Cardiac Surgery
LEARNING OBJECTIVES
After studying this chapter the reader will be able to:
• Correlate cardiac surgical anatomy with correction of select cardiac defects
• Contrast the unique characteristic of the veins and arteries with regard to function and blood flow
• Discuss the properties, purposes, and problems associated with cardiac surgery
• Discuss diagnostic methods for determination of surgical interventions and approaches
• Identify tissue layers involved in opening and closing incisions used in cardiac surgery
• List the pharmacologic and hemostatic agents used during cardiac surgery
Overview
Cardiac surgery (History box) reflects individual and collaborative innovation, risk-taking, and problem-solving. Anastomotic techniques to join blood vessels, advances in the development of atraumatic vascular instruments, endovascular approaches in the repair of great vessels, and improvements in myocardial protection strategies represent a few of the many advances that have led to remarkable achievements in the surgical repair of congenital and acquired cardiovascular diseases (Stoney, 2008). Cardiac surgeries continue to evolve and reflect societal mandates for personal safety, enhanced functional outcomes, institutional efficiency, staff competence, and operational cost-effectiveness (Lytle, 2008). Evidence of these achievements can be seen in the rapid growth of endoscopic and robotic technology, minimally invasive procedures, off-pump techniques, combined (hybrid) surgical and percutaneous interventions, and molecular-level treatments for cardiovascular disease. Many newer procedures require smaller incisions, promote faster healing, and enable patients to achieve their desired functional outcomes more quickly.
These trends have not replaced the need for traditional techniques; rather, they have expanded treatment options for coronary artery disease (CAD), valvular dysfunction, thoracic aneurysms, conduction disturbances, congenital abnormalities (Sommer et al, 2008a, b, c), heart failure, intracardiac masses (Kuchukarian et al, 2007), and end-stage cardiac disease. Therapeutic options may employ laser, radiofrequency, and cryo energies. Stem cell therapy, used to induce cardiac neorevascularization at the cellular level (Menasche, 2009), and mechanical assist devices are effective alternatives to allograft transplant organs, which are in scarce supply. Great strides have been made to reduce mortality from coronary artery disease (Gardner, 2009; American Heart Association, 2009a), but significant challenges associated with the aging population, obesity, diabetes, and other risk factors continue to affect the cardiovascular system and the need for surgical treatment.
Surgical Anatomy
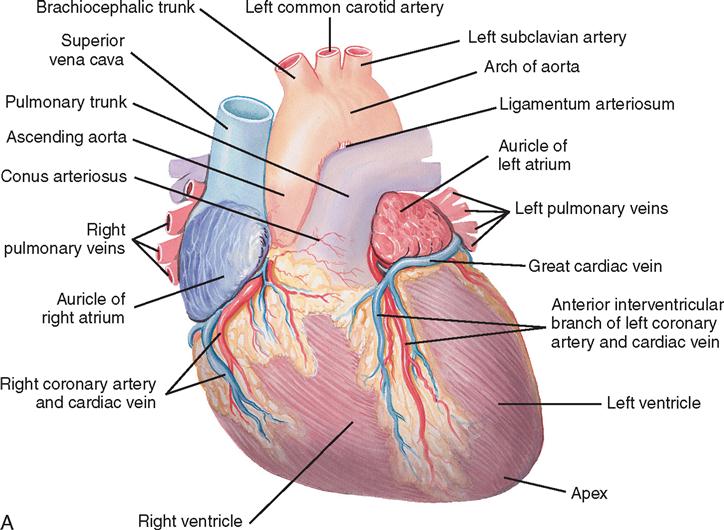
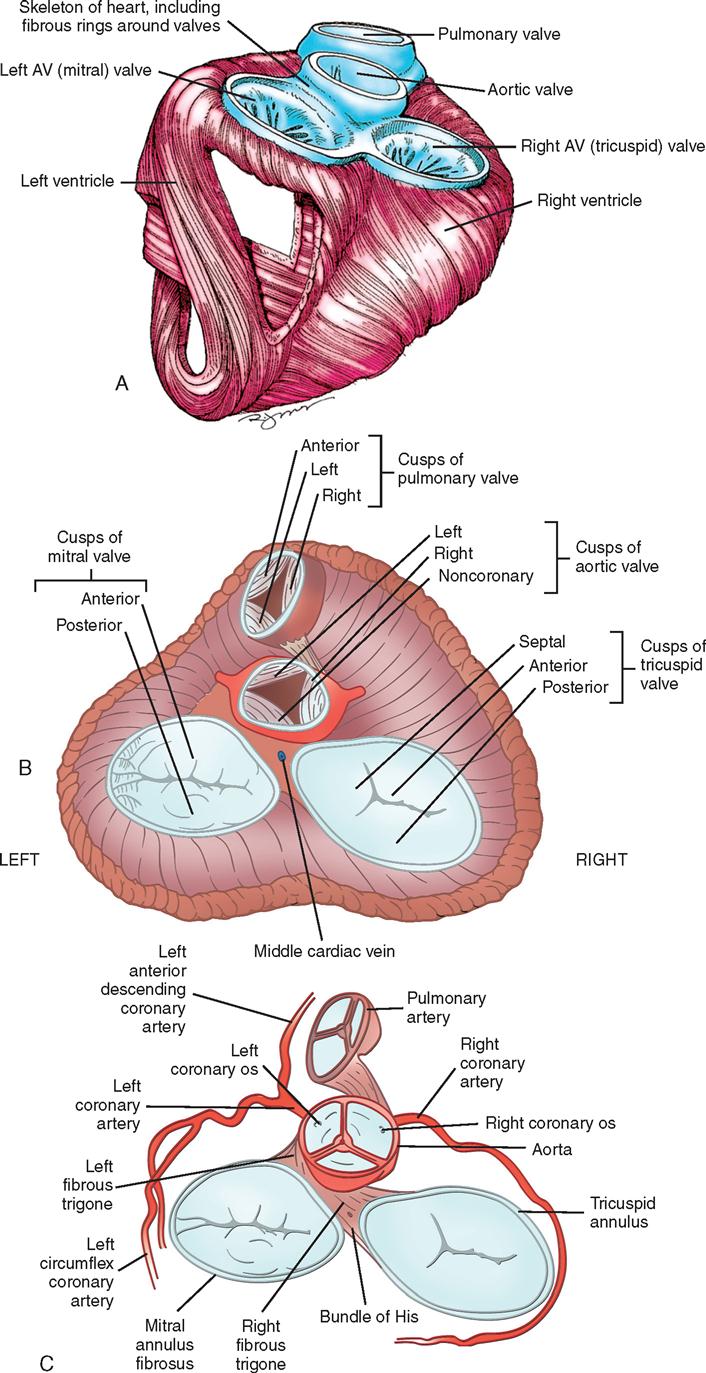
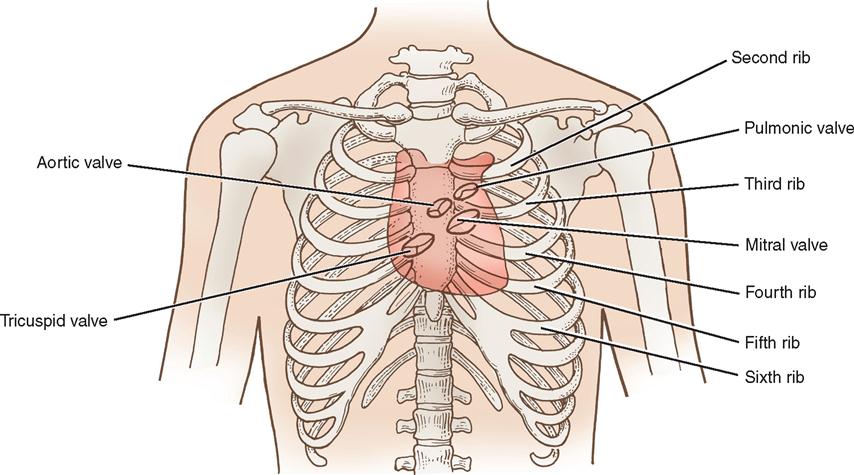
The heart (Figure 16-1) is a four-chamber muscular pump that propels blood into the systemic and pulmonary circulatory systems. It is enclosed in a pericardial sac within the mediastinum, which lies between the lungs, posterior to the sternum, and anterior to the vertebrae, esophagus, and descending portion of the aorta. The diaphragm is positioned below the heart (Figure 16-2). The cardiac wall is composed of three layers: the epicardium, the outer lining; the myocardium, or muscular layer, which is the important functional layer; and the endocardium, the inner lining (Figure 16-3).
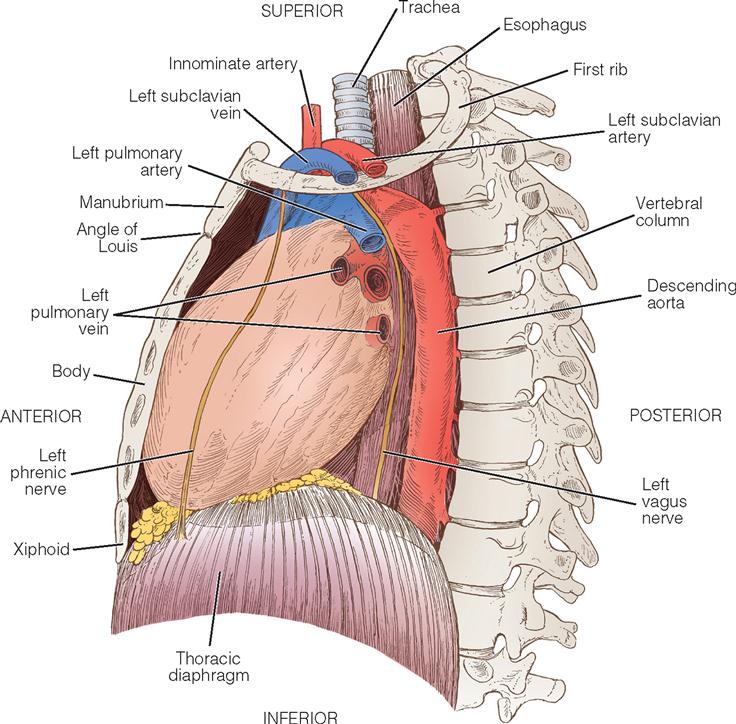
Two thirds of the heart is located to the left of the midline, and the remaining one third is to the right. Although functionally divided into right and left halves, the heart is rotated to the left, with the right side located anteriorly and the left side situated relatively posteriorly. Each half of the heart contains an upper and a lower communicating chamber: the atrium and the ventricle, respectively. The right atrium (RA) receives desaturated blood from the inferior and superior venae cavae and from the coronary circulation by way of the coronary sinus. The left atrium (LA) receives oxygenated blood from the lungs by way of the pulmonary veins. From the atria, blood flows through the atrioventricular (AV) valves into the ventricles.
The left ventricle (LV) pumps blood into the major vessels of the systemic circulatory system by way of the aorta and its main branches to the head, upper extremities, abdominal organs, and lower extremities. The right and left internal (thoracic) mammary arteries, used as grafts during coronary bypass surgery, branch off the subclavian arteries and course behind and parallel to the edges of the sternum. The arteries of the circulatory system subdivide into arterioles and eventually into capillaries, where internal respiration and metabolic exchange occur. From the capillary beds, desaturated blood flows into the venules and veins and finally returns to the RA.
In the pulmonary circulatory system, blood is pumped from the right ventricle (RV) through the pulmonary valve into the main pulmonary artery (PA). The PA divides into the right and left pulmonary arteries, which further subdivide into the arterioles and capillaries of the lungs. External respiration occurs in the capillary beds and the alveoli, where carbon dioxide is exchanged for oxygen. Freshly oxygenated blood from the lungs flows through the pulmonary veins into the left atrium.
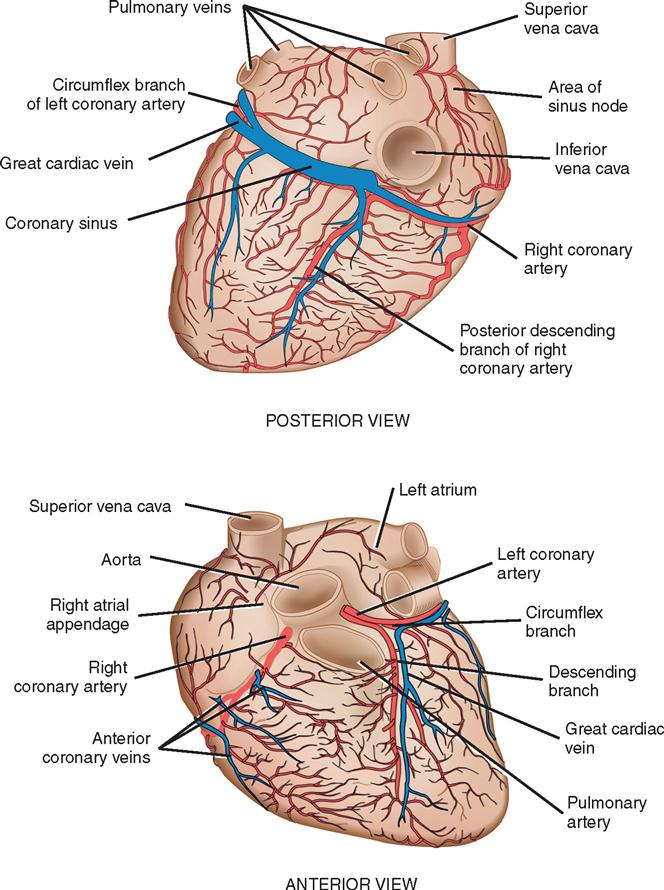
Coronary circulation (Figure 16-4) supplies oxygen and nutrients to, and removes metabolic waste from, the myocardium; internal respiration occurs in the myocytes. The heart receives its blood supply from the left and right coronary arteries, which originate in the sinuses of Valsalva behind the cusps of the aortic valve in the ascending aorta. The left main coronary artery divides into the left anterior descending (LAD) coronary artery and the circumflex coronary artery; along with the right coronary artery (RCA), these arteries represent the three main vessels of the coronary arterial system. In coronary arteries affected by coronary artery disease (CAD), focal or diffuse atherosclerotic plaques develop and progressively enlarge, jeopardizing myocardial blood flow and oxygenation, producing ischemic pain (in many, but not all, cases). Occlusion of an artery by expanding atherosclerotic lesions causes myocardial infarction (MI) and irreversible damage to the region of the myocardium perfused by the obstructed artery. Coronary artery bypass procedures increase blood flow to the affected ischemic areas by attaching a bypass graft conduit to the artery distal to the narrowed portion of the artery. A totally occluded (infarcted) artery does not benefit from bypass surgery because the myocardial injury is irreversible.
The main coronary arteries are situated in the epicardium, which facilitates accessibility during coronary bypass procedures. From these arteries arise the septal perforators and other branches that penetrate the entire myocardium. The cardiac veins empty into the right atrium by way of the coronary sinus; the thebesian veins, prominent in the walls of the right atrium and the right ventricle, open directly into these chambers.
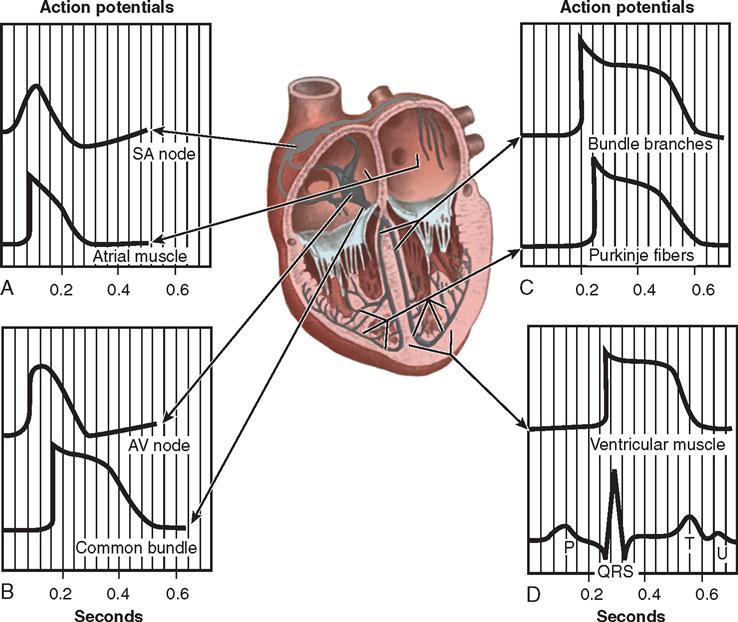
Nerve impulses to the heart travel from the medulla oblongata along the middle cervical nerve, composed of sympathetic fibers, and the vagus nerve, composed of parasympathetic fibers. Sympathetic fibers increase the force and rate of contraction, while parasympathetic fibers control heart rate. Running vertically along the right and left sides of the pericardium are major branches of the phrenic nerve, which innervate the diaphragm and stimulate it to contract. Identifying this nerve is important to protect the diaphragm in procedures in which the lateral pericardium is incised or excised. Within the myocardium itself, certain areas of tissue are modified to form a conduction system (Figure 16-5). The process of excitation and contraction originates in the sinoatrial (SA) node, located in the area where the superior vena cava (SVC) meets the right atrium. The impulse spreads to the atria through internodal pathways and travels to the AV junction (which contains the AV node) located medial to the entrance of the coronary sinus in the right atrium, close to the tricuspid valve. From the AV junction, the impulse spreads to the bundle of His, which extends down the right side of the interventricular septum. The bundle of His divides into the right and left bundle branches, which terminate in a network of fibers called the Purkinje system. Purkinje fibers are spread throughout the inner surface of both ventricles and the papillary muscles, which when stimulated produce contraction of the heart muscle. The location of conduction tissue is clinically significant during surgical repair of atrial or ventricular septal defects.
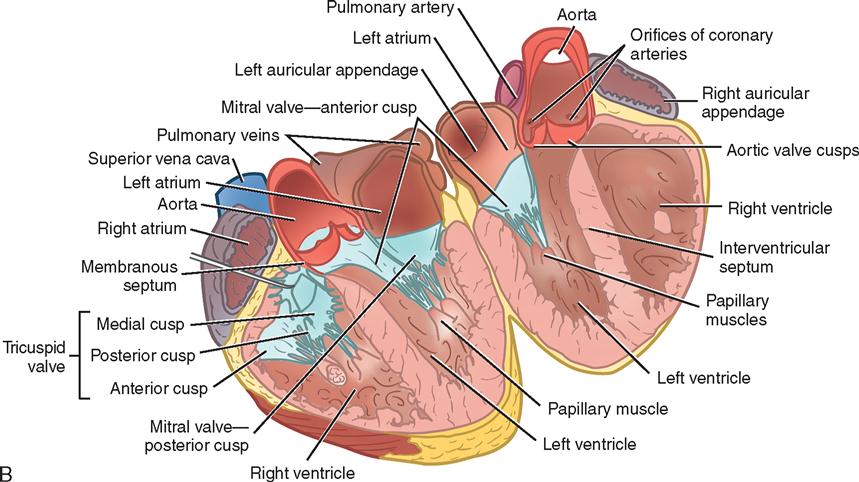
During myocardial contraction and relaxation, spiral fibers of the heart contract and relax (Figure 16-6, A). To prevent regurgitation of blood, the four cardiac valves (Figure 16-6, B and C, and Figure 16-7) open and close to maintain unidirectional blood flow. The AV valves are located between the atria and the ventricles. The right AV valve is called the tricuspid valve and contains three leaflets. The left AV valve, called the mitral valve, consists of two leaflets (see Figure 16-6). Each AV valve is a complex system consisting of a fibrous annulus surrounding the valve orifice, the valve leaflets (or cusps), the chordae tendineae, the atrium, and the papillary muscles, which anchor the valve to the inner ventricular wall (see Figure 16-1). The mitral valve annulus is a dynamic structure with a three-dimensional “saddle” shape, which has stimulated the design of newer prosthetic annuloplasty rings (Fedak et al, 2008). When the ventricle contracts, these muscles and the chordae tendineae, connected to the valve leaflets, prevent the leaflets from everting into the atrium. All parts of the system must function for the valve to work properly. If the shape of the ventricle has been changed by dilatation or hypertrophy, for example, the altered geometry of the ventricle impairs ventricular function. Conditions such as hypertension, myocardial injury, and aortic stenosis promote a pathologic remodeling of the heart that can lead not only to valvular dysfunction but also to heart failure and malignant dysrhythmias (Hill and Olson, 2008).
The semilunar valves are at the outlets of the left and right ventricles. These valves are known as the aortic and pulmonic valves, respectively. They are less complex than the AV valves, and they open and close passively with cyclic fluctuations in the blood pressure and volume that occur during systole and diastole.
Abnormalities such as stenosis, insufficiency, or a combination of both impair the mechanical function of the valves. Stenosed valves have leaflets that are fibrous and stiff, with uneven and adherent margins. Regurgitant, insufficient, or incompetent valves, such as those with leaflet degeneration or perforations, dilated annuli, or ruptured chordae tendineae, produce regurgitation of blood into the originating chamber. These conditions, or a combination of stenosis and insufficiency, strain the myocardium by increasing intracardiac pressure, volume, and workload. The sound of blood flowing through a narrowed or incompetent valve produces an abnormal sound called a murmur.
Any of the four valves may be congenitally deformed. Acquired valvular heart disease most commonly affects the mitral and aortic valves and is believed to be exacerbated by increased stress associated with the higher pressures within the left chambers of the heart.
Surgical Technologist Considerations
The history obtained by the surgical team includes information about the patient’s health status as well as the response to the disease and the recommended intervention or interventions. Patients with cardiac disease may display symptoms including ischemic chest pain (angina pectoris), fatigue, dyspnea, and syncope. Depending on their severity, these subjective symptoms affect the patient’s functional status and ability to engage in activities of daily living.
Atypical ischemic chest pain is more likely in women than in men, and angina may be attributed to vasospastic angina, mitral valve prolapse, or psychologic factors. CAD is unusual in premenopausal women, but after cessation of menses the risk is similar to that of men (McSweeney and Lefler, 2008; Mosca et al, 2007). Estrogen hormone replacement therapy is not recommended in postmenopausal women, according to the Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women: 2007 Update (Mosca et al, 2007) (Evidence for Practice).
A cardiovascular disease risk factor profile (Box 16-1) is helpful to the surgical team when planning for both hospitalization and discharge. For example, the presence of diabetes is notable because diabetes affects the vascular system and may retard healing and predispose the patient to infection. Of special concern are the epidemic growth of type 2 diabetes in particular and the role of hyperglycemia in general. Although type 2 diabetes was formerly considered an adult-onset disease, children are increasingly vulnerable because of a greater incidence of increased body weight, sedentary lifestyle, and accelerated insulin resistance in this population (Warziski et al, 2008). Altered glucose metabolism (in the absence of diagnosed diabetes mellitus) has shown a significant correlation to atherosclerosis (Lamendola, 2008), and control of hyperglycemia during coronary bypass surgery is an important strategy to reduce the risk of adverse clinical outcomes (Lorenz et al, 2005). This information becomes an integral component of assessment by the surgical team, especially in patients at risk.
Inflammation has been increasingly implicated in the development of coronary artery disease; in particular, elevated homocysteine levels (which increase platelet aggregation) and high C-reactive protein (CRP) levels (CRP is a biologic marker for inflammation and is associated with increased risk for CAD) have been implicated (Ruz and Lennie, 2008; Seymour et al, 2009). Targeting low-density lipoprotein (“bad” cholesterol) levels of less than 100 mg/dl is often recommended (Grundy, 2008); some authors also stress the importance of increasing the levels of high-density lipoproteins (“good” cholesterol) (Superko and King, 2008).
Additional recognized risk factors include metabolic syndrome (central obesity, hypertension, insulin resistance, and dyslipidemia) and obesity (Lamendola, 2008). Hypertension and obesity increase the workload of the heart; obesity may also increase the risk for postoperative infection because adipose tissue is poorly vascularized. Patients who are obese and those who are underweight (compared with patients in the high-normal and overweight categories) have a higher risk of death after coronary artery bypass surgery (Jin et al, 2005).
Among the newer biomarkers denoting cardiovascular diseases are the natriuretic peptides—cardiac hormones synthesized and secreted by the atrium (atrial natriuretic peptide [ANP]) and the ventricle (B-type natriuretic peptide [BNP]). Discovered in 1981, the natriuretic peptides have a regulatory effect that causes myocardial relaxation in response to acute increases in ventricular volume. Measurement of circulating peptides is increasingly used to identify heart failure and sudden cardiac death (Korngold et al, 2009; Hlatky et al, 2009). Another biomarker under intensive study as a risk factor is lipoprotein(a) (Lp[a]), which has been identified from genetic studies as a causal link to myocardial infarction. According to the researchers (Kamstrupp et al, 2009), Lp(a) levels need to be tested only once in a lifetime as the levels do not change over time. Optimal therapy has not been established, but niacin has been shown to reduce the level of Lp(a) in some individuals.
The risk for complications and major adverse cardiac events (MACE) after cardiac surgery has also shown some gender differences (Puskas et al, 2007; Edwards et al, 2005). Investigations of risk factors in men and women for sternal wound infection showed that three significant risk factors were identified and occurred at significantly different rates in men and women: smoking, use of a single internal mammary artery (IMA), and age more than 70 years. Smoking and the use of a single IMA for bypass grafting were more common risk factors in men compared with women; women tended to be older than men at the time of surgery (Hussey et al, 2001). Fewer MACE were seen in women undergoing coronary bypass surgery without the use of cardiopulmonary bypass (CPB) compared to surgery with CPB (Puskas et al, 2007); however, malignant ventricular dysrhythmias (e.g., ventricular tachycardia), a calcified aorta, or preoperative renal failure were poor prognostic signs in women (Gray and Sethna, 2008). These findings can be incorporated into patient/family teaching plans with recommendations for lifestyle changes as indicated.
Other risk factors associated with postoperative infection include previous cardiac surgery, extensive length of surgery, use of cardiopulmonary bypass (CPB) and/or blood transfusion, amount of postoperative blood loss, and length of preoperative hospitalization. Female gender, obesity, and arterial occlusive disease of the legs are related to impaired wound healing at the saphenous venectomy site after coronary bypass surgery; bilateral IMA grafts in patients with diabetes, obesity, and postoperative inotropic support have also been implicated (Newby and Douglas, 2008; Pradhan et al, 2008).
A history of rheumatic fever or frequent tonsillitis as a child is significant because the sequelae of rheumatic fever and streptococcal infections can lead to damage of the cardiac valves. Although the incidence of rheumatic fever has declined significantly in wealthy countries, rheumatic heart disease is a significant problem in less industrialized countries (Carapetis, 2007). Given the increasing number of immigrants to the United States, it is expected that treatment for rheumatic heart disease will continue to grow.
Diagnostic Studies.
Most patients referred for surgery have had clinical evaluations including both invasive and noninvasive studies (Box 16-2). After the history and physical assessment, a resting electrocardiogram (ECG) is ordered. An exercise ECG (stress test) is often performed because ST-segment changes indicating myocardial ischemia may be apparent only during or after exercise. In patients with intractable dysrhythmias, electrophysiology (EP) studies may be performed to locate the site of irritable atrial or ventricular foci that can be surgically ablated, excised, or controlled with pharmacologic therapy. EP studies are also performed to determine the need for internal defibrillators or antitachycardia devices. Bradycardia may be an indication for pacemaker insertion.
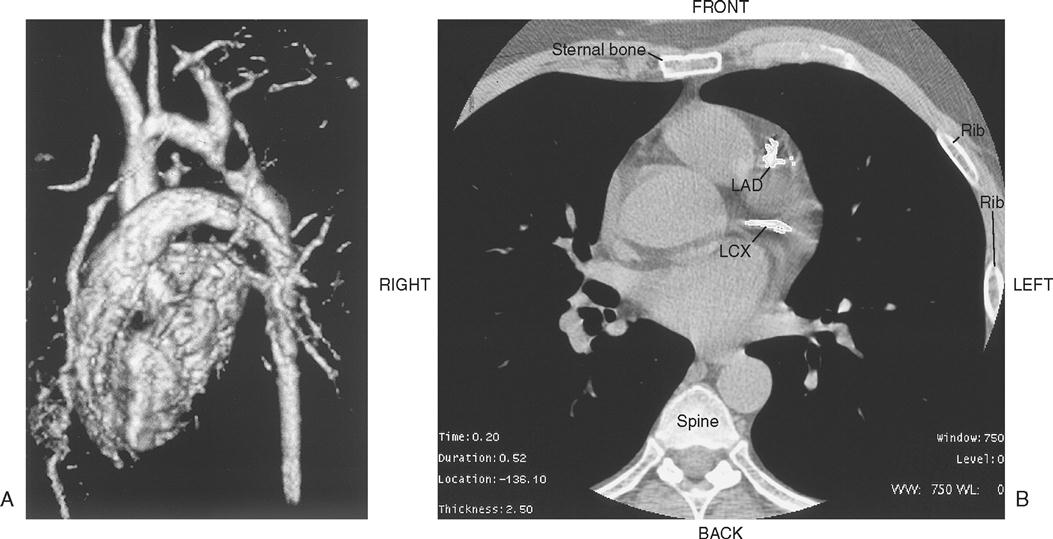
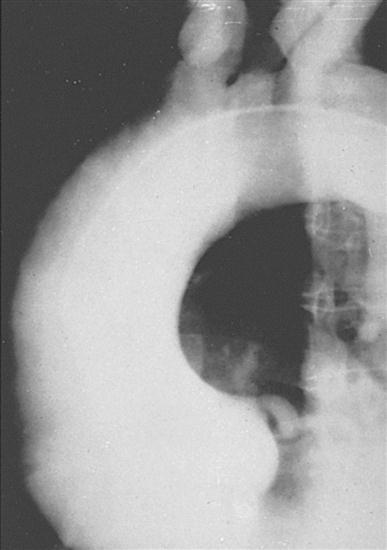
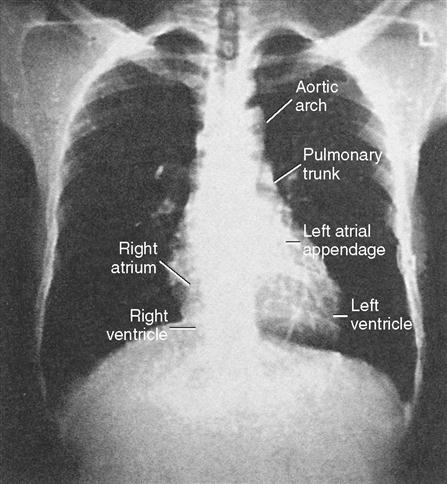
Chest radiography provides information about the size of the cardiac chambers, thoracic aorta, and pulmonary vasculature as well as the presence of calcium in valves, pericardium, coronary arteries, and aorta (Figure 16-8). Lateral chest radiographs of patients with prior sternal operations show the chest wires and extent of pericardial adhesions. Magnetic resonance imaging (MRI) is used to assess myocardial viability and can also be employed to image vascular structures with MRI angiograms that provide great clarity (Figure 16-9, A). In patients with suspected aortic or other vascular abnormalities, a computerized tomography (CT) scan of the chest with intravenous injection of a contrast medium is used to create x-ray serial “slices” of the body area under study (Figure 16-9, B), and CT angiography is especially useful for imaging the aorta and great vessels. CT scans may be contraindicated in very unstable patients because the patient’s position in the tubelike scanner makes patient access difficult. Less frequently performed is arteriography with radiographic contrast material (dye) to determine the size and location of the lesion and the site of the intimal tear in aortic dissections (Figure 16-10); digital subtraction angiography (DSA) provides clear images and requires less contrast material (Libby et al, 2008).
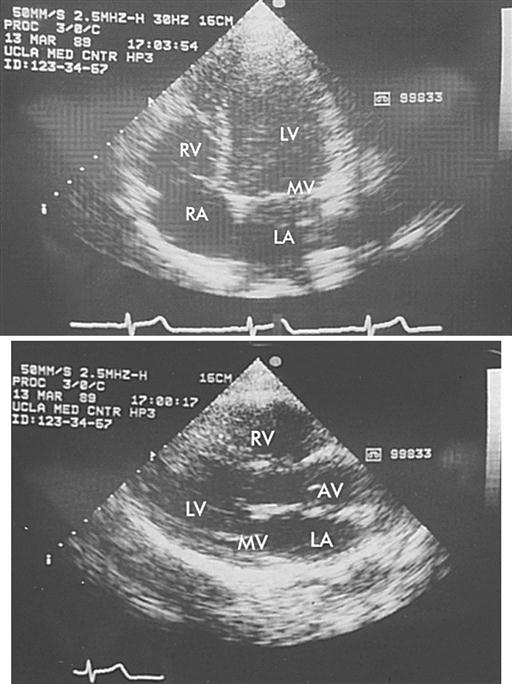
Echocardiography is a noninvasive test that evaluates both the structure and the function of the heart by transmitting sound waves to the heart and measuring those sound waves reflected back to the transducer (Figure 16-11). The sound waves are processed by the transducer, which creates visual images of the structure’s movements. This test is commonly used to assess ventricular and valvular function before, during, and after surgery and to determine the degree of valvular stenosis or regurgitation. It can also demonstrate a tumor, thrombus, or air in the ventricular or atrial cavities. Two-dimensional and color-flow Doppler techniques have greatly enhanced the functional assessment of valvular performance and carotid artery stenoses. Echocardiography is the gold standard for diagnosing mitral stenosis, and it is widely used to assess other valvular disorders and congenital heart disease. Transesophageal echocardiography (TEE) is also employed to evaluate the effectiveness of valve repairs and other surgical procedures.
Radionuclide imaging is employed to illustrate wall motion and blood flow through the heart and to quantify cardiac function. These noninvasive techniques are generally well tolerated by patients, especially when patients may be too unstable to withstand a cardiac catheterization. These techniques may also be used as a complement to catheterization.
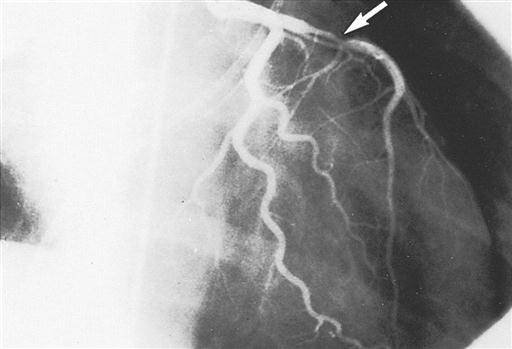
Cardiac catheterization provides definitive information about the extent and location of ischemic heart disease and is an adjunct to echocardiography for diagnosing valvular heart disease (Todd and Phippen, 2009). A radiopaque plastic catheter is inserted retrograde through the aortic valve into the left side of the heart by a percutaneous puncture or a cutdown to the vessels of the brachial artery (Sones technique) or the femoral artery (Judkins technique). The right side of the heart is approached percutaneously by the superior or inferior vena caval route. To perform coronary angiography that demonstrates intracoronary anatomy, a contrast medium is injected into the coronary ostia. Obstructions (Figure 16-12), flow, and distal perfusion can be assessed. Ventriculography illustrates contractile weaknesses of the ventricles as well as shunting and regurgitation of blood. These studies are used to assess the degree of myocardial dysfunction and to plan interventions such as coronary artery bypass grafting, valve repair or replacement, repair of congenital anomalies, and cardiac transplantation. The cardiologist can compute the orifice of a stenosed valve or determine the degree of regurgitation of an incompetent valve.
Ventricular, atrial, and pulmonary pressures are recorded, and cardiac output and ejection fraction are estimated (Box 16-3). Oxygen saturation of cardiac chambers and the ratio of pulmonary to systemic blood flow (Qp/Qs) are calculated for patients with shunts and congenital or acquired defects. Cinearteriograms record the movement of the heart, and cut films or digitized versions of the cines may be displayed during surgery.
The cardiac catheterization laboratory also performs percutaneous coronary interventional (PCI) therapies for evolving and acute myocardial infarctions (Leeson et al, 2008). Coronary thrombolysis with fibrinolytic drugs can dissolve fresh blood clots and reopen, or recanalize, the artery; antiplatelet agents such as aspirin, dipyridamole, and clopidogrel are often prescribed to block platelet aggregation that can lead to restenosis. Percutaneous transluminal coronary angioplasty (PTCA) followed by insertion of intracoronary (bare metal or drug-eluting) stents is often performed to dilate and maintain the patency of the recanalized artery. Laser angioplasty and atherectomy to excise intraluminal plaque may also be employed (Todd and Phippen, 2009). In many instances these interventions may obviate the need for surgical bypass grafting, although the progressive nature of CAD may eventually lead to patients requiring surgical revascularization. OR availability may be recommended for unstable patients undergoing stent insertion and atherectomy, but the need for on-site cardiac surgical availability has been questioned. One study demonstrated that patients undergoing PCI at facilities without surgical backup had rates of morbidity, emergency cardiac surgery, and mortality related to emergency surgery that were similar to those in facilities with surgical backup (Kutcher et al, 2009).
EP studies are performed to diagnose conduction disturbances and to provide therapeutic interventions, such as radiofrequency or cryologic ablation of foci producing atrial fibrillation or accessory pathways seen in Wolff-Parkinson-White syndrome. Insertion of a pacemaker for bradydysrhythmias or an internal cardioverter defibrillator for ventricular tachydysrhythmias is commonly performed in EP laboratories. However, insertion of these devices may require an OR when percutaneous access is unfeasible or when concomitant cardiac surgery is being performed.
Safety Considerations.
The safety of the patient is a primary responsibility of the entire perioperative team. Equipment should be functioning properly and undergo routine testing by the biomedical engineering department. Supplies should be used according to manufacturers’ instructions, and instruments should be regularly scrutinized to ensure that there are no burrs that could injure tissue, that the jaws of vascular and other clamps align properly, and that small items are accounted for at the end of surgery. Toxic material, such as the glutaraldehyde storage solution of bioprosthetic valves, should be thoroughly rinsed before implantation. Monitoring the aseptic practices of team members as well as visitors is an important safety consideration.
Staff safety is also important. Protective personal equipment should be consistently and properly worn. Gloves should be used by the OR staff whenever there is contact with a patient. The effects of electrical, chemical, and other potentially hazardous materials within the OR can be minimized by reinforcing safe practices to decrease the risks of injury.
Special Facilities.
The OR must be large enough to accommodate bulky, highly specialized equipment while maintaining aseptic technique. Multiple electrical outlets, auxiliary lighting, and additional suction outlets should be available. Ceiling-mounted, mobile booms for housing electrosurgical units (ESUs), headlight sources, suction, medical gases, electrical outlets, and other items can reduce floor clutter and enhance the safety of the environment for patients and staff.
A growing number of hybrid suites have been designed that combine the traditional surgical controlled environment and the fluoroscopic imaging capabilities of interventional cardiologic laboratories. A typical hybrid procedure may consist of a surgical coronary anastomosis under direct vision or with a robot that is combined with percutaneously inserted coronary artery stents under fluoroscopy (Jansens et al, 2009).
Instrumentation and Equipment.
A basic back table for CABG will include a basin set with large pitchers, an open heart instrument set, a mammary retractor, a self-retaining sternal retractor, and the camera, light cord, and scope for the endovein harvest. A sternal saw and internal defibrillator paddles are also required. The surgical technologist will also have the cardiovascular fine set that will include tenotomy scissors, Gerald forceps, suture tags, and coronary epicardial retractors for the finer parts of the procedure. Most vascular surgeons have a set of doctor specials that include the forward and backward Potts scissors, their favorite forceps for taking down the mammary, titanium bulldogs, fine rummells, or vascular clamp.
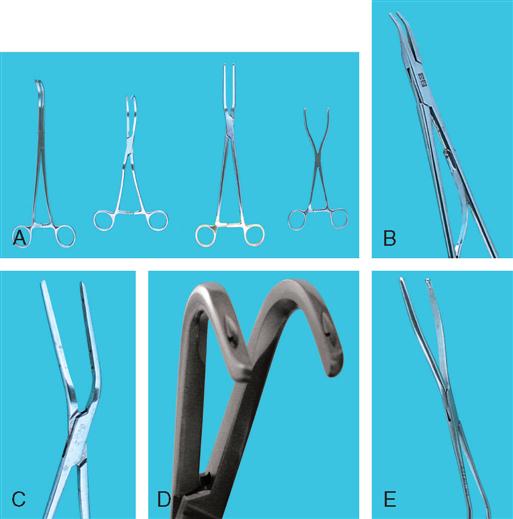
Vascular clamps, which are designed to occlude blood flow partially or completely, must be maintained in good condition if they are to prevent fracture of the delicate tunica intima of the blood vessels and still retain their specific holding qualities. There are many variations in construction of vascular instruments. The jaws may consist of single or double rows of fine, sharp, or blunt teeth or special crosshatching or longitudinal serrations. The working angles of the clamps also vary. All clamps are designed to hold the vessels securely and without trauma (Figure 16-13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree