “triple rule out”—CAD, aortic dissection, PE in the rapid assessment of chest pain.
Cardiac CT (64 slice) has excellent negative predictive value (>90%), slightly diminished positive predictive value (80%). Rapid acquisition but requires lower heart rates for image analysis with a tendency to overestimate disease severity; provides only anatomic, not functional information (i.e., culprit lesion). At present, no consensus for use of CT as a “triple rule out”—CAD, aortic dissection, PE in the rapid assessment of chest pain.
Suggested Readings
Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2007;50:e1–e157.
Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol. 2011;58:1332–1339.
McCaig L, Burt C. National Hospital Ambulatory Medical Care Survey: 2003 Emergency Department Summary. In: Advance Data from Vital and Health Statistics. Atlanta, GA: Centers for Disease Control and Prevention, 2005.
Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain syndromes using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59:2091–2098.
Thygesen K, Alpert JS, White HD; on behalf of the Joint ESCIACCFI AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Cardiol. 2007;50:2173–2195.
CHEST PAIN: NONATHEROSCLEROTIC ISCHEMIA
 Definition
Definition
 Approximately 5% of patients with acute myocardial infarction do not have atherosclerotic coronary disease, increasing to 20% in patients under the age of 35. Necropsy studies in these individuals often demonstrate luminal narrowing, leading to ischemia via several mechanisms: internal narrowing by obstructions or encroachment by adjacent structures.
Approximately 5% of patients with acute myocardial infarction do not have atherosclerotic coronary disease, increasing to 20% in patients under the age of 35. Necropsy studies in these individuals often demonstrate luminal narrowing, leading to ischemia via several mechanisms: internal narrowing by obstructions or encroachment by adjacent structures.
 Ischemia may also result from dynamic changes in an otherwise normal arterial wall (spasm and anomalous arteries) or an imbalance in oxygen supply and demand (type 2 MI).
Ischemia may also result from dynamic changes in an otherwise normal arterial wall (spasm and anomalous arteries) or an imbalance in oxygen supply and demand (type 2 MI).
 Over 50% of fatal MIs without coronary disease likely represent coronary vasospasm.
Over 50% of fatal MIs without coronary disease likely represent coronary vasospasm.
 Who Should Be Suspected?
Who Should Be Suspected?
 Diagnosis is often made by exclusion via cardiac imaging due to overlap of symptom presentation with ACS.
Diagnosis is often made by exclusion via cardiac imaging due to overlap of symptom presentation with ACS.
 Young age (<35 years) and lack of coronary risk factors raise the suspicion of congenital coronary anomalies or congenital coronary aneurysm. A careful history to exclude cocaine use (supported by tox screen if needed) is mandatory in STEMI patients without significant atherosclerotic risk factors, as is rheumatic history.
Young age (<35 years) and lack of coronary risk factors raise the suspicion of congenital coronary anomalies or congenital coronary aneurysm. A careful history to exclude cocaine use (supported by tox screen if needed) is mandatory in STEMI patients without significant atherosclerotic risk factors, as is rheumatic history.
 Coronary spasm has also been described with patients receiving chemotherapeutic drugs such as 5-fluorouracil, and those talking herbal medicines. A careful medical reconciliation should be performed on all chest pain patients and review of “vasospastic potential” performed, and this includes use of estrogen replacement therapy (coronary dissection).
Coronary spasm has also been described with patients receiving chemotherapeutic drugs such as 5-fluorouracil, and those talking herbal medicines. A careful medical reconciliation should be performed on all chest pain patients and review of “vasospastic potential” performed, and this includes use of estrogen replacement therapy (coronary dissection).
 Hypercoagulable/malignancy history should be reviewed, and subtherapeutic INR for patients on Coumadin should be assessed for the possibility of coronary embolism.
Hypercoagulable/malignancy history should be reviewed, and subtherapeutic INR for patients on Coumadin should be assessed for the possibility of coronary embolism.
 Presentation/Findings
Presentation/Findings
Congenital Coronary Anomalies
 Present in 1–2% of the general population but 4% of autopsies for MI. When coronary arteries arise from the contralateral sinus of Valsalva, the anomalous artery may course between the great vessels. States of increased cardiac output may cause either compression or torsion of the proximal coronary artery resulting in ischemia, infarct, or sudden cardiac death.
Present in 1–2% of the general population but 4% of autopsies for MI. When coronary arteries arise from the contralateral sinus of Valsalva, the anomalous artery may course between the great vessels. States of increased cardiac output may cause either compression or torsion of the proximal coronary artery resulting in ischemia, infarct, or sudden cardiac death.
 Diagnosis is made by imaging based on ACS risk profile of presentation. Cardiac catheterization (high-risk patients) demonstrates interarterial course of anomalous vessel. Direct visualization with cardiac CT or MRI is an advantage when considering these modalities for stress testing in younger populations but must be balanced by cost consideration. Surgical bypass for high-risk anatomy is the preferred treatment.
Diagnosis is made by imaging based on ACS risk profile of presentation. Cardiac catheterization (high-risk patients) demonstrates interarterial course of anomalous vessel. Direct visualization with cardiac CT or MRI is an advantage when considering these modalities for stress testing in younger populations but must be balanced by cost consideration. Surgical bypass for high-risk anatomy is the preferred treatment.
Myocardial Bridges (“Tunneled” Epicardial Arteries)
 Congenital in origin: The course of the epicardial coronary artery dives below the myocardium and is compressed in systole. As the majority of coronary blood flow occurs in diastole, tachycardia with resulting shortened diastolic filling period is often required to produce ischemia. Length of the tunneled arterial segment may not play a significant role in risk.
Congenital in origin: The course of the epicardial coronary artery dives below the myocardium and is compressed in systole. As the majority of coronary blood flow occurs in diastole, tachycardia with resulting shortened diastolic filling period is often required to produce ischemia. Length of the tunneled arterial segment may not play a significant role in risk.
 Diagnosis made by direct visualization by angiography or CT/MRI. The presence of a myocardial bridge does not necessarily imply ischemia is present.
Diagnosis made by direct visualization by angiography or CT/MRI. The presence of a myocardial bridge does not necessarily imply ischemia is present.
Coronary Aneurysm
 Congenital (more common in right coronary artery) or acquired (infection/ inflammation): Turbulent flow in the aneurysm may predispose to thrombus formation and ACS. Acquired aneurysm may be the result of atherosclerosis (50%) or syphilis, mycotic emboli, Kawasaki disease, or lupus. Appropriate serologies should be sent when aneurysm is identified by imaging (see Chapter 2, Autoimmune Diseases and Chapter 11, Infectious Diseases).
Congenital (more common in right coronary artery) or acquired (infection/ inflammation): Turbulent flow in the aneurysm may predispose to thrombus formation and ACS. Acquired aneurysm may be the result of atherosclerosis (50%) or syphilis, mycotic emboli, Kawasaki disease, or lupus. Appropriate serologies should be sent when aneurysm is identified by imaging (see Chapter 2, Autoimmune Diseases and Chapter 11, Infectious Diseases).
Embolism
 Coronary artery emboli should be considered in any patient presenting with ACS (usually STEMI) in the setting of atrial fibrillation, active infective endocarditis, prosthetic heart valve, known LV thrombus, or left-sided cardiac tumor (right-sided tumors require a right-to-left shunt to be present). Initial triage is performed as dictated by ACS algorithm, and diagnosis often made angiographically. Coronary embolism most often involves the LAD and may resolve spontaneously with anticoagulation.
Coronary artery emboli should be considered in any patient presenting with ACS (usually STEMI) in the setting of atrial fibrillation, active infective endocarditis, prosthetic heart valve, known LV thrombus, or left-sided cardiac tumor (right-sided tumors require a right-to-left shunt to be present). Initial triage is performed as dictated by ACS algorithm, and diagnosis often made angiographically. Coronary embolism most often involves the LAD and may resolve spontaneously with anticoagulation.
 Embolism or spasm should be considered for angiographically normal arteries in the setting of MI.
Embolism or spasm should be considered for angiographically normal arteries in the setting of MI.
Spontaneous Coronary Artery Dissection
 Occurs from vessel wall hematoma between media and adventitia in the absence of trauma or iatrogenic causes. Most diagnosed with autopsy and occur in LAD or left main artery.
Occurs from vessel wall hematoma between media and adventitia in the absence of trauma or iatrogenic causes. Most diagnosed with autopsy and occur in LAD or left main artery.
 Described in young women with risk factors among whom 25–30% are pregnant or in the postpartum period. Likely etiologies include hormonal impairment of collagen synthesis. Oral contraceptive use also associated with dissection. Thrombolytic therapy should not be given in postpartum patients with STEMI due to increased chance of propagation of hematoma.
Described in young women with risk factors among whom 25–30% are pregnant or in the postpartum period. Likely etiologies include hormonal impairment of collagen synthesis. Oral contraceptive use also associated with dissection. Thrombolytic therapy should not be given in postpartum patients with STEMI due to increased chance of propagation of hematoma.
 Men are more likely to be older and have right coronary involvement with coronary risk factors. Systemic hypertension is not a risk factor for dissection.
Men are more likely to be older and have right coronary involvement with coronary risk factors. Systemic hypertension is not a risk factor for dissection.
 Other associated conditions include cocaine or cyclosporine use, hypertrophic cardiomyopathy, Marfan or Ehlers-Danlos syndrome, and immunemediated diseases such as rheumatic arteritis, autoimmune thyroiditis, hepatitis C infection, sarcoidosis, systemic lupus erythematosus, Kawasaki arteritis, and eosinophilic coronary arteritis.
Other associated conditions include cocaine or cyclosporine use, hypertrophic cardiomyopathy, Marfan or Ehlers-Danlos syndrome, and immunemediated diseases such as rheumatic arteritis, autoimmune thyroiditis, hepatitis C infection, sarcoidosis, systemic lupus erythematosus, Kawasaki arteritis, and eosinophilic coronary arteritis.
 A careful family history of connective tissue disease, lab assessment for eosinophilia, ESR, ANA, and thyroid evaluation should be performed if coronary dissection is suspected.
A careful family history of connective tissue disease, lab assessment for eosinophilia, ESR, ANA, and thyroid evaluation should be performed if coronary dissection is suspected.
Coronary Artery Spam
 Spasm that occurs within epicardial coronary arteries usually occurs at sites with non–flow-limiting luminal narrowing by atherosclerotic plaque. An abundance of smooth muscle cells is usually present in necropsy studies at the known sites of spasm.
Spasm that occurs within epicardial coronary arteries usually occurs at sites with non–flow-limiting luminal narrowing by atherosclerotic plaque. An abundance of smooth muscle cells is usually present in necropsy studies at the known sites of spasm.
 Hyperadrenergic conditions associated with spasm include pheochromocytoma, cocaine, amphetamine, and ecstasy use. This includes dobutamine infusion for stress testing. Epicardial spasm can be seen in inflammatory conditions of thyrotoxicosis, allergic angina, as well as administration of fluorouracil, capecitabine, sumatriptan, and bromocriptine.
Hyperadrenergic conditions associated with spasm include pheochromocytoma, cocaine, amphetamine, and ecstasy use. This includes dobutamine infusion for stress testing. Epicardial spasm can be seen in inflammatory conditions of thyrotoxicosis, allergic angina, as well as administration of fluorouracil, capecitabine, sumatriptan, and bromocriptine.
 Diagnosis of spasm is usually made with angiography. Provocative challenge during catheterization with ergotamine is no longer performed due to risk of MI/death from refractory spasm. Potential offending medications or behaviors should be stopped immediately.
Diagnosis of spasm is usually made with angiography. Provocative challenge during catheterization with ergotamine is no longer performed due to risk of MI/death from refractory spasm. Potential offending medications or behaviors should be stopped immediately.
 Epicardial spasm should be differentiated from Syndrome X, or microvascular spasm. This syndrome carries a benign prognosis but presents with angina-like pain. Stress testing often reveals signs of ischemia with normal epicardial arteries on invasive testing (coronary flow reserve—which can be assessed invasively is often abnormal). Pain is likely due to either microvascular spasm or abnormal pain perception (sympathetic predominance).
Epicardial spasm should be differentiated from Syndrome X, or microvascular spasm. This syndrome carries a benign prognosis but presents with angina-like pain. Stress testing often reveals signs of ischemia with normal epicardial arteries on invasive testing (coronary flow reserve—which can be assessed invasively is often abnormal). Pain is likely due to either microvascular spasm or abnormal pain perception (sympathetic predominance).
Hypertrophic Obstructive Cardiomyopathy
 See Dyspnea/CHF section.
See Dyspnea/CHF section.
Suggested Readings
Angelini P, Trivellato M, Doris J, et al. Myocardial bridges: a review. Prog Cardiovasc Dis. 1983;26:75–88.
Chetlin MD, Virami R. Myocardial infarction in the absence of coronary atherosclerotic disease. In: Virmani R, Forman MB (eds). Nonatherosclerotic ischemic heart disease. New York, NY: Raven Press; 1989:1–30.
CHEST PAIN: INFLAMMATORY
Chest pain may occur due to an inflammatory response to immune-mediated or infectious triggers without necessarily predisposing to ischemic insult. Pericardium, myocardium, or direct coronary artery involvement may occur. Ischemia may occur when coronary arteries are involved as a direct result of the inflammatory process (necrosis and aneurysm formation) or via wall thickening and luminal narrowing, rupture of the vessel wall, or from thrombosis due to hypercoagulable state or accelerated atherosclerosis.
VASCULITIS
 Definition
Definition
 Vasculitis describes a heterogeneous group of disorders that are characterized by leukocyte migration in the vessel wall resulting in damage of blood vessels, which leads to tissue ischemia and necrosis.
Vasculitis describes a heterogeneous group of disorders that are characterized by leukocyte migration in the vessel wall resulting in damage of blood vessels, which leads to tissue ischemia and necrosis.
 Epicardial coronary vasculitis is relatively rare but can be life threatening. Coronary arteries are involved through either direct extension or hematogenous spread.
Epicardial coronary vasculitis is relatively rare but can be life threatening. Coronary arteries are involved through either direct extension or hematogenous spread.
 Cardiac manifestations are rarely predominant in vasculitis and are just as likely to occur as a result of other organ involvement or treatment side effects of this systemic process.
Cardiac manifestations are rarely predominant in vasculitis and are just as likely to occur as a result of other organ involvement or treatment side effects of this systemic process.
 Heart failure due to direct myocardial involvement, ischemic cardiomyopathy, or valvular involvement in vasculitis is more common than ACS-like presentation.
Heart failure due to direct myocardial involvement, ischemic cardiomyopathy, or valvular involvement in vasculitis is more common than ACS-like presentation.
 Size and shape of both arteries and veins are affected due to a primary process or secondary to an underlying pathology.
Size and shape of both arteries and veins are affected due to a primary process or secondary to an underlying pathology.
 Classification By
Classification By
Etiology
 Primary: polyarteritis nodosa, Wegener granulomatosis, giant cell arteritis, hypersensitivity vasculitis (see Chapter 2, Autoimmune Diseases)
Primary: polyarteritis nodosa, Wegener granulomatosis, giant cell arteritis, hypersensitivity vasculitis (see Chapter 2, Autoimmune Diseases)
 Secondary
Secondary
 Infections: bacteria (e.g., septicemia caused by gonococcal organisms or Staphylococcus), mycobacteria, viruses (e.g., CMV, hepatitis B), Rickettsia (e.g., Rocky Mountain spotted fever), spirochetes (e.g., syphilis, Lyme disease)
Infections: bacteria (e.g., septicemia caused by gonococcal organisms or Staphylococcus), mycobacteria, viruses (e.g., CMV, hepatitis B), Rickettsia (e.g., Rocky Mountain spotted fever), spirochetes (e.g., syphilis, Lyme disease)
 Associated with malignancy (e.g., multiple myeloma, lymphomas)
Associated with malignancy (e.g., multiple myeloma, lymphomas)
 Connective tissue diseases (e.g., RA, SLE, Sjögren syndrome)
Connective tissue diseases (e.g., RA, SLE, Sjögren syndrome)
 Diseases that may simulate vasculitis (e.g., ergotamine toxicity, cholesterol embolization, atrial myxoma)
Diseases that may simulate vasculitis (e.g., ergotamine toxicity, cholesterol embolization, atrial myxoma)
Size of Involved Vessel (Noninfectious Vasculitis)
 Large vessel: dissection of aorta (dissecting aneurysm), Takayasu arteritis, giant cell (temporal) arteritis
Large vessel: dissection of aorta (dissecting aneurysm), Takayasu arteritis, giant cell (temporal) arteritis
 Medium-sized vessel: polyarteritis nodosa (or small), Kawasaki disease, primary granulomatous CNS vasculitis
Medium-sized vessel: polyarteritis nodosa (or small), Kawasaki disease, primary granulomatous CNS vasculitis
 Small vessel: ANCA-associated vasculitis (Wegener granulomatosis, Churg- Strauss syndrome, drug-induced, microscopic polyangiitis), immune complex–type vasculitis (Henoch-Schönlein purpura, cryoglobulinemia, rheumatoid vasculitis [or medium], SLE, Sjögren syndrome, Goodpasture syndrome, Behçet syndrome, drug-induced serum sickness), paraneoplastic vasculitis (lymphoproliferative, myeloproliferative, carcinoma), inflammatory bowel disease
Small vessel: ANCA-associated vasculitis (Wegener granulomatosis, Churg- Strauss syndrome, drug-induced, microscopic polyangiitis), immune complex–type vasculitis (Henoch-Schönlein purpura, cryoglobulinemia, rheumatoid vasculitis [or medium], SLE, Sjögren syndrome, Goodpasture syndrome, Behçet syndrome, drug-induced serum sickness), paraneoplastic vasculitis (lymphoproliferative, myeloproliferative, carcinoma), inflammatory bowel disease
 Any size vessel (pseudovasculitis): antiphospholipid syndrome, emboli (e.g., myxomas, cholesterol emboli, bacterial or nonbacterial endocarditis), drugs (e.g., amphetamines)
Any size vessel (pseudovasculitis): antiphospholipid syndrome, emboli (e.g., myxomas, cholesterol emboli, bacterial or nonbacterial endocarditis), drugs (e.g., amphetamines)
 Who Should Be Suspected?
Who Should Be Suspected?
 Patients may present with fatigue, weakness, fever, myalgias, arthralgia, headache, abdominal pain, hypertension, nosebleeds, palpable purpura, and/ or mononeuritis.
Patients may present with fatigue, weakness, fever, myalgias, arthralgia, headache, abdominal pain, hypertension, nosebleeds, palpable purpura, and/ or mononeuritis.
 Coronary artery imaging (angiography, MRI, CT) that reveals a “string-of-pearls” sign sequential proximal coronary aneurysms that is suggestive of a primary or secondary vasculitic process. A focused rheumatic history should be performed in all patients with this angiographic finding.
Coronary artery imaging (angiography, MRI, CT) that reveals a “string-of-pearls” sign sequential proximal coronary aneurysms that is suggestive of a primary or secondary vasculitic process. A focused rheumatic history should be performed in all patients with this angiographic finding.
 Laboratory Findings
Laboratory Findings
The gold standard in the diagnosis of most vasculitides is based on pathologic findings in a biopsy of the involved tissue.
 Hematology: ESR is increased in 90% of cases, often to very high levels; CRP correlates with disease activity even better than ESR. Normochromic anemia of chronic disease, thrombocytosis, and mild leukocytosis occur in 30–40% of patients; eosinophilia may occur but is not a feature. Leukopenia or thrombocytopenia occurs only during cytotoxic therapy.
Hematology: ESR is increased in 90% of cases, often to very high levels; CRP correlates with disease activity even better than ESR. Normochromic anemia of chronic disease, thrombocytosis, and mild leukocytosis occur in 30–40% of patients; eosinophilia may occur but is not a feature. Leukopenia or thrombocytopenia occurs only during cytotoxic therapy.
 Urinalysis: hematuria, proteinuria, and azotemia.
Urinalysis: hematuria, proteinuria, and azotemia.
 Core laboratory: serum globulins (IgG and IgA) are increased in ≤50% of cases. Serum C3 and C4 complement levels may be increased. RF may be present in low titer. ANA positive in vasculitis secondary to connective tissue disorders. ANCA determination provides valuable information and is highly specific for the diagnosis of small-vessel vasculitides, particularly Wegener granulomatosis.
Core laboratory: serum globulins (IgG and IgA) are increased in ≤50% of cases. Serum C3 and C4 complement levels may be increased. RF may be present in low titer. ANA positive in vasculitis secondary to connective tissue disorders. ANCA determination provides valuable information and is highly specific for the diagnosis of small-vessel vasculitides, particularly Wegener granulomatosis.
 Imaging studies: arteriogram, MRI, and ultrasound.
Imaging studies: arteriogram, MRI, and ultrasound.
 Considerations.
Considerations.
 c-ANCA (anti-proteinase 3; coarse diffuse cytoplasmic pattern) is highly specific (>90%) for active Wegener granulomatosis. Sensitivity is >90% in systemic vasculitic phase, approximately 65% in predominantly granulomatous disease of respiratory tract, and approximately 30% during complete remission.
c-ANCA (anti-proteinase 3; coarse diffuse cytoplasmic pattern) is highly specific (>90%) for active Wegener granulomatosis. Sensitivity is >90% in systemic vasculitic phase, approximately 65% in predominantly granulomatous disease of respiratory tract, and approximately 30% during complete remission.
 ELISA titer does not correlate with disease activity; a high titer may persist during remission for years. c-ANCA is also occasionally found in other vasculitides (polyarteritis nodosa, microscopic polyangiitis [e.g., lung, idiopathic crescentic and pauci-immune GN], Churg-Strauss vasculitis).
ELISA titer does not correlate with disease activity; a high titer may persist during remission for years. c-ANCA is also occasionally found in other vasculitides (polyarteritis nodosa, microscopic polyangiitis [e.g., lung, idiopathic crescentic and pauci-immune GN], Churg-Strauss vasculitis).
 p-ANCA (against various proteins [e.g., myeloperoxidase, elastase, lysozyme; perinuclear pattern]) occurs only with fixation in alcohol, not formalin. A positive result should be confirmed by ELISA. The test has poor specificity and 20–60% sensitivity in a variety of autoimmune diseases (microscopic polyangiitis, Churg-Strauss vasculitis, SLE, inflammatory bowel disease, Goodpasture syndrome, Sjögren syndrome, idiopathic GN, chronic infection). However, pulmonary small vessel vasculitis is strongly linked to myeloperoxidase antibodies.
p-ANCA (against various proteins [e.g., myeloperoxidase, elastase, lysozyme; perinuclear pattern]) occurs only with fixation in alcohol, not formalin. A positive result should be confirmed by ELISA. The test has poor specificity and 20–60% sensitivity in a variety of autoimmune diseases (microscopic polyangiitis, Churg-Strauss vasculitis, SLE, inflammatory bowel disease, Goodpasture syndrome, Sjögren syndrome, idiopathic GN, chronic infection). However, pulmonary small vessel vasculitis is strongly linked to myeloperoxidase antibodies.
 Both p-ANCA and c-ANCA may be found in non–immune-mediated polyarteritis and other vasculitides.
Both p-ANCA and c-ANCA may be found in non–immune-mediated polyarteritis and other vasculitides.
 Atypical pattern (neither c-ANCA nor p-ANCA; unknown target antigens) has poor specificity and unknown sensitivity in various conditions (e.g., HIV infection, endocarditis, CF, Felty syndrome, Kawasaki disease, ulcerative colitis, Crohn disease).
Atypical pattern (neither c-ANCA nor p-ANCA; unknown target antigens) has poor specificity and unknown sensitivity in various conditions (e.g., HIV infection, endocarditis, CF, Felty syndrome, Kawasaki disease, ulcerative colitis, Crohn disease).
ANTIPHOSPHOLIPID ANTIBODY SYNDROME
See Chapter 9, Hematologic Disorders.
HENOCH-SCHÖNLEIN PURPURA
 Definition
Definition
 Henoch-Schönlein purpura is a self-limited hypersensitivity systemic vasculitis of the small vessels. It involves the skin and to variable degrees joints, kidneys, and GI tract. The small vessel and renal involvement is caused by IgA deposition.
Henoch-Schönlein purpura is a self-limited hypersensitivity systemic vasculitis of the small vessels. It involves the skin and to variable degrees joints, kidneys, and GI tract. The small vessel and renal involvement is caused by IgA deposition.
 Who Should Be Suspected?
Who Should Be Suspected?
 This condition is seen more commonly in children (90% of cases), but it may affect adults as well.
This condition is seen more commonly in children (90% of cases), but it may affect adults as well.
 In adults, renal disease is common. The renal picture may vary, with minimal urinary abnormalities occurring for years. Patients may present with palpable purpura without thrombocytopenia or a coagulopathy and acute abdominal pain, or with purpura and joint symptoms.
In adults, renal disease is common. The renal picture may vary, with minimal urinary abnormalities occurring for years. Patients may present with palpable purpura without thrombocytopenia or a coagulopathy and acute abdominal pain, or with purpura and joint symptoms.
 Laboratory Findings
Laboratory Findings
Diagnosis is made clinically; there are no pathognomonic laboratory findings.
 Histology: renal or skin biopsy supports the diagnosis; it shows focal segmental necrotizing GN that becomes more diffuse and crescentic with IgA and C3 deposition.
Histology: renal or skin biopsy supports the diagnosis; it shows focal segmental necrotizing GN that becomes more diffuse and crescentic with IgA and C3 deposition.
 Urinalysis: RBCs, casts, and slight protein in 25–50% of patients. The renal picture varies from minimal urinary abnormalities for years to end-stage renal disease within months. Gross hematuria and proteinuria are uncommon.
Urinalysis: RBCs, casts, and slight protein in 25–50% of patients. The renal picture varies from minimal urinary abnormalities for years to end-stage renal disease within months. Gross hematuria and proteinuria are uncommon.
 Hematology: coagulation tests are normal.
Hematology: coagulation tests are normal.
 Core laboratory: BUN and creatinine may be increased.
Core laboratory: BUN and creatinine may be increased.
Suggested Reading
Trapani S, Micheli A, Grisolla F, et al. Henoch Schönlein Purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of the literature. Semin Arthritis Rheum. 2005;35:143–153.
KAWASAKI SYNDROME (MUCOCUTANEOUS LYMPH NODE SYNDROME)
 Definition
Definition
 Kawasaki syndrome is a variant of childhood polyarteritis of unknown etiology, with a high incidence of coronary artery complications.
Kawasaki syndrome is a variant of childhood polyarteritis of unknown etiology, with a high incidence of coronary artery complications.
 Laboratory Findings
Laboratory Findings
 Histology: diagnosis is confirmed by histologic examination of the coronary artery (same as for polyarteritis nodosa).
Histology: diagnosis is confirmed by histologic examination of the coronary artery (same as for polyarteritis nodosa).
 Hematology: anemia (approximately 50% of patients). Leukocytosis (20,000–30,000/μL) with shift to left occurs during 1st week; lymphocytosis appears thereafter, peaking at the end of the 2nd week, and is a hallmark of this illness. Increased ESR.
Hematology: anemia (approximately 50% of patients). Leukocytosis (20,000–30,000/μL) with shift to left occurs during 1st week; lymphocytosis appears thereafter, peaking at the end of the 2nd week, and is a hallmark of this illness. Increased ESR.
 CSF findings: increased mononuclear cells with normal protein and sugar.
CSF findings: increased mononuclear cells with normal protein and sugar.
 Urinalysis: increased mononuclear cells; dipstick negative.
Urinalysis: increased mononuclear cells; dipstick negative.
 Joint fluid findings: increased white blood cell (WBC) count (predominantly PMNs) in patients with arthritis.
Joint fluid findings: increased white blood cell (WBC) count (predominantly PMNs) in patients with arthritis.
 Core laboratory: laboratory changes due to AMI. Acute-phase reactants are increased (e.g., CRP, α-1-antitrypsin); these usually return to normal after 6–8 weeks.
Core laboratory: laboratory changes due to AMI. Acute-phase reactants are increased (e.g., CRP, α-1-antitrypsin); these usually return to normal after 6–8 weeks.
TAKAYASU SYNDROME (ARTERITIS)
 Definition
Definition
 Takayasu syndrome is the term for granulomatous arteritis of the aorta. Temporal arteritis and rheumatic disease may also be associated with aortitis.
Takayasu syndrome is the term for granulomatous arteritis of the aorta. Temporal arteritis and rheumatic disease may also be associated with aortitis.
 Greater incidence in young to middle-aged Asian females. Coronary involvement occurs in 15–25% of cases. Involvement is usually in segments and rarely diffuse.
Greater incidence in young to middle-aged Asian females. Coronary involvement occurs in 15–25% of cases. Involvement is usually in segments and rarely diffuse.
 Average age of onset is 24 years, and the diagnosis should be considered in individuals of <40 years with acute myocardial infarction.
Average age of onset is 24 years, and the diagnosis should be considered in individuals of <40 years with acute myocardial infarction.
 Diagnosis is established by characteristic arteriographic narrowing or occlusion or histologic examination. Laboratory tests are not useful for diagnosis or to guide management.
Diagnosis is established by characteristic arteriographic narrowing or occlusion or histologic examination. Laboratory tests are not useful for diagnosis or to guide management.
 Laboratory Findings
Laboratory Findings
Findings are due to involvement of coronary or renal vessels.
 Hematology: increased ESR is found in approximately 75% of cases during active disease but is normal in only 50% of cases during remission. WBC count is usually normal.
Hematology: increased ESR is found in approximately 75% of cases during active disease but is normal in only 50% of cases during remission. WBC count is usually normal.
 Core laboratory: serum proteins are abnormal, with increased γ globulins (mostly composed of IgM). Female patients have a continuous high level of urinary total estrogens (rather than the usual rise during the luteal phase after a low excretion during the follicular phase).
Core laboratory: serum proteins are abnormal, with increased γ globulins (mostly composed of IgM). Female patients have a continuous high level of urinary total estrogens (rather than the usual rise during the luteal phase after a low excretion during the follicular phase).
THROMBOANGIITIS OBLITERANS (BUERGER DISEASE)
 Thromboangiitis obliterans is very rare and is the vascular inflammation and occlusion of medium and small arteries and veins of limbs; it is related to smoking and occurs mostly in males. Histology shows characteristic inflammatory and proliferative lesions. Coronary involvement is uncommon. Laboratory tests are usually normal.
Thromboangiitis obliterans is very rare and is the vascular inflammation and occlusion of medium and small arteries and veins of limbs; it is related to smoking and occurs mostly in males. Histology shows characteristic inflammatory and proliferative lesions. Coronary involvement is uncommon. Laboratory tests are usually normal.
INFECTIOUS (SECONDARY) VASCULITIS
 Definition
Definition
 Various microorganisms may cause vasculitis of any size vessel by either hematogenous spread or direct extension of cardiac structures involved (pericardium, valves).
Various microorganisms may cause vasculitis of any size vessel by either hematogenous spread or direct extension of cardiac structures involved (pericardium, valves).
 Most important infections of the coronary arteries are syphilis, tuberculosis, and syphilitic arteritis.
Most important infections of the coronary arteries are syphilis, tuberculosis, and syphilitic arteritis.
 Who Should Be Suspected?
Who Should Be Suspected?
 Tuberculosis coronary arteritis occurs mainly in patients with preexisting pericardial or myocardial tuberculosis.
Tuberculosis coronary arteritis occurs mainly in patients with preexisting pericardial or myocardial tuberculosis.
 Syphilitic arteritis can involve the first 3–4 mm of the left and right coronary arteries with an obliterative arteritis.
Syphilitic arteritis can involve the first 3–4 mm of the left and right coronary arteries with an obliterative arteritis.
 When a nonviral infectious angiitis occurs, it is almost always accompanied by myocarditis with abscesses and pericarditis.
When a nonviral infectious angiitis occurs, it is almost always accompanied by myocarditis with abscesses and pericarditis.
 Laboratory Findings
Laboratory Findings
 Core lab blood work, cultures, and PCR analysis should be dictated by systemic clues to the underlying infectious process.
Core lab blood work, cultures, and PCR analysis should be dictated by systemic clues to the underlying infectious process.
THROMBOPHLEBITIS, SEPTIC
 Definition
Definition
 Thrombophlebitis is vascular inflammation due to a blood clot.
Thrombophlebitis is vascular inflammation due to a blood clot.
 Laboratory Findings
Laboratory Findings
Findings are due to associated septicemia, complications (e.g., septic pulmonary infarction), and underlying disease.
 Hematology: increased WBC count (often >20,000/μL), with marked shift to left and toxic changes in neutrophils. DIC may be present.
Hematology: increased WBC count (often >20,000/μL), with marked shift to left and toxic changes in neutrophils. DIC may be present.
 Core laboratory: azotemia.
Core laboratory: azotemia.
 Culture: positive blood culture (Staphylococcus aureus is the most frequent organism; others are Klebsiella, Pseudomonas aeruginosa, enterococci, Candida).
Culture: positive blood culture (Staphylococcus aureus is the most frequent organism; others are Klebsiella, Pseudomonas aeruginosa, enterococci, Candida).
PERICARDITIS (ACUTE) AND PERICARDIAL EFFUSION
 Definition
Definition
 The pericardium is a double-walled sac that surrounds the heart. The inner visceral pericardium is normally separated from the outer, fibrous parietal pericardium by a small volume (15–50 mL) of fluid, a plasma ultrafiltrate. Inflammation of the pericardium results in pericarditis, with or without an associated pericardial effusion.
The pericardium is a double-walled sac that surrounds the heart. The inner visceral pericardium is normally separated from the outer, fibrous parietal pericardium by a small volume (15–50 mL) of fluid, a plasma ultrafiltrate. Inflammation of the pericardium results in pericarditis, with or without an associated pericardial effusion.
 Common causes of pericardial inflammation include infection, uremia, trauma, malignancy, hypersensitivity, and autoimmune diseases. Viral infections (coxsackie- and echovirus) are by far the most common and are usually self-limited.
Common causes of pericardial inflammation include infection, uremia, trauma, malignancy, hypersensitivity, and autoimmune diseases. Viral infections (coxsackie- and echovirus) are by far the most common and are usually self-limited.
 Cardiac tamponade is more likely to present as dyspnea in its mild form with additional precordial discomfort and hypotension/shock more likely with severe tamponade.
Cardiac tamponade is more likely to present as dyspnea in its mild form with additional precordial discomfort and hypotension/shock more likely with severe tamponade.
 When presenting as chest pain, myocarditis is often due to concomitant pericarditis. Myocardial involvement alone more often presents as dyspnea and dilated cardiomyopathy (see Dyspnea section), although younger patients are more likely to present.
When presenting as chest pain, myocarditis is often due to concomitant pericarditis. Myocardial involvement alone more often presents as dyspnea and dilated cardiomyopathy (see Dyspnea section), although younger patients are more likely to present.
 Who Should Be Suspected?
Who Should Be Suspected?
 Any recent trauma victim in shock, post-MI patients, patients with comorbid conditions predisposed to effusion (neoplasm, chronic inflammatory disease), patients with chest pain after a viral prodrome.
Any recent trauma victim in shock, post-MI patients, patients with comorbid conditions predisposed to effusion (neoplasm, chronic inflammatory disease), patients with chest pain after a viral prodrome.
 Typical signs and symptoms of acute pericarditis include chest pain (often pleuritic and worse with inspiration and supine position), pericardial friction rub (pathognomonic), ECG changes (e.g., ST elevation, PR depression), and pericardial effusion.
Typical signs and symptoms of acute pericarditis include chest pain (often pleuritic and worse with inspiration and supine position), pericardial friction rub (pathognomonic), ECG changes (e.g., ST elevation, PR depression), and pericardial effusion.
 Not all patients will manifest all of these features; the presence or absence of an effusion does not exclude the diagnosis.
Not all patients will manifest all of these features; the presence or absence of an effusion does not exclude the diagnosis.
 Diagnostic and Laboratory Findings
Diagnostic and Laboratory Findings
 Echocardiography: Most useful imaging technique for the evaluation of acute pericarditis and is critical for patients if tamponade is suspected. Small pericardial effusions, undetectable by routine examinations, may be detected, providing support for the diagnosis of pericardial disease. Typically >1 cm of effusion is required for safe performance of pericardiocentesis. Dopplerderived flow-velocity measures of mitral and tricuspid flow may assist in diagnosing tamponade, but it is ultimately a clinical diagnosis based on inspiratory decline in systolic arterial pressure exceeding 10 mm Hg (pulsus paradoxus), which can be also seen in COPD and pulmonary embolism. The absence of any chamber collapse on echocardiography has a high negative predictive value for tamponade (92%), although the positive predictive value is low (58%). Abnormalities of right heart venous return (expiratory diastolic reversal) are more predictive but cannot be obtained in one third of patients.
Echocardiography: Most useful imaging technique for the evaluation of acute pericarditis and is critical for patients if tamponade is suspected. Small pericardial effusions, undetectable by routine examinations, may be detected, providing support for the diagnosis of pericardial disease. Typically >1 cm of effusion is required for safe performance of pericardiocentesis. Dopplerderived flow-velocity measures of mitral and tricuspid flow may assist in diagnosing tamponade, but it is ultimately a clinical diagnosis based on inspiratory decline in systolic arterial pressure exceeding 10 mm Hg (pulsus paradoxus), which can be also seen in COPD and pulmonary embolism. The absence of any chamber collapse on echocardiography has a high negative predictive value for tamponade (92%), although the positive predictive value is low (58%). Abnormalities of right heart venous return (expiratory diastolic reversal) are more predictive but cannot be obtained in one third of patients.
 Electrocardiography: ECG abnormalities may support a diagnosis or suggest alternative diagnoses, such as myocardial infarction or early repolarization abnormalities. There are several important distinguishing characters of pericarditis ECGs from that of STEMI patients. There is upward concavity of ST elevations (compared with downward for ischemic) that rarely exceeds 5 mm with PR-segment depression (not in aVR) that is not present with repolarization abnormalities. T-wave inversions may persist with tuberculuous, uremic, or neoplastic pericarditis. Electrical alternans suggests large effusion.
Electrocardiography: ECG abnormalities may support a diagnosis or suggest alternative diagnoses, such as myocardial infarction or early repolarization abnormalities. There are several important distinguishing characters of pericarditis ECGs from that of STEMI patients. There is upward concavity of ST elevations (compared with downward for ischemic) that rarely exceeds 5 mm with PR-segment depression (not in aVR) that is not present with repolarization abnormalities. T-wave inversions may persist with tuberculuous, uremic, or neoplastic pericarditis. Electrical alternans suggests large effusion.
 Chest x-ray: Generally normal but may detect specific abnormalities, like increased cardiac silhouette with effusions (water-bottle heart), pleural effusion, or evidence of underlying etiology (TB, fungal disease, pneumonia, neoplasm).
Chest x-ray: Generally normal but may detect specific abnormalities, like increased cardiac silhouette with effusions (water-bottle heart), pleural effusion, or evidence of underlying etiology (TB, fungal disease, pneumonia, neoplasm).
 Chest CT and MRI detect effusions with high sensitivity and specificity and may provide useful information pertinent to performing pericardiocentesis (hematocrit of effusion, loculations, pericardial thickening). Often, concern of tamponade makes echocardiography the imaging modality of choice for clinically tenuous patients due to its mobility.
Chest CT and MRI detect effusions with high sensitivity and specificity and may provide useful information pertinent to performing pericardiocentesis (hematocrit of effusion, loculations, pericardial thickening). Often, concern of tamponade makes echocardiography the imaging modality of choice for clinically tenuous patients due to its mobility.
 Tuberculin skin test or interferon-gamma release assay: Evaluation to rule out TB is recommended for all patients. Additional diagnostic testing for TB, like AFB cultures, should be performed on patients at increased risk on the basis of epidemiologic and clinical factors.
Tuberculin skin test or interferon-gamma release assay: Evaluation to rule out TB is recommended for all patients. Additional diagnostic testing for TB, like AFB cultures, should be performed on patients at increased risk on the basis of epidemiologic and clinical factors.
 Cultures: Cultures of blood and other potentially infected specimens should be submitted for patients with significant fever, signs of sepsis, or systemic or local infection.
Cultures: Cultures of blood and other potentially infected specimens should be submitted for patients with significant fever, signs of sepsis, or systemic or local infection.
 Histology: Pericardiocentesis (and occasionally pericardial biopsy) should be performed for patients with clinically significant tamponade or persistent effusions. Pericardiocentesis is recommended for patients in whom pyogenic, tuberculous, or malignant pericardial disease is suspected. As most forms of pericarditis are viral in etiology, the diagnostic yield of routine pericardiocentesis has a low diagnostic yield (7%).
Histology: Pericardiocentesis (and occasionally pericardial biopsy) should be performed for patients with clinically significant tamponade or persistent effusions. Pericardiocentesis is recommended for patients in whom pyogenic, tuberculous, or malignant pericardial disease is suspected. As most forms of pericarditis are viral in etiology, the diagnostic yield of routine pericardiocentesis has a low diagnostic yield (7%).
 Recommended tests for pericardial fluid include
Recommended tests for pericardial fluid include
 Histopathologic and cytologic examination of tissue and fluid.
Histopathologic and cytologic examination of tissue and fluid.
 Bacterial and mycobacterial stains and culture.
Bacterial and mycobacterial stains and culture.
 Triglyceride concentration for chylous fluid.
Triglyceride concentration for chylous fluid.
 Adenosine deaminase and M. tuberculosis PCR, if tuberculous pericarditis is suspected.
Adenosine deaminase and M. tuberculosis PCR, if tuberculous pericarditis is suspected.
 Other specific diagnostic tests, like fungal cultures or PCR, are performed based on clinical suspicion.
Other specific diagnostic tests, like fungal cultures or PCR, are performed based on clinical suspicion.
 Core laboratory: CBC, electrolytes, tests of renal function and thyroid function, and plasma troponin concentration. ANA titers, anti-dsDNA, and serum complement are recommended for patients when an autoimmune cause is suspected. Note: protein, glucose, LDH, RBC count, and WBC count cannot distinguish exudative from transudative effusions and are usually noncontributory in establishing a diagnosis.
Core laboratory: CBC, electrolytes, tests of renal function and thyroid function, and plasma troponin concentration. ANA titers, anti-dsDNA, and serum complement are recommended for patients when an autoimmune cause is suspected. Note: protein, glucose, LDH, RBC count, and WBC count cannot distinguish exudative from transudative effusions and are usually noncontributory in establishing a diagnosis.
 Serology: HIV should be considered. Pericardial disease is relatively common in HIV-infected patients. Furthermore, HIV infection predisposes patients to mycobacterial infections. Viral diagnostic testing, including serology, has low diagnostic yield and is not routinely recommended.
Serology: HIV should be considered. Pericardial disease is relatively common in HIV-infected patients. Furthermore, HIV infection predisposes patients to mycobacterial infections. Viral diagnostic testing, including serology, has low diagnostic yield and is not routinely recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


