EMBRYOLOGY
The pancreas appears in the fourth week of fetal life from the caudal part of the foregut as dorsal and ventral pancreatic buds. Both anlagen rotate to the right and fuse near the point of origin of the ventral pancreas. Later, as the duodenum rotates, the pancreas shifts to the left. In the adult, only the caudal portion of the head and the uncinate process are derived from the ventral pancreas. The cranial part of the head and all of the body and tail are derived from the dorsal pancreas. Most of the dorsal pancreatic duct joins with the duct of the ventral pancreas to form the main pancreatic duct (duct of Wirsung); a small part persists as the accessory duct (duct of Santorini). In 5%-10% of people, the ventral and dorsal pancreatic ducts do not fuse, and most regions of the pancreas drain through the duct of Santorini and the orifice of the minor papilla. In this case, only the small ventral pancreas drains with the common bile duct through the papilla of Vater.
ANATOMY
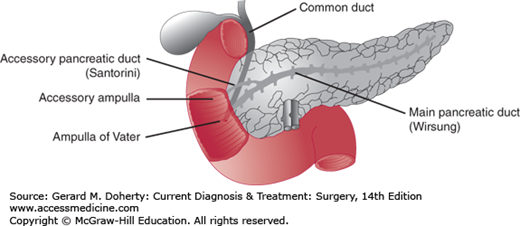
The pancreas is a thin elliptic organ that lies within the retroperitoneum in the upper abdomen (Figures 26–1 and 26–2). In the adult, it is 12-15 cm long and weighs 70-100 g. The gland can be divided into three portions—head, body, and tail. The head of the pancreas is intimately adherent to the medial portion of the duodenum and lies in front of the inferior vena cava and superior mesenteric vessels. A small tongue of tissue called the uncinate process lies behind the superior mesenteric vessels as they emerge from the retroperitoneum. Anteriorly, the stomach and the first portion of the duodenum lie partly in front of the pancreas. The common bile duct passes through a posterior groove in the head of the pancreas adjacent to the duodenum. The body of the pancreas is in contact posteriorly with the aorta, the left crus of the diaphragm, the left adrenal gland, and the left kidney. The tail of the pancreas lies in the hilum of the spleen. The main pancreatic duct (the duct of Wirsung) courses along the gland from the tail to the head and joins the common bile duct just before entering the duodenum at the ampulla of Vater. The accessory pancreatic duct (the duct of Santorini) enters the duodenum 2-2.5 cm proximal to the ampulla of Vater (Figure 26–1).
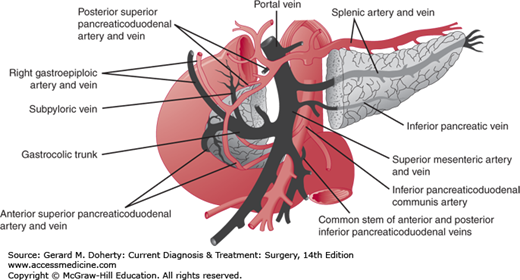
The blood supply of the pancreas is derived from branches of the celiac and superior mesenteric arteries (Figure 26–2). The superior pancreaticoduodenal artery arises from the gastroduodenal artery, runs parallel to the duodenum, and eventually meets the inferior pancreaticoduodenal artery, a branch of the superior mesenteric artery, to form an arcade. The splenic artery provides tributaries that supply the body and tail of the pancreas. The main branches are termed the dorsal pancreatic, pancreatica magna, and caudal pancreatic arteries. The venous supply of the gland parallels the arterial supply. Lymphatic drainage is into the peripancreatic nodes located along the veins.
The innervation of the pancreas is derived from the vagal and splanchnic nerves. The efferent fibers pass through the celiac plexus from the celiac branch of the right vagal nerve to terminate in ganglia located in the interlobular septa of the pancreas. Postganglionic fibers from these synapses innervate the acini, the islets, and the ducts. The visceral afferent fibers from the pancreas also travel in the vagal and splanchnic nerves, but those that mediate pain are confined to the latter. Sympathetic fibers to the pancreas pass from the splanchnic nerves through the celiac plexus and innervate the pancreatic vasculature.
PHYSIOLOGY
The external secretion of the pancreas consists of a clear, alkaline (pH 7.0-8.3) solution of 1-2 L/d containing digestive enzymes. Secretion is stimulated by the hormones secretin and cholecystokinin (CCK) and by parasympathetic vagal discharge. Secretin and CCK are synthesized, stored, and released from duodenal mucosal cells in response to specific stimuli. Acid in the lumen of the duodenum causes the release of secretin, and luminal digestion products of fat and protein cause the release of CCK.
The water and electrolyte secretion is formed by the centroacinar and intercalated duct cells principally in response to secretin stimulation. The secretion is modified by exchange processes and active secretion in the ductal collecting system. The cations sodium and potassium are present in the same concentrations as in plasma. The anions bicarbonate and chloride vary in concentration according to the rate of secretion: with increasing rate of secretion, the bicarbonate concentration increases and chloride concentration falls, so that the sum of the two is the same throughout the secretory range. Pancreatic juice helps neutralize gastric acid in the duodenum and adjusts luminal pH to the level that gives optimal activity of pancreatic enzymes.
Pancreatic enzymes are synthesized, stored (as zymogen granules), and released by the acinar cells of the gland, principally in response to CCK and vagal stimulation. Pancreatic enzymes are proteolytic, lipolytic, and amylolytic. Lipase and amylase are stored and secreted in active forms. The proteolytic enzymes are secreted as inactive precursors and are activated by the duodenal enzyme enterokinase. Other enzymes secreted by the pancreas include ribonucleases and phospholipase A. Phospholipase A is secreted as an inactive proenzyme activated in the duodenum by trypsin. It catalyzes the conversion of biliary lecithin to lysolecithin.
Turnover of protein in the pancreas exceeds that of any other organ in the body. Intravenously injected amino acids are incorporated into enzyme protein and may appear in the pancreatic juice within 1 hour. Three mechanisms prevent autodigestion of the pancreas by its proteolytic enzymes: (1) the enzymes are stored in acinar cells as zymogen granules, where they are separated from other cell proteins; (2) the enzymes are secreted in an inactive form; and (3) inhibitors of proteolytic enzymes are present in pancreatic juice and pancreatic tissue.
The function of the endocrine pancreas is to facilitate storage of foodstuffs by release of insulin after a meal and to provide a mechanism for their mobilization by release of glucagon during periods of fasting. Insulin and glucagon, as well as pancreatic polypeptide and somatostatin, are produced by the islets of Langerhans.
Insulin, a polypeptide (MW 5734) consisting of 51 amino acid residues, is formed in the beta cells of the pancreas via the precursor proinsulin. Insulin secretion is stimulated by rising or high serum concentrations of metabolic substrates such as glucose, amino acids, and perhaps short-chain fatty acids. The major normal stimulus for insulin release appears to be glucose. The release and synthesis of insulin are stimulated by activation of specific glucoreceptors located on the surface membrane of the beta cell. Insulin release is also stimulated by calcium, glucagon, secretin, CCK, vasoactive intestinal polypeptide (VIP), and gastrin, all of which sensitize the receptors on the beta cell to glucose. Epinephrine, tolbutamide, and chlorpropamide release insulin by acting on the adenylyl cyclase system.
Glucagon, a polypeptide (MW 3485) consisting of 29 amino acid residues, is formed in the α cells of the pancreas. The release of glucagon is stimulated by a low blood glucose concentration, amino acids, catecholamines, sympathetic nervous discharge, and CCK. It is suppressed by hyperglycemia and insulin.
The principal functions of insulin are to stimulate anabolic reactions involving carbohydrates, fats, proteins, and nucleic acids. Insulin decreases glycogenolysis, lipolysis, proteolysis, gluconeogenesis, ureagenesis, and ketogenesis. Glucagon stimulates glycogenolysis from the liver and proteolysis and lipolysis in adipose tissue as well as in the liver. With the increase in lipolysis, there is an increase in ketogenesis and gluconeogenesis. Glucagon increases cAMP in the liver, heart, skeletal muscle, and adipose tissue. The short-term regulation of gluconeogenesis depends on the balance between insulin and glucagon. Studies on insulin and glucagon suggest that the hormones exert their effects via receptors on the cell membrane. Before entering the systemic circulation, blood draining from the islets of Langerhans perfuses the pancreatic acini, and this exposure to high levels of hormones is thought to influence acinar function.
ANNULAR PANCREAS
Annular pancreas is a rare congenital condition in which a ring of pancreatic tissue from the head of the pancreas surrounds the descending duodenum. The abnormality usually presents in infancy as duodenal obstruction with postprandial vomiting. There is bile in the vomitus if the constriction is distal to the entrance of the common bile duct. X-rays show a dilated stomach and proximal duodenum (double bubble sign) and little or no air in the rest of the small bowel.
After correction of fluid and electrolyte imbalance, the obstructed segment should be bypassed by a duodenojejunostomy or other similar procedure. No attempt should be made to resect the obstructing pancreas, because a pancreatic fistula or acute pancreatitis often develops postoperatively.
Occasionally, annular pancreas will present in adult life with similar symptoms.
PANCREATITIS
Pancreatitis is a common nonbacterial inflammatory disease caused by activation, interstitial liberation, and autodigestion of the pancreas by its own enzymes. The process may or may not be accompanied by permanent morphologic and functional changes in the gland. Much is known about the causes of pancreatitis, but despite the accumulation of much experimental data, understanding of the pathogenesis of this disorder is still incomplete.
In acute pancreatitis, there is sudden upper abdominal pain, nausea and vomiting, and elevated serum amylase. Chronic pancreatitis is characterized by chronic pain, pancreatic calcification on x-ray, and exocrine (steatorrhea) or endocrine (diabetes mellitus) insufficiency. Attacks of acute pancreatitis often occur in patients with chronic pancreatitis. Acute relapsing pancreatitis is defined as multiple attacks of pancreatitis without permanent pancreatic scarring, a picture most often associated with biliary pancreatitis. The unsatisfactory term chronic relapsing pancreatitis, denoting recurrent acute attacks superimposed on chronic pancreatitis, will not be used in this chapter. Alcoholic pancreatitis often behaves in this way. The term subacute pancreatitis has also been used by some to denote the minor acute attacks that typically appear late in alcoholic pancreatitis.
Most cases of pancreatitis are caused by gallstone disease or alcoholism; a few result from hypercalcemia, trauma, hyperlipidemia, and genetic predisposition; and the remainder are idiopathic. Important differences exist in the manifestations and natural history of the disease as produced by these various factors.
About 40% of cases of pancreatitis are associated with gallstone disease, which, if untreated, usually gives rise to additional acute attacks. For unknown reasons, even repeated attacks of acute biliary pancreatitis seldom produce chronic pancreatitis. Eradication of the biliary disease nearly always prevents recurrent pancreatitis. The etiologic mechanism most likely consists of transient obstruction of the ampulla of Vater and pancreatic duct by a gallstone. Choledocholithiasis is found in only 25% of cases, but because over 90% of patients excrete a gallstone in feces passed within 10 days after an acute attack, it is assumed that most attacks are caused by a gallstone or biliary sludge traversing the common duct and ampulla of Vater. Other possible steps in pathogenesis initiated by passage of the gallstone are discussed below.
In the United States, alcoholism accounts for about 40% of cases of pancreatitis. Characteristically, the patients have been heavy users of hard liquor or wine; the condition is relatively infrequent in countries where beer is the most popular alcoholic beverage. Most commonly, 6 years or more of alcoholic excess precede the initial attack of pancreatitis, and even with the first clinical manifestations, signs of chronic pancreatitis can be detected if the gland is examined microscopically. Thus, alcoholic pancreatitis is often considered to be synonymous with chronic pancreatitis no matter what the clinical findings.
Acute administration of alcohol stimulates pancreatic secretion and induces spasm in the sphincter of Oddi. This has been compared to experiments that produce acute pancreatitis by combining partial ductal obstruction and secretory stimulation. If the patient can be persuaded to stop drinking, acute attacks may be prevented, but parenchymal damage continues to occur owing to persistent ductal obstruction and fibrosis.
Hyperparathyroidism and other disorders accompanied by hypercalcemia are occasionally complicated by acute pancreatitis. With time, chronic pancreatitis and ductal calculi appear. The increased calcium concentrations in pancreatic juice that result from hypercalcemia may prematurely activate proteases. They may also facilitate precipitation of calculi in the ducts.
In some patients—especially alcoholics—hyperlipidemia appears transiently during an acute attack of pancreatitis; in others with primary hyperlipidemia (especially those associated with elevated chylomicrons and very low density lipoproteins), pancreatitis seems to be a direct consequence of the metabolic abnormality. Hyperlipidemia during an acute attack of pancreatitis is usually associated with normal serum amylase levels, because the lipid interferes with the chemical determination for amylase; urinary output of amylase may still be high. One should inspect the serum of every patient with acute abdominal pain, because if it is lactescent, pancreatitis will almost always be the correct diagnosis. If a primary lipid abnormality is present, dietary control reduces the chances of additional attacks of pancreatitis as well as other complications.
In this condition, attacks of abdominal pain usually begin in childhood. Some affected families also have aminoaciduria, but this is not a universal finding. Diabetes mellitus and steatorrhea are uncommon. Chronic calcific pancreatitis develops eventually in most patients, and many patients become candidates for operation for chronic pain. Pancreatic carcinoma is more frequent in patients with familial pancreatitis.
In certain populations where dietary protein intake is markedly deficient, the incidence of chronic pancreatitis is high. The reason for this association is obscure, especially in view of the observation that pancreatitis afflicts alcoholics with higher dietary protein and fat intake than those who consume less protein and fat.
Most cases of postoperative pancreatitis follow common bile duct exploration, especially if sphincterotomy was performed. Two practices, now largely abandoned, were often responsible: (1) use of a common duct T tube with a long arm passing through the sphincter of Oddi and (2) dilation of the sphincter to 5-7 mm during common duct exploration. Operations on the pancreas, including pancreatic biopsy, are another cause. A few cases follow gastric surgery or even operations remote from the pancreas. Pancreatitis is particularly common after cardiac surgery with cardiopulmonary bypass, where the risk factors are preoperative renal failure, valve surgery, postoperative hypotension, and (particularly) the perioperative administration of calcium chloride (> 800 mg calcium chloride per square meter of body surface area). Pancreatitis may also complicate endoscopic retrograde pancreatography or endoscopic sphincterotomy.
Rarely, pancreatitis follows Billroth II gastrectomy, owing to acute obstruction of the afferent loop and reflux of duodenal secretions under high pressure into the pancreatic ducts. The condition has been recreated experimentally in dogs (Pfeffer loop preparation).
Drugs are probably responsible for more cases of acute pancreatitis than is generally suspected. The most commonly incriminated drugs are corticosteroids, estrogen-containing contraceptives, azathioprine, thiazide diuretics, and tetracyclines. Pancreatitis associated with use of estrogens is usually the result of drug-induced hypertriglyceridemia. The mechanisms involved in the case of other drugs are unknown.
Chronic partial obstruction of the pancreatic duct may be congenital or may follow healing after injury or inflammation. Over time, the parenchyma drained by the obstructed duct is replaced by fibrous tissue, and chronic pancreatitis develops. Sometimes there are episodes of acute pancreatitis as well.
Pancreas divisum may predispose to a kind of obstructive pancreatitis. If this anomaly is present and further narrowing of the opening of the minor papilla occurs (eg, by an inflammatory process), the orifice may be inadequate to handle the flow of pancreatic juice. The diagnosis of pancreas divisum may be made by endoscopic retrograde cholangio-pancreatography (ERCP). If a patient with the anomaly is found to have documented episodes of acute pancreatitis and no other cause is found, it is reasonable to assume that the anomaly is the cause.
Surgical sphincteroplasty of the minor papilla or the insertion of a stent has been proposed as treatment, but results have been suboptimal. This may be due to the presence of irreversible parenchymal changes and the persistence of chronic inflammation. In patients with obvious changes of chronic pancreatitis, surgical treatment should consist of pancreatic resection or drainage.
In about 15% of patients, representing the third largest group after biliary and alcoholic pancreatitis, there is no identifiable cause of the condition. If investigated in greater than usual detail (eg, duodenal drainage examination for cholesterol crystals), many of these patients will be found to have gallstones or biliary sludge undetectable by ultrasound scans. Recent data have linked mutations of the cystic fibrosis gene to idiopathic pancreatitis.
Viral infections and scorpion stings may cause pancreatitis.
The concept that pancreatitis is due to enzymatic digestion of the gland is supported by the finding of proteolytic enzymes in ascitic fluid and increased amounts of phospholipase A and lysolecithins in pancreatic tissue from patients with acute pancreatitis. Experimentally, pancreatitis can be created readily if activated enzymes are injected into the pancreatic ducts under pressure. Trypsin has not been found in excessive amounts in pancreatic tissue from affected humans, possibly because of inactivation by trypsin inhibitors. Nevertheless, although the available evidence is inconclusive, the autodigestion theory is almost universally accepted. Other proposed factors are vascular insufficiency, lymphatic congestion, and activation of the kallikrein-kinin system.
For many years, trypsin and other proteases were held to be the principal injurious agents, but recent evidence has emphasized phospholipase A, lipase, and elastase as perhaps of greater importance. Trypsin ordinarily does not attack living cells, and even when trypsin is forced into the interstitial spaces, the resulting pancreatitis does not include coagulation necrosis, which is so prominent in human pancreatitis.
Phospholipase A, in the presence of small amounts of bile salts, attacks free phospholipids (eg, lecithin) and those bound in cellular membranes to produce extremely potent lyso-compounds. Lysolecithin, which would result from the action of phospholipase A on biliary lecithin, or phospholipase A itself, plus bile salts, is capable of producing severe necrotizing pancreatitis. Trypsin is important in this scheme, because small amounts are needed to activate phospholipase A from its inactive precursor.
Elastase, which is both elastolytic and proteolytic, is secreted in an inactive form. Because it can digest the walls of blood vessels, elastase has been thought to be important in the pathogenesis of hemorrhagic pancreatitis.
If autodigestion is the final common pathway in pancreatitis, earlier steps must account for the presence of active enzymes and their reaction products in the ducts and their escape into the interstitium. The following are the most popular theories that attempt to link the known etiologic factors with autodigestion.
In animals, ligation of the pancreatic duct generally produces mild edema of the pancreas that resolves within a week. Thereafter, atrophy of the secretory apparatus occurs. On the other hand, partial or intermittent ductal obstruction, which more closely mimics what seems to happen in humans, can produce frank pancreatitis if the gland is simultaneously stimulated to secrete. The major shortcoming of these experiments has been the difficulty encountered in attempting to cause severe pancreatitis in this way. However, since the human pancreas manufactures ten times as much phospholipase A as does the dog or rat pancreas, the consequences of obstruction in humans conceivably could be more serious.
Flow between the biliary and pancreatic ducts requires a common channel connecting these two systems with the duodenum. Although these ducts converge in 90% of humans, only 10% have a common channel long enough to permit biliary-pancreatic reflux if the ampulla contained a gallstone. Experimentally, pancreatitis produced by pancreatic duct obstruction alone is similar in severity to pancreatitis following obstruction of a common channel, so biliary reflux is discounted as an etiologic factor in this disease.
The above theories do not explain activation of pancreatic enzymes, a process that normally takes place through the action of enterokinase in the duodenum. In experimental animals, if the segment of duodenum into which the pancreatic duct empties is surgically converted to a closed loop, reflux of duodenal juice initiates severe pancreatitis (Pfeffer loop). Pancreatitis associated with acute afferent loop obstruction after Billroth II gastrectomy is probably the result of similar factors. Other than in this specific example, there is no direct evidence for duodenal reflux in the pathogenesis of pancreatitis in humans.
Just as the gastric mucosa must serve as a barrier to maintain high concentrations of acid, so must the epithelium of the pancreatic duct prevent diffusion of luminal enzymes into the pancreatic parenchyma. Experiments in cats have shown that the barrier function of the pancreatic duct is vulnerable to several injurious agents, including alcohol and bile acids. Furthermore, the effects of alcohol can occur even after oral ingestion, because alcohol is secreted in the pancreatic juice. Injury to the barrier renders the duct permeable to molecules as large as MW 20,000, and enzymes from the lumen may be able to enter the gland and produce pancreatitis.
Some studies have shown that a very early event in several forms of experimental pancreatitis, including that due to pancreatic duct obstruction, consists of zymogen activation within acinar cells by lysosomal hydrolases (eg, cathepsin B). This may represent the long-sought unifying explanation. Other factors must be postulated, however, to account for the variations in severity of the disease. In biliary pancreatitis, transient obstruction of the ampulla of Vater by a gallstone is most likely the first event. Alcoholic pancreatitis probably has several causes, including partial ductal obstruction, secretory stimulation, acute effects on the ductal barrier, and toxic actions of alcohol on parenchymal cells.
Severe acute pancreatitis may be complicated by multiple organ failure, principally respiratory insufficiency (acute respiratory distress syndrome), myocardial depression, renal insufficiency, and gastric stress ulceration. The pathogenesis of these complications is similar in many respects to that of multiple organ failure in sepsis, and in fact, sepsis due to pancreatic abscess formation is a contributing factor in some of the most severe cases of acute pancreatitis. During acute pancreatitis, pancreatic proteases, bacterial endotoxins, and other active agents are liberated into the systemic circulation. The endotoxin probably originates from bacteria that translocate through an abnormally permeable intestinal mucosa. Within the circulation, the proteases and the endotoxin activate the complement system (especially C5) and kinins. Complement activation leads to granulocyte aggregation and accumulation of aggregates in the pulmonary capillaries. The granulocytes release neutrophil elastase, superoxide anion, hydrogen peroxide, and hydroxide radicals, which in concert with bradykinin exert local toxic effects on the pulmonary epithelium that result in increased permeability. Arachidonate metabolites (eg, PGE2, PGI2, leukotriene B4) may also be involved in some way. Analogous events are thought to occur in other organs.
ESSENTIALS OF DIAGNOSIS
Abrupt onset of epigastric pain, frequently with back pain
Nausea and vomiting
Elevated serum or urinary amylase
Cholelithiasis or alcoholism (many patients)
While edematous and hemorrhagic pancreatitis are manifestations of the same pathologic processes and the general principles of treatment are the same, hemorrhagic pancreatitis has more complications and a higher death rate. In edematous pancreatitis, the glandular tissue and surrounding retroperitoneal structures are engorged with interstitial fluid, and the pancreas is infiltrated with inflammatory cells that surround small foci of parenchymal necrosis. Hemorrhagic pancreatitis is characterized by bleeding into the parenchyma and surrounding retroperitoneal structures and extensive pancreatic necrosis. In both forms, the peritoneal surfaces may be studded with small calcifications representing areas of fat necrosis.
The acute attack frequently begins with severe epigastric pain that radiates through to the back. The pain is unrelenting and usually associated with vomiting and retching. In severe cases, the patient may collapse from shock.
Depending on the severity of the disease, there may be profound dehydration, tachycardia, and postural hypotension. Myocardial function is depressed in severe pancreatitis, presumably because of circulating factors that affect cardiac performance. Examination of the abdomen reveals decreased or absent bowel sounds and tenderness that may be generalized but more often is localized to the epigastrium. Temperature is usually normal or slightly elevated in uncomplicated pancreatitis. Clinical evidence of pleural effusion may be present, especially on the left. If an abdominal mass is found, it probably represents a swollen pancreas (phlegmon) or, later in the illness, a pseudocyst or abscess. In 1%-2% of patients, bluish discoloration is present in the flank (Grey Turner sign) or periumbilical area (Cullen sign), indicating hemorrhagic pancreatitis with dissection of blood retroperitoneally into these areas.
The hematocrit may be elevated as a consequence of dehydration or low as a result of abdominal blood loss in hemorrhagic pancreatitis. There is usually a moderate leukocytosis, but total white blood cell counts over 12,000/mL are unusual in the absence of suppurative complications. Liver function studies are usually normal, but there may be a mild elevation of the serum bilirubin concentration (usually < 2 mg/dL).
The serum amylase concentration rises to more than three times normal within 6 hours after the onset of an acute episode and generally remains elevated for several days. Values in excess of 1000 IU/dL occur early in the attack in 95% of patients with biliary pancreatitis and 85% of patients with acute alcoholic pancreatitis. Those with the most severe disease are more apt to have amylase levels below 1000 IU/dL.
Elevated serum lipase is detectable early and for several days after the acute attack. Since the lipase level tends to be higher in alcoholic pancreatitis and the amylase level higher in gallstone pancreatitis, the lipase/amylase ratio has been suggested as a means to help distinguishing the two.
Elevated amylase levels may occur in other acute abdominal conditions, such as gangrenous cholecystitis, small bowel obstruction, mesenteric infarction, and perforated ulcer, though levels rarely exceed 500 IU/dL. Episodes of acute pancreatitis may occur without rises in serum amylase; this is the rule if hyperlipidemia is present. Furthermore, high levels may return to normal before blood is drawn.
The methods most commonly used for measuring amylase in the serum detect pancreatic amylase, salivary amylase, and macroamylase. However, hyperamylasemia is sometimes present in patients with abdominal pain when the elevated amylase levels consist entirely of salivary amylase or macroamylase and the pancreas is not inflamed.
In severe pancreatitis, the serum calcium concentration may fall as a result of calcium being complexed with fatty acids (liberated from retroperitoneal fat by lipase) and impaired reabsorption from bone owing to the action of calcitonin (liberated by high levels of glucagon). Relative hypoparathyroidism and hypoalbuminemia have also been implicated.
In about two-thirds of cases, a plain abdominal film is abnormal. The most frequent finding is isolated dilation of a segment of gut (sentinel loop) consisting of jejunum, transverse colon, or duodenum adjacent to the pancreas. Gas distending the right colon that abruptly stops in the mid or left transverse colon (colon cutoff sign) is due to colonic spasm adjacent to the pancreatic inflammation. Both of these findings are relatively nonspecific. Glandular calcification may be evident, signifying chronic pancreatitis. An upper gastrointestinal series may show a widened duodenal loop, swollen ampulla of Vater, and, occasionally, evidence of gastric irritability. Chest films may reveal pleural effusion on the left side. Occasionally, radiopaque gallstones will be apparent on plain x-rays.
Ultrasound study may demonstrate gallstones early in the attack and may be used as a baseline for sequential examinations of the pancreas.
A CT scan of the pancreas using intravenous contrast media should be obtained for one of three reasons: (1) diagnostic uncertainty, (2) confirmation/evaluation of severity based upon other markers or clinical suspicion, or (3) evaluation in the setting of clinical deterioration or failure to respond to therapy. The radiologic findings may be consistent with any of the following: relatively normal appearing pancreas, pancreatic phlegmon, pancreatic phlegmon with extension of the inflammatory process to adjacent extrapancreatic spaces, pancreatic necrosis, or pancreatic pseudocyst, or abscess formation.
Several weeks after the pancreatitis has subsided, ERCP may be of value in patients with a tentative diagnosis of idiopathic pancreatitis (ie, those who have no history of alcoholism and no evidence of gallstones on ultrasound and oral cholecystogram). This examination demonstrates gallstones or changes of chronic pancreatitis in about 40% of such patients.
To some extent, acute pancreatitis is a diagnosis of exclusion, for other acute upper abdominal conditions such as acute cholecystitis, penetrating or perforated duodenal ulcer, high small bowel obstruction, acute appendicitis, and mesenteric infarction must always be seriously considered. In most cases, the distinction is possible on the basis of the clinical picture, laboratory findings, and CT scans. The critical point is that the diseases with which acute pancreatitis is most likely to be confused are often lethal if not treated surgically.
Chronic hyperamylasemia occurs rarely without any relation to pancreatic disease. Some cases are associated with renal failure, chronic sialadenitis, salivary tumors, ovarian tumors, or liver disease, but often there is no explanation. Analysis of serum amylase isoenzymes is the only way to determine whether the amylase originates from salivary glands or pancreas. Macroamylasemia is a chronic hyperamylasemia in which normal amylase (usually salivary) is bound to a large serum glycoprotein or immunoglobulin molecule and is therefore not excreted into urine. The diagnosis rests on the combination of hyperamylasemia and low urinary amylase. Macroamylasemia has been found in patients with other diseases such as malabsorption, alcoholism, and cancer. Many patients have abdominal pain, but the relationship of the pain and the macroamylasemia is uncertain.
The principal complications of acute pancreatitis are abscess and pseudocyst formation. These are discussed in separate sections. Gastrointestinal bleeding may occur from adjacent inflamed stomach or duodenum, ruptured pseudocyst, or peptic ulcer. Intraperitoneal bleeding may occur spontaneously from the celiac or splenic artery or from the spleen following acute splenic vein thrombosis. Involvement of the transverse colon or duodenum by the inflammatory process may result in partial obstruction, hemorrhage, necrosis, or fistula formation.
Early identification of patients at greatest risk of complications allows them to be managed more aggressively, which appears to decrease the mortality rate. The criteria of severity that have been found to be reliable are based either on the systemic manifestations of the disease as reflected in the clinical and laboratory findings or on the local changes in the pancreas as reflected by the findings on CT scan. Ranson used the former approach to develop the staging criteria listed in Table 26–1. Just the single finding of fluid sequestration (ie, fluid administered minus urine output) exceeding 2 L/d for more than 2 days is a reasonably accurate dividing line between severe (life-threatening) and mild-to-moderate disease. The local changes in the pancreas as shown on CT scans may be even more revealing. The presence of any of the following indicates a high risk of local infection in the pancreatic bed: involvement of extrapancreatic spaces in the inflammatory process, pancreatic necrosis (areas in the pancreas that do not enhance with intravenous contrast media), and early signs of abscess formation (eg, gas bubbles in the tissue).
Criteria Present Initially Age > 55 years White blood cell count > 16,000/μL Blood glucose > 200 mg/dL SERUM LDH > 350 IU/L AST (SGOT) > 250 IU/dL Criteria Developing During First 24 Hours Hematocrit fall > 10% BUN rise > 8 mg/dL Serum Ca2+ < 8 mg/dL Arterial PO2 < 60 mm Hg Base deficit > 4 meq/L Estimated fluid sequestration > 600 mL |
The goals of medical therapy are reduction of pancreatic secretory stimuli and correction of fluid and electrolyte derangements.
Oral intake is withheld. A nasogastric tube is often inserted to aspirate gastric secretions, although the latter has no specific therapeutic effect. Oral feeding should be resumed only after the patient appears much improved, appetite has returned, and serum amylase levels have dropped to normal. Premature resumption of eating may result in exacerbation of disease.
Patients with acute pancreatitis sequester fluid in the retroperitoneum and bowel, and large volumes of intravenous fluids are necessary to maintain circulating blood volume and renal function. In severe hemorrhagic pancreatitis, blood transfusions may also be required. The adequacy of fluid replacement is the single most important aspect of medical therapy. In fact, undertreatment with fluids may actually contribute to the progression of pancreatitis. Fluid replacement may be judged most accurately by monitoring the volume and concentration of the urine.
Antibiotics are not useful in mild cases of acute pancreatitis. However, some studies have shown benefit of antibiotics that penetrate pancreatic tissue for patients with severe pancreatitis. Imipenem is the most commonly used antibiotic, though its use is not universally supported even in patients with severe disease. Antibiotics should also be used for treatment of specific operative complications.
In severe attacks of acute pancreatitis, hypocalcemia may require parenteral calcium replacement in amounts determined by serial calcium measurements. Recognition of hypocalcemia is important because it may produce cardiac dysrhythmias. Hypomagnesemia is also common, especially in alcoholics, and magnesium should also be replaced as indicated by serum levels.