Definition and Use
 Smears of patient specimens are stained and examined for the presence of mycobacteria. They may provide early evidence of TB or other mycobacterial diseases. Certain dyes bind to the thick, mycolic acid–rich cell walls of mycobacteria. The lipids of the cell wall make the cells resistant to decolorization with acid–alcohol solutions. AFB staining should be undertaken for most specimens that are submitted for mycobacterial culture.
Smears of patient specimens are stained and examined for the presence of mycobacteria. They may provide early evidence of TB or other mycobacterial diseases. Certain dyes bind to the thick, mycolic acid–rich cell walls of mycobacteria. The lipids of the cell wall make the cells resistant to decolorization with acid–alcohol solutions. AFB staining should be undertaken for most specimens that are submitted for mycobacterial culture.
 Methods:
Methods:
 There are two types of AFB stains: chromogenic (carbol-fuchsin stains [hot: Ziehl-Neelsen; cold: Kinyoun]) and fluorogenic (auramine O + rhodamine). After staining, the smear is destained with an acid–alcohol solution, typically HCl in ethanol. Mycobacteria retain the stain.
There are two types of AFB stains: chromogenic (carbol-fuchsin stains [hot: Ziehl-Neelsen; cold: Kinyoun]) and fluorogenic (auramine O + rhodamine). After staining, the smear is destained with an acid–alcohol solution, typically HCl in ethanol. Mycobacteria retain the stain.
 In chromogenic methods, slides are examined using a 100× oil immersion objective with light microscopy. Nonmycobacterial cells are counterstained with methylene blue. Mycobacteria appear red, whereas other bacteria and background are stained blue.
In chromogenic methods, slides are examined using a 100× oil immersion objective with light microscopy. Nonmycobacterial cells are counterstained with methylene blue. Mycobacteria appear red, whereas other bacteria and background are stained blue.
 In fluorogenic methods, auramine-stained slides are examined by fluorescence microscopy using a 25× or 40× objective. Mycobacteria are yellow-orange against a dark background. The improved signal-to-noise of auramine fluorochrome staining, allowing scanning with lower power objective, results in examination of a greater area of the slide at a given time, and therefore greater sensitivity. Any detected organisms should be confirmed by examination for typical morphology using the 100× objective. Some laboratories confirm positive fluorochrome smears with a carbol-fuchsin–based stain.
In fluorogenic methods, auramine-stained slides are examined by fluorescence microscopy using a 25× or 40× objective. Mycobacteria are yellow-orange against a dark background. The improved signal-to-noise of auramine fluorochrome staining, allowing scanning with lower power objective, results in examination of a greater area of the slide at a given time, and therefore greater sensitivity. Any detected organisms should be confirmed by examination for typical morphology using the 100× objective. Some laboratories confirm positive fluorochrome smears with a carbol-fuchsin–based stain.
 Specimens should be collected and transported to the laboratory according to recommendations for mycobacterial cultures.
Specimens should be collected and transported to the laboratory according to recommendations for mycobacterial cultures.
 Turnaround time: <24 hours
Turnaround time: <24 hours
 Interpretation
Interpretation
 Expected results: Negative. Detection of mycobacteria requires 10,000 or more organisms per milliliter or gram of sample for consistent detection. Sensitivity may be improved by concentration of specimen, such as by centrifugation, and by examination of multiple specimens. Rapidly growing mycobacteria, such as Mycobacterium fortuitum, have relatively thin layers of cell wall mycolic acid and may decolorize with acid–alcohol decolorizing solutions. These organisms may be stained using a weaker acid in aqueous solution.
Expected results: Negative. Detection of mycobacteria requires 10,000 or more organisms per milliliter or gram of sample for consistent detection. Sensitivity may be improved by concentration of specimen, such as by centrifugation, and by examination of multiple specimens. Rapidly growing mycobacteria, such as Mycobacterium fortuitum, have relatively thin layers of cell wall mycolic acid and may decolorize with acid–alcohol decolorizing solutions. These organisms may be stained using a weaker acid in aqueous solution.
 Positive results: Positive specimens are very likely (>90%) to yield growth of mycobacteria in culture. In a minority of patients, usually with cavitary or extensive tuberculosis, sputum AFB stains may remain persistently positive for weeks after patients have converted to negative cultures. Nonviable organisms may be detected by AFB stains. Nocardia and related species are weakly acid fast and may give false-positive results if staining protocols are not followed closely.
Positive results: Positive specimens are very likely (>90%) to yield growth of mycobacteria in culture. In a minority of patients, usually with cavitary or extensive tuberculosis, sputum AFB stains may remain persistently positive for weeks after patients have converted to negative cultures. Nonviable organisms may be detected by AFB stains. Nocardia and related species are weakly acid fast and may give false-positive results if staining protocols are not followed closely.
 Limitations
Limitations
 Standardized protocols, such as those published by the American Thoracic Society, should be followed carefully to ensure sensitive detection and accurate interpretation of smears.
Standardized protocols, such as those published by the American Thoracic Society, should be followed carefully to ensure sensitive detection and accurate interpretation of smears.
 Common pitfalls: Care must be taken to avoid contaminating slides with acid-fast organisms. Common causes of slide contamination are use of tap water for solution preparation, carryover between slides with immersion oil, and use of common staining chambers.
Common pitfalls: Care must be taken to avoid contaminating slides with acid-fast organisms. Common causes of slide contamination are use of tap water for solution preparation, carryover between slides with immersion oil, and use of common staining chambers.
ACID-FAST STAIN, MODIFIED
 Definition and Use
Definition and Use
 This stain may be used for detection of Nocardia in patient specimens or culture isolates when nocardiosis is suspected on the basis of clinical presentation or because of typical morphology in culture isolates. The Gram stain is very sensitive for detection of Nocardia in patient specimens.
This stain may be used for detection of Nocardia in patient specimens or culture isolates when nocardiosis is suspected on the basis of clinical presentation or because of typical morphology in culture isolates. The Gram stain is very sensitive for detection of Nocardia in patient specimens.
 The modified acid-fast stain is typically used to confirm nocardioform organisms detected by Gram stain. The modified acid-fast stain is useful for differentiating Nocardia (positive) from Streptomyces (negative), especially in culture isolates. The modified acid-fast stain is similar to the carbol-fuchsin– based acid-fast stains (Ziehl-Neelsen or Kinyoun stains) except that a less active decolorizer is used (1% H2 SO4 or 3% HCl in aqueous solution). Specimens should be collected and transported as appropriate for routine bacterial cultures for the specimen type.
The modified acid-fast stain is typically used to confirm nocardioform organisms detected by Gram stain. The modified acid-fast stain is useful for differentiating Nocardia (positive) from Streptomyces (negative), especially in culture isolates. The modified acid-fast stain is similar to the carbol-fuchsin– based acid-fast stains (Ziehl-Neelsen or Kinyoun stains) except that a less active decolorizer is used (1% H2 SO4 or 3% HCl in aqueous solution). Specimens should be collected and transported as appropriate for routine bacterial cultures for the specimen type.
 Turnaround time: 24–72 hours
Turnaround time: 24–72 hours
 Interpretation
Interpretation
 Expected results: Negative. Negative stains do not rule out nocardiosis. Rapidly growing mycobacteria, such as M. fortuitum, may be negative by routine acid-fast staining but positive by modified acid-fast staining.
Expected results: Negative. Negative stains do not rule out nocardiosis. Rapidly growing mycobacteria, such as M. fortuitum, may be negative by routine acid-fast staining but positive by modified acid-fast staining.
 Positive results with Nocardia: Delicate, branching filaments that retain the carbol-fuchsin stain.
Positive results with Nocardia: Delicate, branching filaments that retain the carbol-fuchsin stain.
 Limitations
Limitations
 Nocardia may stain poorly in direct staining of patient specimens. Other species of aerobic actinomycetes, such as Rhodococcus equi and occasionally coryneform bacteria, may be modified acid-fast stain positive.
Nocardia may stain poorly in direct staining of patient specimens. Other species of aerobic actinomycetes, such as Rhodococcus equi and occasionally coryneform bacteria, may be modified acid-fast stain positive.
Suggested Readings
Al-Moamary M, Black W, Bessuille E, et al. The significance of the persistent presence of acid-fast bacilli in sputum smears in pulmonary tuberculosis. Chest 1999;116:726–731.
Winn WC Jr, Allen SD, Janda WM, et al. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006.
AEROBIC CULTURE
 Definition and Use
Definition and Use
 Aerobic cultures are indicated for the detection of common aerobic bacterial pathogens in patient specimens taken from sites with signs and symptoms of bacterial infection (e.g., swelling, redness, heat, pus, or exudate). Site-specific bacterial cultures (e.g., sputum culture, genital culture) are recommended, if available. Specimens may be inoculated on several types of aerobic culture plates and broth media and may include selective and enriched media. Typical media for aerobic cultures include
Aerobic cultures are indicated for the detection of common aerobic bacterial pathogens in patient specimens taken from sites with signs and symptoms of bacterial infection (e.g., swelling, redness, heat, pus, or exudate). Site-specific bacterial cultures (e.g., sputum culture, genital culture) are recommended, if available. Specimens may be inoculated on several types of aerobic culture plates and broth media and may include selective and enriched media. Typical media for aerobic cultures include
 Supportive media to isolate nonfastidious organisms, like sheep blood agar (SBA).
Supportive media to isolate nonfastidious organisms, like sheep blood agar (SBA).
 Enriched media to isolate organisms with special nutritional requirements, like chocolate agar.
Enriched media to isolate organisms with special nutritional requirements, like chocolate agar.
 Selective media to suppress the growth of specific types of bacteria. Selective media are often formulated so that colonies of different types of organisms that are able to grow on the media have different appearances. MacConkey is an example: Selective: nonfastidious gram-negative bacilli are able to grow. Differential: lactose fermenters are distinguished from lactose nonfermenters.
Selective media to suppress the growth of specific types of bacteria. Selective media are often formulated so that colonies of different types of organisms that are able to grow on the media have different appearances. MacConkey is an example: Selective: nonfastidious gram-negative bacilli are able to grow. Differential: lactose fermenters are distinguished from lactose nonfermenters.
 Solid versus Broth Media
Solid versus Broth Media
 Culture media may be prepared in a solid or broth phase.
Culture media may be prepared in a solid or broth phase.
 Solid media (culture plates) are inoculated with a small amount of specimen. Mixed cultures are recognized by differences in colony morphology. The amount of each type of organism (and relative proportions in mixed cultures) can be estimated (e.g., rare, light, moderate, or heavy).
Solid media (culture plates) are inoculated with a small amount of specimen. Mixed cultures are recognized by differences in colony morphology. The amount of each type of organism (and relative proportions in mixed cultures) can be estimated (e.g., rare, light, moderate, or heavy).
 Pyogenic infections are usually associated with growth of a single (or predominant) pathogen in moderate or heavy amounts.
Pyogenic infections are usually associated with growth of a single (or predominant) pathogen in moderate or heavy amounts.
 Broth media can be inoculated with a larger volume of specimen than agar plates, which may improve detection of infections with low concentrations of pathogens, but the amount of bacteria in the specimen cannot be estimated from broth cultures.
Broth media can be inoculated with a larger volume of specimen than agar plates, which may improve detection of infections with low concentrations of pathogens, but the amount of bacteria in the specimen cannot be estimated from broth cultures.
 Broth media may allow detection of some relatively aerotolerant anaerobic pathogens. Broth cultures have been associated with an increased rate of contamination.
Broth media may allow detection of some relatively aerotolerant anaerobic pathogens. Broth cultures have been associated with an increased rate of contamination.
 Expected results: No pathogen isolated
Expected results: No pathogen isolated
 Turnaround time: 48–72 hours
Turnaround time: 48–72 hours
 In positive cultures, additional time is required for isolation, identification, susceptibility testing, and further characterization, as appropriate.
In positive cultures, additional time is required for isolation, identification, susceptibility testing, and further characterization, as appropriate.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 Standard precautions apply. Ensure that material from the site of infection is collected.
Standard precautions apply. Ensure that material from the site of infection is collected.
 Decontaminate skin or mucous membranes that must be crossed to obtain the specimen.
Decontaminate skin or mucous membranes that must be crossed to obtain the specimen.
 Use appropriate sterile supplies to collect the specimen. Place the specimen in a sterile, leak-proof container for transport. Ensure that the lid is firmly tightened, but avoid overtightening. Use specific transport medium and/or procedures as required for suspected pathogens (described below) or if transport to the lab will be prolonged (>2 hours). Apply a label to the specimen with information to identify the patient and type of specimen, as described below. Transport the specimen to the lab as quickly as possible, avoiding extremes of temperature. Note that collection protocols for some types of specimens require specific training and/or certification of the health care professional performing the collection. Examples include collection of bone marrow and CSF specimens.
Use appropriate sterile supplies to collect the specimen. Place the specimen in a sterile, leak-proof container for transport. Ensure that the lid is firmly tightened, but avoid overtightening. Use specific transport medium and/or procedures as required for suspected pathogens (described below) or if transport to the lab will be prolonged (>2 hours). Apply a label to the specimen with information to identify the patient and type of specimen, as described below. Transport the specimen to the lab as quickly as possible, avoiding extremes of temperature. Note that collection protocols for some types of specimens require specific training and/or certification of the health care professional performing the collection. Examples include collection of bone marrow and CSF specimens.
 Limitations
Limitations
 Anaerobic culture is recommended for infections at sites likely to be infected by anaerobic pathogens. Examples include pelvic infections, intra-abdominal infections, abscesses, and traumatic and surgical wounds. Certain aerobic pathogens, such as Legionella species, require special processing or culture techniques for detection.
Anaerobic culture is recommended for infections at sites likely to be infected by anaerobic pathogens. Examples include pelvic infections, intra-abdominal infections, abscesses, and traumatic and surgical wounds. Certain aerobic pathogens, such as Legionella species, require special processing or culture techniques for detection.
 Common pitfalls:
Common pitfalls:
 Specimens may be collected from sites that are not the primary site of active infection (even though there may be signs of inflammation at the site).
Specimens may be collected from sites that are not the primary site of active infection (even though there may be signs of inflammation at the site).
 Inadequate site preparation may result in false-positive cultures due to specimen contamination with endogenous flora. Contaminated specimens may also mask the recognition of slow-growing or fastidious pathogens in the culture.
Inadequate site preparation may result in false-positive cultures due to specimen contamination with endogenous flora. Contaminated specimens may also mask the recognition of slow-growing or fastidious pathogens in the culture.
ANAEROBIC CULTURE
 Definition and Use
Definition and Use
 Anaerobic cultures are indicated for evaluation of patient specimens taken from sites with signs and symptoms of bacterial infection (e.g., swelling, redness, heat, pus, or exudate). Infections associated with anaerobic pathogens include surgical and traumatic wounds, sinusitis and pararespiratory infections, pelvic and intra-abdominal infections, osteomyelitis, myositis, gangrene, and necrotic wounds, abscesses, and actinomycosis and infections associated with fistula formation.
Anaerobic cultures are indicated for evaluation of patient specimens taken from sites with signs and symptoms of bacterial infection (e.g., swelling, redness, heat, pus, or exudate). Infections associated with anaerobic pathogens include surgical and traumatic wounds, sinusitis and pararespiratory infections, pelvic and intra-abdominal infections, osteomyelitis, myositis, gangrene, and necrotic wounds, abscesses, and actinomycosis and infections associated with fistula formation.
 Anaerobic cultures are used for the detection of common anaerobic bacterial pathogens from patient specimens. Site-specific aerobic bacterial cultures (e.g., tissue culture, abscess culture, wound culture) are recommended, if available. Specimens are inoculated on several types of anaerobic culture media. (see “Aerobic Culture” for a general discussion about media). Media should be fresh and prereduced. Typical media for aerobic cultures include
Anaerobic cultures are used for the detection of common anaerobic bacterial pathogens from patient specimens. Site-specific aerobic bacterial cultures (e.g., tissue culture, abscess culture, wound culture) are recommended, if available. Specimens are inoculated on several types of anaerobic culture media. (see “Aerobic Culture” for a general discussion about media). Media should be fresh and prereduced. Typical media for aerobic cultures include
 Supportive agar media, like Schaedler agar or CDC anaerobic blood agar
Supportive agar media, like Schaedler agar or CDC anaerobic blood agar
 Selective/differential agar media:
Selective/differential agar media:
 Phenylethyl alcohol or CNA agar for anaerobic gram-positive pathogens
Phenylethyl alcohol or CNA agar for anaerobic gram-positive pathogens
 Kanamycin–vancomycin–laked blood agar, for anaerobic gram-negative bacilli
Kanamycin–vancomycin–laked blood agar, for anaerobic gram-negative bacilli
 Bacteroide bile–esculin agar, for Bacteroides fragilis group
Bacteroide bile–esculin agar, for Bacteroides fragilis group
 Egg yolk agar, for characterization of Clostridium species
Egg yolk agar, for characterization of Clostridium species
 Cycloserine–cefoxitin–egg yolk–fructose agar (CCFA), for Clostridium difficile
Cycloserine–cefoxitin–egg yolk–fructose agar (CCFA), for Clostridium difficile
 Broth, like enriched thioglycolate medium or chopped meat broth
Broth, like enriched thioglycolate medium or chopped meat broth
 Turnaround time: Incubation for 5–7 days
Turnaround time: Incubation for 5–7 days
 Additional time is required for positive cultures for additional testing required for isolation, confirmation as anaerobic (aerotolerance testing), identification, susceptibility testing, and further characterization, as needed. Anaerobic infections are frequently polymicrobial; final results may require several weeks for full laboratory evaluation, if needed.
Additional time is required for positive cultures for additional testing required for isolation, confirmation as anaerobic (aerotolerance testing), identification, susceptibility testing, and further characterization, as needed. Anaerobic infections are frequently polymicrobial; final results may require several weeks for full laboratory evaluation, if needed.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 See “Aerobic Culture” for a general discussion of collection and transport instructions
See “Aerobic Culture” for a general discussion of collection and transport instructions
 Because of the anaerobic endogenous flora, specimens from the following sites should not be submitted for anaerobic culture: sputum or bronchoscopically collected lower respiratory specimens, swabs from skin or mucosal surfaces, specimens from the GI tract (including fistulae, stoma surfaces, and so on), superficial ulcers or eschars, including decubitus ulcers, vaginal or cervical swabs or urine (except suprapubic aspirate urine).
Because of the anaerobic endogenous flora, specimens from the following sites should not be submitted for anaerobic culture: sputum or bronchoscopically collected lower respiratory specimens, swabs from skin or mucosal surfaces, specimens from the GI tract (including fistulae, stoma surfaces, and so on), superficial ulcers or eschars, including decubitus ulcers, vaginal or cervical swabs or urine (except suprapubic aspirate urine).
 When submitting samples, ensure that sufficient specimen is collected for all of the diagnostic testing required (e.g., aerobic, fungal, and/or mycobacterial cultures and stains). Minimize exposure to atmospheric oxygen and transport in an anaerobic transport system. Do not refrigerate or freeze specimens for anaerobic culture. Note the following: Specimens collected and transported for anaerobic culture are also acceptable for aerobic bacterial, fungal, or mycobacterial culture, provided a sufficient volume of specimen is provided.
When submitting samples, ensure that sufficient specimen is collected for all of the diagnostic testing required (e.g., aerobic, fungal, and/or mycobacterial cultures and stains). Minimize exposure to atmospheric oxygen and transport in an anaerobic transport system. Do not refrigerate or freeze specimens for anaerobic culture. Note the following: Specimens collected and transported for anaerobic culture are also acceptable for aerobic bacterial, fungal, or mycobacterial culture, provided a sufficient volume of specimen is provided.
 Interpretation
Interpretation
 Expected results: No anaerobic pathogen isolated.
Expected results: No anaerobic pathogen isolated.
 Limitations
Limitations
 Anaerobic infections are frequently polymicrobial. Initial isolation and aerotolerance testing may require repeated subculture of the primary culture media. Many anaerobic pathogens are slow growing and biochemically indolent, making identification, susceptibility testing, and further characterization of isolates in the laboratory much slower than most aerobic bacterial pathogens. Therefore, patient care decisions must often be made before results of testing are available, limiting the clinical utility of extensive workup of mixed anaerobic cultures.
Anaerobic infections are frequently polymicrobial. Initial isolation and aerotolerance testing may require repeated subculture of the primary culture media. Many anaerobic pathogens are slow growing and biochemically indolent, making identification, susceptibility testing, and further characterization of isolates in the laboratory much slower than most aerobic bacterial pathogens. Therefore, patient care decisions must often be made before results of testing are available, limiting the clinical utility of extensive workup of mixed anaerobic cultures.
 Common pitfalls
Common pitfalls
 Anaerobic culture may be significantly compromised by collection and transport conditions that are not strictly anaerobic or because of refrigeration during transport. Inadequate site preparation may result in false-positive cultures due to specimen contamination with endogenous flora. Contaminated cultures may also mask the recognition of slow-growing or fastidious anaerobic pathogens in the culture.
Anaerobic culture may be significantly compromised by collection and transport conditions that are not strictly anaerobic or because of refrigeration during transport. Inadequate site preparation may result in false-positive cultures due to specimen contamination with endogenous flora. Contaminated cultures may also mask the recognition of slow-growing or fastidious anaerobic pathogens in the culture.
BACTERIAL ANTIGEN DETECTION
 Definition and Use
Definition and Use
 This test in intended for the rapid initial detection of Streptococcus pneumoniae, Haemophilus influenzae type b, group B beta-hemolytic Streptococcus (GBS), or Neisseria meningitidis in CSF. The indication for this test is limited. Published reports have demonstrated limited sensitivity for the detection of patients with meningitis caused by common pathogens, and test results rarely result in changes to the management or therapy of patients. There may be some utility in patients who have been treated with antibiotics prior to CSF collection. There is some evidence that the performance for initial detection of GBS meningitis in neonates is acceptable.
This test in intended for the rapid initial detection of Streptococcus pneumoniae, Haemophilus influenzae type b, group B beta-hemolytic Streptococcus (GBS), or Neisseria meningitidis in CSF. The indication for this test is limited. Published reports have demonstrated limited sensitivity for the detection of patients with meningitis caused by common pathogens, and test results rarely result in changes to the management or therapy of patients. There may be some utility in patients who have been treated with antibiotics prior to CSF collection. There is some evidence that the performance for initial detection of GBS meningitis in neonates is acceptable.
 Latex particles are coated with antibodies directed against specific antigens of the pathogens noted above. Agglutination should occur if the antigen is present in CSF, as either a free antigen or intact bacterial cells. Specimens are collected and transported according to directions for CSF culture.
Latex particles are coated with antibodies directed against specific antigens of the pathogens noted above. Agglutination should occur if the antigen is present in CSF, as either a free antigen or intact bacterial cells. Specimens are collected and transported according to directions for CSF culture.
 Turnaround time: <4 hours
Turnaround time: <4 hours
 Interpretation
Interpretation
 Expected results: Negative; no agglutination means that a CSF infection caused by specific pathogen is less likely. Positive agglutination for specific latex reagent: CSF infection caused by the specific pathogen is more likely.
Expected results: Negative; no agglutination means that a CSF infection caused by specific pathogen is less likely. Positive agglutination for specific latex reagent: CSF infection caused by the specific pathogen is more likely.
 Limitations
Limitations
 The sensitivity and specificity are too low to be recommended for routine use. Results are unlikely to change patient therapy or management.
The sensitivity and specificity are too low to be recommended for routine use. Results are unlikely to change patient therapy or management.
Suggested Readings
Perkins MD, Mirrett S, Reller LB. Rapid bacterial antigen detection is not clinically useful. J Clin Microbiol. 1995;30(06):1486–1491.
Ringelmann R, Heym B, Kniehl E. Role of immunologic tests in diagnosis of bacterial meningitis. Antibiot Chemother. 1992;45:68–78.
BLOOD CULTURE, FUNGAL
 Definition and Use
Definition and Use
 Fungal blood cultures are used for detection of bloodstream infection caused by fungi, especially when dimorphic species and uncommon pathogens are suspected. Identification, susceptibility, and further testing can be performed on culture isolates. The culture is indicated primarily for patients with cancer, extensive therapy with broad-spectrum antibiotics, trauma, and HIV and other immunocompromising conditions and symptoms that suggest sepsis, like fever, chills, malaise, hypotension, poor perfusion, toxicity, tachycardia, and hyperventilation. Biphasic and lysis–centrifugation methods have demonstrated improved isolation of dimorphic and filamentous fungi.
Fungal blood cultures are used for detection of bloodstream infection caused by fungi, especially when dimorphic species and uncommon pathogens are suspected. Identification, susceptibility, and further testing can be performed on culture isolates. The culture is indicated primarily for patients with cancer, extensive therapy with broad-spectrum antibiotics, trauma, and HIV and other immunocompromising conditions and symptoms that suggest sepsis, like fever, chills, malaise, hypotension, poor perfusion, toxicity, tachycardia, and hyperventilation. Biphasic and lysis–centrifugation methods have demonstrated improved isolation of dimorphic and filamentous fungi.
 Turnaround time: 4 weeks
Turnaround time: 4 weeks
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 Inoculate blood culture system according to the manufacturer’s recommendations. Alert the laboratory if infection due to Malassezia furfur is suspected. Special culture processing is needed for isolation of this lipophilic yeast. Transport to the laboratory at room temperature.
Inoculate blood culture system according to the manufacturer’s recommendations. Alert the laboratory if infection due to Malassezia furfur is suspected. Special culture processing is needed for isolation of this lipophilic yeast. Transport to the laboratory at room temperature.
 Interpretation
Interpretation
 Expected results: No growth
Expected results: No growth
 Most commonly isolated pathogens in positive cultures:
Most commonly isolated pathogens in positive cultures:
 Yeasts: Candida albicans, nonalbicans Candida species, and Cryptococcus neoformans. (Candida and other commonly isolated yeasts may be efficiently detected using routine blood cultures.)
Yeasts: Candida albicans, nonalbicans Candida species, and Cryptococcus neoformans. (Candida and other commonly isolated yeasts may be efficiently detected using routine blood cultures.)
 Dimorphic fungus: Histoplasma capsulatum.
Dimorphic fungus: Histoplasma capsulatum.
 Mold: Fusarium and Scedosporium species.
Mold: Fusarium and Scedosporium species.
 Limitation
Limitation
 Aspergillus species are rarely isolated by blood culture even in the presence of acute systemic infection.
Aspergillus species are rarely isolated by blood culture even in the presence of acute systemic infection.
Suggested Reading
CLSI. Principles and Procedures for Blood Cultures; Approved Guideline. CLSI document M47-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
BLOOD CULTURE, MYCOBACTERIAL
 Definition and Use
Definition and Use
 The mycobacterial blood culture is used for the detection of bloodstream infection due to Mycobacterium species. Mycobacteremia is most commonly seen in patients with AIDS, although it may occur in other congenital and acquired immunocompromising conditions, including patients taking chronic corticosteroid therapy, and malignancies. Growth of mycobacteria in culture requires the use of specialized, supplemented media with prolonged incubation time. Lysis of blood cells improves detection, by releasing phagocytized organisms, and is used in most methods (e.g., lysis–centrifugation methods).
The mycobacterial blood culture is used for the detection of bloodstream infection due to Mycobacterium species. Mycobacteremia is most commonly seen in patients with AIDS, although it may occur in other congenital and acquired immunocompromising conditions, including patients taking chronic corticosteroid therapy, and malignancies. Growth of mycobacteria in culture requires the use of specialized, supplemented media with prolonged incubation time. Lysis of blood cells improves detection, by releasing phagocytized organisms, and is used in most methods (e.g., lysis–centrifugation methods).
 Turnaround time: 4–8 weeks
Turnaround time: 4–8 weeks
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 Collect 5–10 mL of blood in sodium polyanethol sulfonate (SPS) or heparin, or directly inoculate specific mycobacterial blood culture media. Inoculate the blood culture media or collection system according to the manufacturer’s instructions. Transport to the laboratory at room temperature.
Collect 5–10 mL of blood in sodium polyanethol sulfonate (SPS) or heparin, or directly inoculate specific mycobacterial blood culture media. Inoculate the blood culture media or collection system according to the manufacturer’s instructions. Transport to the laboratory at room temperature.
 Interpretation
Interpretation
 Expected results: No growth
Expected results: No growth
 Positive results: Mycobacterium avium complex (MAC) is the most commonly isolated mycobacterial pathogen. M. tuberculosis may be isolated at the time of hematogenous spread associated with severe primary or reactivation disease. Rapidly growing mycobacteria, such as M. fortuitum, have been associated with chronic indwelling vascular catheters and other prosthetic material.
Positive results: Mycobacterium avium complex (MAC) is the most commonly isolated mycobacterial pathogen. M. tuberculosis may be isolated at the time of hematogenous spread associated with severe primary or reactivation disease. Rapidly growing mycobacteria, such as M. fortuitum, have been associated with chronic indwelling vascular catheters and other prosthetic material.
 Limitations
Limitations
 Some mycobacterial infections are rarely associated with mycobacteremia. EDTA or acid citrate dextrose (ACD)-anticoagulated blood should not be used for mycobacterial blood culture inoculum.
Some mycobacterial infections are rarely associated with mycobacteremia. EDTA or acid citrate dextrose (ACD)-anticoagulated blood should not be used for mycobacterial blood culture inoculum.
Suggested Reading
CLSI. Principles and Procedures for Blood Cultures; Approved Guideline. CLSI document M47-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
BLOOD CULTURE, ROUTINE
 Definition and Use
Definition and Use
 The routine blood culture is used for detection of bloodstream infections (BSIs) due to common aerobic and anaerobic bacterial and yeast pathogens. Potentially pathogenic isolates are identified, and susceptibility testing is performed, as appropriate. Special testing is required for the detection of mycobacteria, parasites, viruses, and certain fungal pathogens.
The routine blood culture is used for detection of bloodstream infections (BSIs) due to common aerobic and anaerobic bacterial and yeast pathogens. Potentially pathogenic isolates are identified, and susceptibility testing is performed, as appropriate. Special testing is required for the detection of mycobacteria, parasites, viruses, and certain fungal pathogens.
 Indications:
Indications:
 Sepsis syndrome, fever, chills, malaise, hypotension, poor perfusion, toxicity, tachycardia, hyperventilation.
Sepsis syndrome, fever, chills, malaise, hypotension, poor perfusion, toxicity, tachycardia, hyperventilation.
 Evaluation of serious localized infections, such as pneumonia, UTIs, and meningitis. Classic signs and symptoms may be absent in infants, the elderly and patients with certain medical or surgical conditions.
Evaluation of serious localized infections, such as pneumonia, UTIs, and meningitis. Classic signs and symptoms may be absent in infants, the elderly and patients with certain medical or surgical conditions.
 Methods:
Methods:
 Most commercially available blood culture systems recommend inoculation of blood into two broth media: one aerobic and one anaerobic. Lysis– centrifugation methods may be used for routine detection of BSIs due to bacteria or yeast but are more typically used for detection of mycobacteremia or fungemia.
Most commercially available blood culture systems recommend inoculation of blood into two broth media: one aerobic and one anaerobic. Lysis– centrifugation methods may be used for routine detection of BSIs due to bacteria or yeast but are more typically used for detection of mycobacteremia or fungemia.
 Turnaround time: Generally, incubation for 5–7 days. Most true-positive blood cultures become positive within 24–48 hours after inoculation.
Turnaround time: Generally, incubation for 5–7 days. Most true-positive blood cultures become positive within 24–48 hours after inoculation.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 Decontamination of the collection site is the most important factor in preventing false-positive (contaminated) blood cultures. Inoculate media according to the manufacturer’s instructions. Usually, 8–10 mL of blood is inoculated into each blood culture bottle. A smaller inoculum volume, based on weight or age, is recommended for small children. Submission of two or three independently drawn (different venipuncture sites) blood cultures is recommended for the initial evaluation of patients with suspected BSI. Transport blood cultures to the laboratory at room temperature.
Decontamination of the collection site is the most important factor in preventing false-positive (contaminated) blood cultures. Inoculate media according to the manufacturer’s instructions. Usually, 8–10 mL of blood is inoculated into each blood culture bottle. A smaller inoculum volume, based on weight or age, is recommended for small children. Submission of two or three independently drawn (different venipuncture sites) blood cultures is recommended for the initial evaluation of patients with suspected BSI. Transport blood cultures to the laboratory at room temperature.
 Interpretation
Interpretation
 Expected results: No growth
Expected results: No growth
 Positive results: Bacteremia or fungemia present. Positive blood cultures must be carefully evaluated to assess the possibility of false-positive culture, usually due to specimen contamination at the time of collection. Organisms, like viridans Streptococci, that are most commonly isolated as contaminants may also cause true BSI, usually in patients with some compromise in immune function. Interpret all positive blood cultures in the context of number of positive blood cultures as well as clinical and laboratory signs and symptoms. In patients with clinically relevant BSIs (true positive), the pathogen is typically isolated from a majority of cultures/bottles. In patients with contaminated blood cultures (false positive), a common contaminant is typically isolated in a single culture or bottle, whereas other cultures are drawn during evaluation remain negative.
Positive results: Bacteremia or fungemia present. Positive blood cultures must be carefully evaluated to assess the possibility of false-positive culture, usually due to specimen contamination at the time of collection. Organisms, like viridans Streptococci, that are most commonly isolated as contaminants may also cause true BSI, usually in patients with some compromise in immune function. Interpret all positive blood cultures in the context of number of positive blood cultures as well as clinical and laboratory signs and symptoms. In patients with clinically relevant BSIs (true positive), the pathogen is typically isolated from a majority of cultures/bottles. In patients with contaminated blood cultures (false positive), a common contaminant is typically isolated in a single culture or bottle, whereas other cultures are drawn during evaluation remain negative.
 Negative results: Bacteremia and fungemia at the time of specimen collection are unlikely. False-negative results may be seen in patients with prior antimicrobial therapy. False-negative results may be caused by inoculation of blood culture bottles with less than the recommended volume of blood. Because clinically significant bacteremia may be intermittent, collection of two or three blood cultures is recommended to rule out bacteremia.
Negative results: Bacteremia and fungemia at the time of specimen collection are unlikely. False-negative results may be seen in patients with prior antimicrobial therapy. False-negative results may be caused by inoculation of blood culture bottles with less than the recommended volume of blood. Because clinically significant bacteremia may be intermittent, collection of two or three blood cultures is recommended to rule out bacteremia.
 Limitations
Limitations
 The significance of positive blood cultures must be evaluated in terms of several factors, including patient signs and symptoms, the intrinsic pathogenicity of the blood culture isolate, number of positive cultures, number of isolates in culture (mixed cultures typically represent contamination), and cultures positive at other infected sites for the blood culture isolate.
The significance of positive blood cultures must be evaluated in terms of several factors, including patient signs and symptoms, the intrinsic pathogenicity of the blood culture isolate, number of positive cultures, number of isolates in culture (mixed cultures typically represent contamination), and cultures positive at other infected sites for the blood culture isolate.
 Routine blood cultures are optimized for detection of the pathogens most frequently associated with BSIs. Clinically relevant BSIs may be associated with pathogens for which special blood cultures are required (e.g., mycobacteria, dimorphic fungi, fastidious bacteria).
Routine blood cultures are optimized for detection of the pathogens most frequently associated with BSIs. Clinically relevant BSIs may be associated with pathogens for which special blood cultures are required (e.g., mycobacteria, dimorphic fungi, fastidious bacteria).
 Common pitfalls:
Common pitfalls:
 Decreased sensitivity because of such factors as a low volume of blood inoculated into blood culture media. Decreased specificity because of contamination due to poor preparation of collection site.
Decreased sensitivity because of such factors as a low volume of blood inoculated into blood culture media. Decreased specificity because of contamination due to poor preparation of collection site.
Suggested Readings
CLSI. Principles and Procedures for Blood Cultures; Approved Guideline. CLSI document M47-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
Katidis AG, et al. Pediatric Infect Dis J. 1996;15:615–620.
Yakoe DS, Anderson J, Chambers R, et al. Simplified surveillance for nosocomial bloodstream infections. Inf Control Hosp Epidemiol. 1998;19:657–660.
BLOOD PARASITE EXAMINATION
 Definition and Use
Definition and Use
 This test is used to detect parasites circulating in peripheral blood. It should be ordered in patients when infection caused by Plasmodium species (malaria), Babesia species (babesiosis), Trypanosoma species (sleeping sickness, Chagas disease), or certain microfilaria species or systemic infection with Leishmania species is suspected. Thin and thick blood smears are prepared from free-flowing capillary blood or EDTA-anticoagulated blood. Smears are inspected after staining with Giemsa, Wright, or Wright-Giemsa stain. For positive smears, the level of parasitemia should be determined for each specimen.
This test is used to detect parasites circulating in peripheral blood. It should be ordered in patients when infection caused by Plasmodium species (malaria), Babesia species (babesiosis), Trypanosoma species (sleeping sickness, Chagas disease), or certain microfilaria species or systemic infection with Leishmania species is suspected. Thin and thick blood smears are prepared from free-flowing capillary blood or EDTA-anticoagulated blood. Smears are inspected after staining with Giemsa, Wright, or Wright-Giemsa stain. For positive smears, the level of parasitemia should be determined for each specimen.
 Turnaround time:
Turnaround time:
 Preliminary examination should be performed “STAT” if malaria is suspected (turnaround time <4 hours). Final report for positive smears: <24 hours.
Preliminary examination should be performed “STAT” if malaria is suspected (turnaround time <4 hours). Final report for positive smears: <24 hours.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 Preparation of smears at the bedside, from free-flowing capillary blood if possible, yields the best morphology. Alternatively, EDTA-anticoagulated blood may be collected. For microfilariae, the diurnal circulation of some species must be taken into account in timing specimen collection (Loa loa: 10 AM–2 PM; Wuchereria or Brugia species: 8 PM– 4 AM). Transport specimens to the laboratory and prepare smears as soon as possible. In general, collect specimens on each of 3 successive days. Collect specimens every 6–8 hours (until positive) for optimal detection in suspected cases. Blood should be examined in treated patients after 24, 48, and 72 hours to determine effectiveness of therapy.
Preparation of smears at the bedside, from free-flowing capillary blood if possible, yields the best morphology. Alternatively, EDTA-anticoagulated blood may be collected. For microfilariae, the diurnal circulation of some species must be taken into account in timing specimen collection (Loa loa: 10 AM–2 PM; Wuchereria or Brugia species: 8 PM– 4 AM). Transport specimens to the laboratory and prepare smears as soon as possible. In general, collect specimens on each of 3 successive days. Collect specimens every 6–8 hours (until positive) for optimal detection in suspected cases. Blood should be examined in treated patients after 24, 48, and 72 hours to determine effectiveness of therapy.
 Interpretation
Interpretation
 Expected results: Negative. Sensitive detection of parasitemia may require the examination of several specimens, as recommended above.
Expected results: Negative. Sensitive detection of parasitemia may require the examination of several specimens, as recommended above.
 Positive result: Disease caused by specific parasite identified.
Positive result: Disease caused by specific parasite identified.
 Limitations
Limitations
 Low level of parasitemia may require the examination of multiple specimens for detection. Examination of smears prepared from buffy coat preparations may improve the sensitivity of detection for some parasites, like microfilaria and trypanosomes. The efficient detection of microfilaria requires specimen collection during the specific hours when circulation of the parasite is expected.
Low level of parasitemia may require the examination of multiple specimens for detection. Examination of smears prepared from buffy coat preparations may improve the sensitivity of detection for some parasites, like microfilaria and trypanosomes. The efficient detection of microfilaria requires specimen collection during the specific hours when circulation of the parasite is expected.
 Common pitfalls: Include the collection of too few specimens for examination.
Common pitfalls: Include the collection of too few specimens for examination.
 Other Considerations
Other Considerations
 In effectively treated patients, the level of parasitemia should drop very quickly. In patients with drug-resistant parasites, the level may remain stable, or even increase.
In effectively treated patients, the level of parasitemia should drop very quickly. In patients with drug-resistant parasites, the level may remain stable, or even increase.
Suggested Readings
Garcia LS. Diagnostic Parasitology, 5th ed. Washington, DC: ASM Press; 2007.
NCCLS document M15-A. Laboratory Diagnosis of Blood-borne Parasitic Diseases; Approved Guideline. 2000. Clinical and Laboratory Standards Institute.
BODY FLUID CULTURE
 Definition
Definition
 Sterile fluid-filled spaces are present at a number of anatomic sites and are subject to infection. Examples of sterile fluids include peritoneal, pleural, pericardial, and synovial/joint fluid. Infections associated with CSF are life threatening and associated with a different etiology of bacterial pathogens, so these cultures are typically processed differently than other sterile fluids. Urine is also processed with different culture techniques because of its connection with the external environment, via the urethra, and pathogenesis of infection. A broad etiology of bacterial pathogens may cause infections of sterile sites, and culture methods are optimized for recovery of organisms present in low concentrations.
Sterile fluid-filled spaces are present at a number of anatomic sites and are subject to infection. Examples of sterile fluids include peritoneal, pleural, pericardial, and synovial/joint fluid. Infections associated with CSF are life threatening and associated with a different etiology of bacterial pathogens, so these cultures are typically processed differently than other sterile fluids. Urine is also processed with different culture techniques because of its connection with the external environment, via the urethra, and pathogenesis of infection. A broad etiology of bacterial pathogens may cause infections of sterile sites, and culture methods are optimized for recovery of organisms present in low concentrations.
 Use
Use
 Collect sterile fluid cultures from sites associated with signs and symptoms of inflammation, including redness, swelling, pain, heat, fluid accumulation, and pus formation.
Collect sterile fluid cultures from sites associated with signs and symptoms of inflammation, including redness, swelling, pain, heat, fluid accumulation, and pus formation.
 Method: Supportive and enriched solid agar (SBA and chocolate agar) and broth media (blood culture media) are typically inoculated; selective/differential agar media, such as MacConkey agar (gram-negative bacilli), CNA, or phenylethyl alcohol agar (gram-positive organisms), should be inoculated for specimens likely to show polymicrobial infection (e.g., peritonitis) or contamination by endogenous flora (e.g., cul-de-sac aspirates). Anaerobic media should be inoculated if there is a significant possibility of anaerobic pathogens. If infection with an uncommon, fastidious pathogen is suspected, the laboratory should be informed so that special cultures may be inoculated.
Method: Supportive and enriched solid agar (SBA and chocolate agar) and broth media (blood culture media) are typically inoculated; selective/differential agar media, such as MacConkey agar (gram-negative bacilli), CNA, or phenylethyl alcohol agar (gram-positive organisms), should be inoculated for specimens likely to show polymicrobial infection (e.g., peritonitis) or contamination by endogenous flora (e.g., cul-de-sac aspirates). Anaerobic media should be inoculated if there is a significant possibility of anaerobic pathogens. If infection with an uncommon, fastidious pathogen is suspected, the laboratory should be informed so that special cultures may be inoculated.
 Turnaround time: Cultures are incubated for up to 7 days. Additional time is required for isolation, identification, susceptibility testing, and further characterization, as needed.
Turnaround time: Cultures are incubated for up to 7 days. Additional time is required for isolation, identification, susceptibility testing, and further characterization, as needed.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 Fluid aspiration is performed after preparation of the puncture site in a manner consistent with a preparation of a surgical site. Submission of specimens from drainage devices is discouraged because of the high incidence of contamination with endogenous flora; direct collection of the sterile fluid is recommended.
Fluid aspiration is performed after preparation of the puncture site in a manner consistent with a preparation of a surgical site. Submission of specimens from drainage devices is discouraged because of the high incidence of contamination with endogenous flora; direct collection of the sterile fluid is recommended.
 Collection of the maximum amount of fluid from the infected site is recommended. Blood culture bottles may be inoculated and is recommended for patients with spontaneous bacterial peritonitis, but a small amount of fluid should be retained for Gram stain and for special culture inoculation, if needed.
Collection of the maximum amount of fluid from the infected site is recommended. Blood culture bottles may be inoculated and is recommended for patients with spontaneous bacterial peritonitis, but a small amount of fluid should be retained for Gram stain and for special culture inoculation, if needed.
 Swabs should not be used for fluid collection.
Swabs should not be used for fluid collection.
 Place fluid into sterile transport tubes; small-volume specimens, or several milliliters from large-volume specimens, should be placed into an anaerobic transport tube. Note: Specimens transported under anaerobic conditions are acceptable for inoculation of cultures for aerobic bacterial, mycobacterial, and fungal cultures.
Place fluid into sterile transport tubes; small-volume specimens, or several milliliters from large-volume specimens, should be placed into an anaerobic transport tube. Note: Specimens transported under anaerobic conditions are acceptable for inoculation of cultures for aerobic bacterial, mycobacterial, and fungal cultures.
 Submission of several specimens prior to antibiotic therapy may significantly improve sensitivity of culture detection.
Submission of several specimens prior to antibiotic therapy may significantly improve sensitivity of culture detection.
 The use of anticoagulants is discouraged because of possible inhibition of some pathogens. If anticoagulation is required, heparin or SPS is recommended.
The use of anticoagulants is discouraged because of possible inhibition of some pathogens. If anticoagulation is required, heparin or SPS is recommended.
 Transport specimens at room temperature; do not refrigerate or freeze specimens.
Transport specimens at room temperature; do not refrigerate or freeze specimens.
 Interpretation
Interpretation
 Expected results: No growth. Infection is not excluded by a negative culture, especially after initiation antibiotic therapy. Uncommon, fastidious pathogens may not be isolated in culture without inoculation of special media.
Expected results: No growth. Infection is not excluded by a negative culture, especially after initiation antibiotic therapy. Uncommon, fastidious pathogens may not be isolated in culture without inoculation of special media.
 Positive cultures indicate infection of the sterile site, but cultures that may be contaminated with endogenous flora must be interpreted with caution in the context of quantity or bacterial growth, purity of culture, Gram stain findings, and clinical signs and symptoms. Infected peritoneal fluid may yield numerous aerobic and anaerobic pathogens. Extensive identification and susceptibility testing are usually not clinically useful: final results are often not available until well into therapy, and empirical treatment is usually effective.
Positive cultures indicate infection of the sterile site, but cultures that may be contaminated with endogenous flora must be interpreted with caution in the context of quantity or bacterial growth, purity of culture, Gram stain findings, and clinical signs and symptoms. Infected peritoneal fluid may yield numerous aerobic and anaerobic pathogens. Extensive identification and susceptibility testing are usually not clinically useful: final results are often not available until well into therapy, and empirical treatment is usually effective.
Suggested Readings
Atkins BL, Athanasou N, Deeks JJ, et al.; the Osiris Collaborative Study Group. Prospective evaluation of criteria for microbiologic diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol. 1998;36:2932–2939.
Baselski V, Beavis KG, Bell M, et al. Clinical Microbiology Procedures Handbook, 3rd ed. Editor in Chief: Lynne S. Garcia, Washington, DC: ASM Press; 2010.
Bernard L, Pron B, Vaugnat A, et al.; the Groupe d’Etude sur l’Osteite. The value of suction drainage fluid culture during aseptic and septic orthopedic surgery: a prospective study of 901 patients. Clin Infect Dis. 2002;34:46–49.
BORDETELLA PERTUSSIS CULTURE (RULE OUT)
 Definition and Use
Definition and Use
 This test is used to detect acute infection caused by the slow-growing, fastidious pathogen B. pertussis, the cause of pertussis or whooping cough. Nasopharyngeal specimens should be submitted for B. pertussis culture; aspirates are preferred. Anterior nares or throat specimens are unacceptable. Transport medium (e.g., Regan-Lowe) is recommended. Specimens are typically inoculated onto an enriched, selective agar, like Regan-Lowe.
This test is used to detect acute infection caused by the slow-growing, fastidious pathogen B. pertussis, the cause of pertussis or whooping cough. Nasopharyngeal specimens should be submitted for B. pertussis culture; aspirates are preferred. Anterior nares or throat specimens are unacceptable. Transport medium (e.g., Regan-Lowe) is recommended. Specimens are typically inoculated onto an enriched, selective agar, like Regan-Lowe.
 Turnaround time: Most cultures are positive in 7–10 days, although some cultures are incubated for up to 14 days. Additional time is required for final identification and further characterization.
Turnaround time: Most cultures are positive in 7–10 days, although some cultures are incubated for up to 14 days. Additional time is required for final identification and further characterization.
 Interpretation
Interpretation
 Expected results: Negative. A negative culture does not exclude the diagnosis of pertussis, especially when a specimen is collected after the early, acute phase of infection.
Expected results: Negative. A negative culture does not exclude the diagnosis of pertussis, especially when a specimen is collected after the early, acute phase of infection.
 Positive results: Confirm the diagnosis of pertussis.
Positive results: Confirm the diagnosis of pertussis.
 Limitations
Limitations
 The sensitivity of culture for B. pertussis falls significantly after the first 7–14 days after onset of symptoms. Poor specimen collection, submission of specimens other than nasopharyngeal specimens, and submission of specimens during the chronic phase of disease are associated with poor sensitivity of culture.
The sensitivity of culture for B. pertussis falls significantly after the first 7–14 days after onset of symptoms. Poor specimen collection, submission of specimens other than nasopharyngeal specimens, and submission of specimens during the chronic phase of disease are associated with poor sensitivity of culture.
 Other Considerations
Other Considerations
 PCR methods have been described for diagnosis of pertussis. Cross-reactions have been described (e.g., Bordetella holmesii) and may limit the utility of molecular diagnostic testing. The sensitivity of PCR is greatest in the early acute phase of infection, but B. pertussis DNA may be detectable for weeks after resolution of acute disease. A number of serologic assays are commercially available, including assays for IgM and IgA. Variable sensitivity and specificity have limited the clinical utility of these assays.
PCR methods have been described for diagnosis of pertussis. Cross-reactions have been described (e.g., Bordetella holmesii) and may limit the utility of molecular diagnostic testing. The sensitivity of PCR is greatest in the early acute phase of infection, but B. pertussis DNA may be detectable for weeks after resolution of acute disease. A number of serologic assays are commercially available, including assays for IgM and IgA. Variable sensitivity and specificity have limited the clinical utility of these assays.
BORDETELLA PERTUSSIS SEROLOGY IgG
 Definition
Definition
 Pertussis is a respiratory tract infection caused by the gram-negative coccobacillus Bordetella pertussis. It is characterized clinically by a severe and prolonged cough. Coughing fits may be paroxysmal and, usually in infants, followed by an inspirational “whoop.” A clinical diagnosis will form the basis of most pertussis diagnosis and treatment decisions. The CDC provides the following clinical case definition for pertussis: A cough illness lasting at least 2 weeks with one of the following: paroxysms of coughing, inspiratory “whoop,” or post-tussive vomiting, without other apparent cause. This test is used to detect acute infection caused by the slow-growing, fastidious pathogen B. pertussis, the cause of pertussis or whooping cough. Because of the contagiousness of the infection, specific laboratory testing may be needed to confirm the diagnosis when pertussis is suspected clinically. Laboratory diagnosis of pertussis is complicated by the limitation of available tests. Options for diagnostic and confirmatory testing, when required, depend on the age of the patient and the phase of illness.
Pertussis is a respiratory tract infection caused by the gram-negative coccobacillus Bordetella pertussis. It is characterized clinically by a severe and prolonged cough. Coughing fits may be paroxysmal and, usually in infants, followed by an inspirational “whoop.” A clinical diagnosis will form the basis of most pertussis diagnosis and treatment decisions. The CDC provides the following clinical case definition for pertussis: A cough illness lasting at least 2 weeks with one of the following: paroxysms of coughing, inspiratory “whoop,” or post-tussive vomiting, without other apparent cause. This test is used to detect acute infection caused by the slow-growing, fastidious pathogen B. pertussis, the cause of pertussis or whooping cough. Because of the contagiousness of the infection, specific laboratory testing may be needed to confirm the diagnosis when pertussis is suspected clinically. Laboratory diagnosis of pertussis is complicated by the limitation of available tests. Options for diagnostic and confirmatory testing, when required, depend on the age of the patient and the phase of illness.
 Expected result: Negative.
Expected result: Negative.
 Use
Use
 Aids in the detection of B. pertussis infection. Serologic testing for B. pertussis infection involves the detection of antibodies to pertussis antigens using standardized assays. Pertussis toxin (PT) and filamentous hemagglutinin (FHA) are the most widely used antigens and to a lesser extent pertactin (PRN) and fimbriae (FIM). Only PT is specific for B. pertussis; FHA and pertactin antigens cross-react with antibodies arising from infection by other Bordetella species and possibly by other bacteria. Serum antibodies have been measured by ELISA, complement fixation, agglutination, and toxin neutralization; ELISA is the detection method of choice due to its wide availability and ease of performance.
Aids in the detection of B. pertussis infection. Serologic testing for B. pertussis infection involves the detection of antibodies to pertussis antigens using standardized assays. Pertussis toxin (PT) and filamentous hemagglutinin (FHA) are the most widely used antigens and to a lesser extent pertactin (PRN) and fimbriae (FIM). Only PT is specific for B. pertussis; FHA and pertactin antigens cross-react with antibodies arising from infection by other Bordetella species and possibly by other bacteria. Serum antibodies have been measured by ELISA, complement fixation, agglutination, and toxin neutralization; ELISA is the detection method of choice due to its wide availability and ease of performance.
 Although B. pertussis serology is most useful in epidemiologic investigations or vaccine trials, it does have some utility in the diagnosis of pertussis in some patients, particularly in adolescents, adults, and previously vaccinated individuals. Serologic testing may also be useful for patients with cough >2–3 weeks in duration. Antibodies can be detected against B. pertussis antigens 1–2 weeks after the onset of the symptoms of pertussis in nonvaccinated individuals. Both IgG and IgA isotypes are produced in response to infection, whereas IgG is the predominant isotype detected after vaccination. However, no single antigen or isotype can be used to distinguish between infection and a response to vaccination with certainty. IgM responses are usually not measured for pertussis and have questionable diagnostic significance.
Although B. pertussis serology is most useful in epidemiologic investigations or vaccine trials, it does have some utility in the diagnosis of pertussis in some patients, particularly in adolescents, adults, and previously vaccinated individuals. Serologic testing may also be useful for patients with cough >2–3 weeks in duration. Antibodies can be detected against B. pertussis antigens 1–2 weeks after the onset of the symptoms of pertussis in nonvaccinated individuals. Both IgG and IgA isotypes are produced in response to infection, whereas IgG is the predominant isotype detected after vaccination. However, no single antigen or isotype can be used to distinguish between infection and a response to vaccination with certainty. IgM responses are usually not measured for pertussis and have questionable diagnostic significance.
 The most reliable serologic approach to diagnosis of pertussis is with simultaneous testing of paired acute and convalescent sera. A significant increase (fourfold or greater) in IgG or IgA antibody titers to PT or FHA, comparing convalescent to acute sample, suggests recent B. pertussis infection in patients with a clinical illness compatible with pertussis. Paired sera, however, are not practical in most clinical settings. Single-sample serology tests for antipertussis toxin IgG must be collected at least 2 weeks after symptom onset. A high antibody titer >2 years following vaccination supports the diagnosis of pertussis.
The most reliable serologic approach to diagnosis of pertussis is with simultaneous testing of paired acute and convalescent sera. A significant increase (fourfold or greater) in IgG or IgA antibody titers to PT or FHA, comparing convalescent to acute sample, suggests recent B. pertussis infection in patients with a clinical illness compatible with pertussis. Paired sera, however, are not practical in most clinical settings. Single-sample serology tests for antipertussis toxin IgG must be collected at least 2 weeks after symptom onset. A high antibody titer >2 years following vaccination supports the diagnosis of pertussis.
 Interpretation
Interpretation
 Positive: IgG antibody to B. pertussis detected, which may indicate a current or past exposure/immunization to B. pertussis.
Positive: IgG antibody to B. pertussis detected, which may indicate a current or past exposure/immunization to B. pertussis.
 Limitations
Limitations
 The CDC does not currently accept serology as laboratory verification of pertussis; cases that meet the clinical case definition with a positive serology but a negative culture or PCR are considered probable cases. Single serology tests are used for the diagnosis of pertussis by the state laboratory in Massachusetts and in selected countries in the European Union.
The CDC does not currently accept serology as laboratory verification of pertussis; cases that meet the clinical case definition with a positive serology but a negative culture or PCR are considered probable cases. Single serology tests are used for the diagnosis of pertussis by the state laboratory in Massachusetts and in selected countries in the European Union.
 The IgG serology test results are not interpretable in children younger than 11 years of age because of interference due to persistent antibody formed by childhood vaccination. The test also cannot be interpreted in older patients who have received the Tdap vaccination in the previous 3 years.
The IgG serology test results are not interpretable in children younger than 11 years of age because of interference due to persistent antibody formed by childhood vaccination. The test also cannot be interpreted in older patients who have received the Tdap vaccination in the previous 3 years.
BORRELIA BURGDORFERI (LYME DISEASE)—ANTIBODY SCREEN
 Definition
Definition
 A sensitive serum serology screening test for the detection of IgG and/or IgM antibodies to Borrelia burgdorferi.
A sensitive serum serology screening test for the detection of IgG and/or IgM antibodies to Borrelia burgdorferi.
 Use
Use
 This test is used if Lyme disease is suspected in at-risk patients. Testing is not necessary if a patient presents with a tick bite and erythema migrans.
This test is used if Lyme disease is suspected in at-risk patients. Testing is not necessary if a patient presents with a tick bite and erythema migrans.
 Interpretation
Interpretation
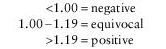
Note: Current CDC recommendations state that equivocal and positive results should be confirmed with Western blot prior to reporting screen results. If testing is negative, consider other tick-borne diseases (i.e., Babesia, Ehrlichia).
 Limitations
Limitations
 Should not be used to screen general population. False-negative results can occur if the patient is tested too early; repeat testing in 2–4 weeks. The IgG antibody response is usually not detectable until 4–6 weeks after infection; the IgM antibody response usually not detected during the first 2 weeks of infection, peaking 3–6 weeks following infection. False-positive results may occur from other spirochetal diseases, autoimmune diseases, or other infections (EBV, HIV, syphilis, infectious mononucleosis, etc.). IgG antibodies can be detected as early as 2 weeks, and both IgM and IgG antibodies can remain detectable for years. Diagnosis depends on clinical features, combined with available laboratory tests.
Should not be used to screen general population. False-negative results can occur if the patient is tested too early; repeat testing in 2–4 weeks. The IgG antibody response is usually not detectable until 4–6 weeks after infection; the IgM antibody response usually not detected during the first 2 weeks of infection, peaking 3–6 weeks following infection. False-positive results may occur from other spirochetal diseases, autoimmune diseases, or other infections (EBV, HIV, syphilis, infectious mononucleosis, etc.). IgG antibodies can be detected as early as 2 weeks, and both IgM and IgG antibodies can remain detectable for years. Diagnosis depends on clinical features, combined with available laboratory tests.
Suggested Reading
FDA Public Health Advisory: Assays for Antibodies to Borrelia burgdorferi; Limitations, Use, and Interpretation for Supporting a Clinical Diagnosis of Lyme Disease. July 7, 1997. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/UCM062429
BORRELIA BURGDORFERI (LYME DISEASE)—WESTERN BLOT*
 Definition
Definition
 The western blot assay for antibodies to Borrelia burgdorferi, the etiologic agent of Lyme disease, is a qualitative method of categorizing specific immuno-reactivities in serum or plasma to B. burgdorferi proteins that have been formatted according to molecular weight into discrete bands on nitrocellulose strips.
The western blot assay for antibodies to Borrelia burgdorferi, the etiologic agent of Lyme disease, is a qualitative method of categorizing specific immuno-reactivities in serum or plasma to B. burgdorferi proteins that have been formatted according to molecular weight into discrete bands on nitrocellulose strips.
 Normal range: Negative.
Normal range: Negative.
 Use
Use
 The western blot assay for B. burgdorferi is used as a second-tier test to characterize the specificity of an individual’s immune response to the component proteins of B. burgdorferi by identifying the presence, relative level, and pattern of reactivities to the complete set of the bacterial proteins. The assay is used routinely to provide supportive serologic evidence of infection following a more sensitive but less specific screening test (such as EIA) for general reactivity to B. burgdorferi. Both IgM and IgG reactivities to the bacterial proteins are assayed to provide information on the evolution of the immune response relative to the stage of infection (i.e., early localized, early disseminated, or late disseminated). A caveat to this use is that IgM testing is not recommended in patients with symptoms lasting greater than 1–2 months. For such patients, IgG testing alone should be performed.
The western blot assay for B. burgdorferi is used as a second-tier test to characterize the specificity of an individual’s immune response to the component proteins of B. burgdorferi by identifying the presence, relative level, and pattern of reactivities to the complete set of the bacterial proteins. The assay is used routinely to provide supportive serologic evidence of infection following a more sensitive but less specific screening test (such as EIA) for general reactivity to B. burgdorferi. Both IgM and IgG reactivities to the bacterial proteins are assayed to provide information on the evolution of the immune response relative to the stage of infection (i.e., early localized, early disseminated, or late disseminated). A caveat to this use is that IgM testing is not recommended in patients with symptoms lasting greater than 1–2 months. For such patients, IgG testing alone should be performed.
 Interpretation
Interpretation
 Reactivity scores: Specimen reactions with protein bands are first scored in terms of relative reaction intensity versus a cutoff control or minimally positive (“+”) band reaction intensity by a positive control specimen.
Reactivity scores: Specimen reactions with protein bands are first scored in terms of relative reaction intensity versus a cutoff control or minimally positive (“+”) band reaction intensity by a positive control specimen.
 Test interpretation (IgM class reactivities)
Test interpretation (IgM class reactivities)
 Positive: Reactivity scores of “+” or greater for at least two of three clinically significant proteins at the early stage of the disease (2–3 weeks after infection): 41, 39, 23 kDa
Positive: Reactivity scores of “+” or greater for at least two of three clinically significant proteins at the early stage of the disease (2–3 weeks after infection): 41, 39, 23 kDa
 Negative: Absence of any band reactivity on the test strip or reactivity for only one of the three clinically significant proteins
Negative: Absence of any band reactivity on the test strip or reactivity for only one of the three clinically significant proteins
 Test interpretation (IgG class reactivities)
Test interpretation (IgG class reactivities)
 Positive: Reactivity scores of “+” or greater for at least 5 of 10 clinically significant proteins at the later stages of the disease (weeks to months after infection): 93, 66, 58, 45, 41, 39, 30, 28, 23, 18 kDa
Positive: Reactivity scores of “+” or greater for at least 5 of 10 clinically significant proteins at the later stages of the disease (weeks to months after infection): 93, 66, 58, 45, 41, 39, 30, 28, 23, 18 kDa
 Negative: Absence of any band reactivity on the test strip or reactivity for <5 of the 10 clinically significant proteins
Negative: Absence of any band reactivity on the test strip or reactivity for <5 of the 10 clinically significant proteins
 Limitations
Limitations
 Minimum specimen volume is 40 μL (20 μL each for the IgM and IgG tests).
Minimum specimen volume is 40 μL (20 μL each for the IgM and IgG tests).
 Like any second-tier test, the positive predictive value for a western blot assay is a function of the a priori likelihood of the disease by clinical and epidemiologic criteria, whereas the negative predictive value is not as well defined because of the variability of the immune response among infected individuals. Cross-reactive diseases are most frequently evidenced by reactivity to the 41-kDa flagellar protein and at much lower frequency to the 66-kDa heat shock protein. Specimens from patients diagnosed with Ehrlichia or Babesia infections can show other Borrelia-specific bands.
Like any second-tier test, the positive predictive value for a western blot assay is a function of the a priori likelihood of the disease by clinical and epidemiologic criteria, whereas the negative predictive value is not as well defined because of the variability of the immune response among infected individuals. Cross-reactive diseases are most frequently evidenced by reactivity to the 41-kDa flagellar protein and at much lower frequency to the 66-kDa heat shock protein. Specimens from patients diagnosed with Ehrlichia or Babesia infections can show other Borrelia-specific bands.
Suggested Reading
Branda JA, Aguero-Rosenfeld ME, Ferraro MJ, et al. 2-tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VisE band as the second-tier test. Clin Infect Dis. 2010;50:20–26.
BRONCHIAL CULTURE (BAL OR BRUSH), QUANTITATIVE
 Definition
Definition
 Quantitative bacterial cultures of specimens collected bronchoscopically (BAL or protected brush) are usually submitted for the evaluation for ventilator-associated pneumonia (VAP). The diagnosis of VAP is challenging, requiring a combination of clinical, imaging, and laboratory studies. Cultures are assessed in comparison to thresholds established by the laboratory in collaboration with clinicians.
Quantitative bacterial cultures of specimens collected bronchoscopically (BAL or protected brush) are usually submitted for the evaluation for ventilator-associated pneumonia (VAP). The diagnosis of VAP is challenging, requiring a combination of clinical, imaging, and laboratory studies. Cultures are assessed in comparison to thresholds established by the laboratory in collaboration with clinicians.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 Protected brush and BAL specimens are collected by a trained physician using standard procedures.
Protected brush and BAL specimens are collected by a trained physician using standard procedures.
 Brush:
Brush:
 The brush is inserted through a plugged catheter via the biopsy channel of the bronchoscope. After expulsion of the plug, the brush is used to collect cells and secretions from the distal airways.
The brush is inserted through a plugged catheter via the biopsy channel of the bronchoscope. After expulsion of the plug, the brush is used to collect cells and secretions from the distal airways.
 The brush end should be removed, using sterile technique, and placed in a small volume (1 mL) of nonbacteriostatic saline for transport.
The brush end should be removed, using sterile technique, and placed in a small volume (1 mL) of nonbacteriostatic saline for transport.
 BAL:
BAL:
 BAL specimens are collected by a trained physician using standard procedures. The procedure and placement of the tip may be done under direct visualization or “blindly” through an endotracheal tube (mini-BAL).
BAL specimens are collected by a trained physician using standard procedures. The procedure and placement of the tip may be done under direct visualization or “blindly” through an endotracheal tube (mini-BAL).
 The bronchoscope should be wedged in the terminal airways to ensure sampling of alveolar contents; return from the procedure should be 10–100 mL, sampling approximately 1 mL of alveolar secretions.
The bronchoscope should be wedged in the terminal airways to ensure sampling of alveolar contents; return from the procedure should be 10–100 mL, sampling approximately 1 mL of alveolar secretions.
 Samples should be transported to the laboratory as quickly as possible, using standard protocols for bacterial cultures.
Samples should be transported to the laboratory as quickly as possible, using standard protocols for bacterial cultures.
 Use
Use
 Method:
Method:
 Known volumes of the specimen (or specimen dilutions) are inoculated onto solid agar media, including SBA, chocolate, and MacConkey agar (and other media as required for uncommon pathogens, such as Legionella); quantitative results are reported on the basis of the number of colonies isolated.
Known volumes of the specimen (or specimen dilutions) are inoculated onto solid agar media, including SBA, chocolate, and MacConkey agar (and other media as required for uncommon pathogens, such as Legionella); quantitative results are reported on the basis of the number of colonies isolated.
 Protected brush: The brush is vigorously agitated in the saline transport fluid to release trapped microorganisms. The saline is then used to prepare dilutions for media inoculation.
Protected brush: The brush is vigorously agitated in the saline transport fluid to release trapped microorganisms. The saline is then used to prepare dilutions for media inoculation.
 BAL: A measured aliquot of BAL fluid is used to prepare dilutions for media inoculation.
BAL: A measured aliquot of BAL fluid is used to prepare dilutions for media inoculation.
 After incubation, the concentration of each type of organism is calculated using the colony count on the solid media, volume inoculated onto the solid media, and the dilution of the original specimen. Cultures are interpreted on the basis of the isolate identification, quantity of isolate in culture, and the presence of other flora, especially endogenous flora of low pathogenicity.
After incubation, the concentration of each type of organism is calculated using the colony count on the solid media, volume inoculated onto the solid media, and the dilution of the original specimen. Cultures are interpreted on the basis of the isolate identification, quantity of isolate in culture, and the presence of other flora, especially endogenous flora of low pathogenicity.
 Turnaround time: Incubation for 48 hours. Additional time is required for pathogen isolation, identification, susceptibility testing, and further characterization, if needed.
Turnaround time: Incubation for 48 hours. Additional time is required for pathogen isolation, identification, susceptibility testing, and further characterization, if needed.
 Interpretation
Interpretation
 Expected results: A low quantity of endogenous upper respiratory flora is often seen.
Expected results: A low quantity of endogenous upper respiratory flora is often seen.
 Positive results: In patients with pneumonia, growth of a respiratory pathogen is expected at a concentration of >103 CFU/mL for bronchial brush. Growth of a respiratory pathogen is expected at a concentration of >104 CFU/mL for visually guided BAL or >105–106 for blind mini-BAL.
Positive results: In patients with pneumonia, growth of a respiratory pathogen is expected at a concentration of >103 CFU/mL for bronchial brush. Growth of a respiratory pathogen is expected at a concentration of >104 CFU/mL for visually guided BAL or >105–106 for blind mini-BAL.
 Negative results: False-negative cultures may be caused by prior antimicrobial therapy. Detection of pneumonia caused by certain fastidious pathogens may require inoculation of special media. Heavy contamination of the specimen with endogenous flora may mask the growth of the causative pathogen.
Negative results: False-negative cultures may be caused by prior antimicrobial therapy. Detection of pneumonia caused by certain fastidious pathogens may require inoculation of special media. Heavy contamination of the specimen with endogenous flora may mask the growth of the causative pathogen.
 Limitations
Limitations
 Quantitative culture of protected brush specimens has only moderate to good performance, with the PPV and NPV of 74% and 85%, respectively. Quantitative culture of BAL has only moderate to good performance, with a PPV of 83–91% and NPV of 87–89%. The presence of intracellular organisms in >5% of cells is associated with higher specificities. Tissue histopathology and quantitative culture of biopsy are considered the “gold standard” for diagnosis.
Quantitative culture of protected brush specimens has only moderate to good performance, with the PPV and NPV of 74% and 85%, respectively. Quantitative culture of BAL has only moderate to good performance, with a PPV of 83–91% and NPV of 87–89%. The presence of intracellular organisms in >5% of cells is associated with higher specificities. Tissue histopathology and quantitative culture of biopsy are considered the “gold standard” for diagnosis.
 Common pitfalls:
Common pitfalls:
 The predictive value of cultures is markedly decreased for patients with any antibiotic therapy prior to the procedure.
The predictive value of cultures is markedly decreased for patients with any antibiotic therapy prior to the procedure.
 Candida species are common contaminants and should not routinely be identified to species level.
Candida species are common contaminants and should not routinely be identified to species level.
Suggested Readings
Carroll KC. Laboratory diagnosis of lower respiratory tract infections: controversy and conundrums. J Clin Microbiol. 2002;40:3115–3120.
Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19:637–657.
BRUCELLA CULTURE (RULE OUT)
 Definition
Definition
 Human infection may be caused by several species of the genus Brucella. These organisms are fastidious, slow-growing gram-negative bacilli capable of producing severe localized and systemic infection. Infections have typically been acquired by zoonotic transmission, primarily related to livestock and dairy industries. There is great concern regarding the use of this organism for a bioterror-related attack. The organism is easily transmissible, so it is critical that the laboratory be informed whenever brucellosis is suspected.
Human infection may be caused by several species of the genus Brucella. These organisms are fastidious, slow-growing gram-negative bacilli capable of producing severe localized and systemic infection. Infections have typically been acquired by zoonotic transmission, primarily related to livestock and dairy industries. There is great concern regarding the use of this organism for a bioterror-related attack. The organism is easily transmissible, so it is critical that the laboratory be informed whenever brucellosis is suspected.
 Use
Use
 This culture is used to isolate Brucella species from clinical specimens. Because of the risk of laboratory-acquired infection and because isolation of Brucella species may represent a sentinel event in a bioterror attack, most clinical microbiology laboratories limit the workup of suspected isolates to simple tests to rule out suspicious colonies, referring isolates that fail to “rule out” to their local public health laboratory for identification and further characterization. Final results for testing, therefore, may be delayed compared to common bacterial isolates.
This culture is used to isolate Brucella species from clinical specimens. Because of the risk of laboratory-acquired infection and because isolation of Brucella species may represent a sentinel event in a bioterror attack, most clinical microbiology laboratories limit the workup of suspected isolates to simple tests to rule out suspicious colonies, referring isolates that fail to “rule out” to their local public health laboratory for identification and further characterization. Final results for testing, therefore, may be delayed compared to common bacterial isolates.
 Method: Specimens are inoculated onto a blood agar (such as Brucella blood agar), chocolate agar, and Thayer-Martin agar (if contamination with endogenous flora is suspected). Specimens for Brucella are also inoculated onto MacConkey agar.
Method: Specimens are inoculated onto a blood agar (such as Brucella blood agar), chocolate agar, and Thayer-Martin agar (if contamination with endogenous flora is suspected). Specimens for Brucella are also inoculated onto MacConkey agar.
 Turnaround time: Isolation and preliminary identification for routine cultures are usually available in 3–7 days. Additional time is required for transfer to the local public health laboratory, confirmation of identification, and further testing.
Turnaround time: Isolation and preliminary identification for routine cultures are usually available in 3–7 days. Additional time is required for transfer to the local public health laboratory, confirmation of identification, and further testing.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 The organisms primarily infect the reticuloendothelial system, so bone marrow and blood are the specimens of choice for patient evaluation. Specimens from other infected tissue or sites should also be submitted for culture. Serologic testing is recommended for diagnosis in patients with suspected brucellosis.
The organisms primarily infect the reticuloendothelial system, so bone marrow and blood are the specimens of choice for patient evaluation. Specimens from other infected tissue or sites should also be submitted for culture. Serologic testing is recommended for diagnosis in patients with suspected brucellosis.
 Interpretation
Interpretation
 Expected results: Negative.
Expected results: Negative.
 Positive: Isolation of Brucella in culture is diagnostic for brucellosis.
Positive: Isolation of Brucella in culture is diagnostic for brucellosis.
 Limitations
Limitations
 Brucella may be difficult to detect by Gram stain in primary specimens.
Brucella may be difficult to detect by Gram stain in primary specimens.
 Common pitfalls: Because brucellosis may present after a prolonged incubation period, or present with nonspecific symptoms and an indolent onset, the diagnosis may not be considered until progression into the chronic phase of illness. Clinicians may fail to request specific cultures for brucellosis, or alert the laboratory of their clinical suspicion.
Common pitfalls: Because brucellosis may present after a prolonged incubation period, or present with nonspecific symptoms and an indolent onset, the diagnosis may not be considered until progression into the chronic phase of illness. Clinicians may fail to request specific cultures for brucellosis, or alert the laboratory of their clinical suspicion.
 Other Considerations
Other Considerations
 Brucellosis is a reportable disease. Patients with a diagnosis of brucellosis must be reported to the local department of health.
Brucellosis is a reportable disease. Patients with a diagnosis of brucellosis must be reported to the local department of health.
CEREBROSPINAL FLUID (CSF) CULTURE
 Definition and Use
Definition and Use
 CSF culture is used for specific diagnosis of bacterial meningitis. Patients commonly present with severe headache, fever, neck stiffness, and meningeal signs, mental status changes, and signs of systemic toxicity.
CSF culture is used for specific diagnosis of bacterial meningitis. Patients commonly present with severe headache, fever, neck stiffness, and meningeal signs, mental status changes, and signs of systemic toxicity.
 Method
Method
 CSF is inoculated onto sheep blood and chocolate agar, incubated aerobically. Broth media may be inoculated. Special media or culture conditions may be used for non–community-acquired meningitis, such as infections associated with trauma and prosthetic implants.
CSF is inoculated onto sheep blood and chocolate agar, incubated aerobically. Broth media may be inoculated. Special media or culture conditions may be used for non–community-acquired meningitis, such as infections associated with trauma and prosthetic implants.
 Turnaround time: Cultures are incubated for 96 hours. Additional time is required for isolate identification, susceptibility testing, and further characterization, as needed.
Turnaround time: Cultures are incubated for 96 hours. Additional time is required for isolate identification, susceptibility testing, and further characterization, as needed.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 CSF is collected by needle aspiration after preparation of the puncture site in a manner consistent with a surgical site preparation.
CSF is collected by needle aspiration after preparation of the puncture site in a manner consistent with a surgical site preparation.
 Fluid is transported in a sterile container or tube with a tight-fitting lid.
Fluid is transported in a sterile container or tube with a tight-fitting lid.
 CSF should be transported at room temperature; do not refrigerate or freeze for transport.
CSF should be transported at room temperature; do not refrigerate or freeze for transport.
 Specimens submitted for bacterial culture are also acceptable for fungal or mycobacterial stains and culture, antigen testing, and VDRL, if sufficient volume of fluid is submitted.
Specimens submitted for bacterial culture are also acceptable for fungal or mycobacterial stains and culture, antigen testing, and VDRL, if sufficient volume of fluid is submitted.
 Interpretation
Interpretation
 Expected results: No growth. False-negative cultures may be caused by low pathogen concentration in CSF, especially when low-volume samples are submitted, or prior antibiotic therapy.
Expected results: No growth. False-negative cultures may be caused by low pathogen concentration in CSF, especially when low-volume samples are submitted, or prior antibiotic therapy.
 Positive results: Positive CSF culture supports a specific diagnosis of meningitis. False-positive cultures may be caused by contamination with endogenous skin flora. For most bacterial pathogens, CSF samples in patients with acute bacterial meningitis usually show increased WBCs (PMNs predominate), increased protein, and decreased glucose.
Positive results: Positive CSF culture supports a specific diagnosis of meningitis. False-positive cultures may be caused by contamination with endogenous skin flora. For most bacterial pathogens, CSF samples in patients with acute bacterial meningitis usually show increased WBCs (PMNs predominate), increased protein, and decreased glucose.
 Limitations
Limitations
 A broad etiology, which may require a number of different tests for diagnosis, may be considered for patients presenting with signs and symptoms of meningitis. The volume of CSF submitted is often insufficient for optimal sensitivity for the range of tests requested.
A broad etiology, which may require a number of different tests for diagnosis, may be considered for patients presenting with signs and symptoms of meningitis. The volume of CSF submitted is often insufficient for optimal sensitivity for the range of tests requested.
CHLAMYDIA TRACHOMATIS, AMPLIFIED NUCLEIC ACID DETECTION
See: Sexually Transmitted Infections, Molecular Diagnosis (Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis)
CHLAMYDIA TRACHOMATIS CULTURE
 Use
Use
 C. trachomatis is an obligate intracellular pathogen, and this culture may be used to diagnose C. trachomatis infections. Although tests based on nucleic acid amplification have emerged as the most sensitive methods for diagnosis of Chlamydia genital infections, Chlamydia culture is still required for specimen types for which molecular diagnostic tests have not been validated. Chlamydia cultures should also be performed in cases that may have legal implications, such as rape and child abuse.
C. trachomatis is an obligate intracellular pathogen, and this culture may be used to diagnose C. trachomatis infections. Although tests based on nucleic acid amplification have emerged as the most sensitive methods for diagnosis of Chlamydia genital infections, Chlamydia culture is still required for specimen types for which molecular diagnostic tests have not been validated. Chlamydia cultures should also be performed in cases that may have legal implications, such as rape and child abuse.
 Method:
Method:
 Infected cells from patient specimens are inoculated onto cultured eukaryotic cells, most commonly McCoy cells.
Infected cells from patient specimens are inoculated onto cultured eukaryotic cells, most commonly McCoy cells.
 Cultures are incubated for 48–72 hours.
Cultures are incubated for 48–72 hours.
 Positive cultures are now most commonly detected by staining fixed monolayers with specific anti–C. trachomatis antibodies; positive cultures
Positive cultures are now most commonly detected by staining fixed monolayers with specific anti–C. trachomatis antibodies; positive cultures
show staining of intracellular inclusions. The sensitivity of cultures for C. trachomatis detection is improved by blind subculture of a primary culture after the initial incubation.
 Turnaround time: Cultures are incubated for 72 hours. An additional 48–72 hours are required if primary cultures are subcultured prior to final examination.
Turnaround time: Cultures are incubated for 72 hours. An additional 48–72 hours are required if primary cultures are subcultured prior to final examination.
 Special Collection and Transport Instructions
Special Collection and Transport Instructions
 It is critical to collect infected epithelial cells from infected sites using toxicity-tested swabs or other device. Swabs may be premoistened with sterile nonbacteriostatic saline before specimen collection. Scrapings or biopsy specimens may be submitted for some specimen types. (See specimens below.)
It is critical to collect infected epithelial cells from infected sites using toxicity-tested swabs or other device. Swabs may be premoistened with sterile nonbacteriostatic saline before specimen collection. Scrapings or biopsy specimens may be submitted for some specimen types. (See specimens below.)
 Place specimens into a Chlamydia transport medium, such as 2-SP, and transport to the laboratory at 4°C. Deliver to the laboratory as quickly as possible.
Place specimens into a Chlamydia transport medium, such as 2-SP, and transport to the laboratory at 4°C. Deliver to the laboratory as quickly as possible.
 Specimens commonly submitted for Chlamydia culture come from the following sites:
Specimens commonly submitted for Chlamydia culture come from the following sites:
 Cervix: Remove excess mucus from the exocervix. Insert a new swab approximately 1 cm into the cervical canal and gently rotate for 10–15 seconds.
Cervix: Remove excess mucus from the exocervix. Insert a new swab approximately 1 cm into the cervical canal and gently rotate for 10–15 seconds.
 Urethra: Clean the distal urethra and meatus with a swab. Insert a new thinshafted swab 2–4 cm into the urethra and gently rotate for 10–15 seconds.
Urethra: Clean the distal urethra and meatus with a swab. Insert a new thinshafted swab 2–4 cm into the urethra and gently rotate for 10–15 seconds.
 Conjunctiva: Remove excess purulent discharge with a swab. With a new swab, gently rotate over the affected conjunctival surface.
Conjunctiva: Remove excess purulent discharge with a swab. With a new swab, gently rotate over the affected conjunctival surface.
 Anus: Insert a premoistened swab into the anorectal juncture and rotate gently. The swab should not be heavily stained with feces.
Anus: Insert a premoistened swab into the anorectal juncture and rotate gently. The swab should not be heavily stained with feces.
 Fallopian tube or epididymis: Place aspirate into an equal volume of Chlamydia transport media.
Fallopian tube or epididymis: Place aspirate into an equal volume of Chlamydia transport media.
 Respiratory tract (neonate): Place aspirate or wash into an equal volume of Chlamydia transport media.
Respiratory tract (neonate): Place aspirate or wash into an equal volume of Chlamydia transport media.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


