INTRODUCTION
The purpose of this Chapter is to provide some basic information about transfusion medicine (TM). In order to provide care to patients, training and/or experience in TM is necessary. Please refer to the suggested reading for additional information. Purposefully omitted in this Chapter is a discussion of blood collection and donor as well as therapeutic apheresis.
 PRETRANSFUSION TESTING
PRETRANSFUSION TESTING
The decision to transfuse blood must be conveyed to a blood bank as a written or electronic order by a physician. In emergent situations, a verbal request for blood may be appropriate, but this should be documented and followed by a written or electronic order as soon as possible. In order to provide compatible blood to a patient, a pretransfusion sample is required for blood bank testing. The sample must be labeled with the patient’s name as well as a second unique identifier such as the patient’s date of birth (DOB) or a medical record number. Most institutions also require additional information on the specimen such as the identity of the phlebotomist and the date that the sample was drawn. Due to the potentially lethal consequences of incorrect patient identification during sample acquisition or errors during testing, it is preferred that two blood bank specimens be drawn and tested from a patient at different times. The results of the testing should also be compared to any historical blood bank data available for the patient.
Pretransfusion testing needs to be performed using the patient’s red blood cells (RBC) and either plasma or serum. Usually, hemolyzed or lipemic samples are not accepted by blood banks as testing such samples may yield inaccurate results. Once an appropriate specimen is received, testing is performed to determine the patient’s ABO group and Rh (D) type followed by screening of the patient’s plasma for unexpected red cell antibodies. After the testing is completed, a suitable unit of donor blood is selected for the patient and checked for compatibility by performing a crossmatch.
AGGLUTINATION (DAT AND IAT)
The majority of testing performed in blood banks involves checking for the presence of agglutination of patient, donor, or reagent red cells. The goal of this testing is to predict compatibility of blood products upon transfusion. Agglutination is “clumping” of red cells caused by the binding of antibody to antigens on the red cell membrane. This usually occurs in two phases: (1) sensitization of the red cells by the antibody binding to antigens and (2) lattice formation that results in macroscopic agglutination. Centrifugation is usually necessary to bring red cells in close proximity for agglutination as there is a net negative charge on the surface of red cells that prevents their aggregation and agglutination. Additionally, as it is possible for IgG antibodies to result in red cell sensitization without agglutination, it is often necessary to add a secondary antibody to cause lattice formation. The secondary antibody required is an antibody to human globulins, specifically IgG and complement. This antihuman globulin (AHG) or Coombs reagent can be used to perform a direct antiglobulin test (DAT) as well as an indirect antiglobulin test (IAT). When performing a DAT, AHG is added to a patient’s RBCs that are suspected to be sensitized. This is unlike an IAT where AHG is added to a suspension of reagent (or donor) red cells and the patient’s plasma that is suspected to contain an antibody to the reagent (or donor) red cells. In either test, the addition of AHG will result in agglutination if the red cells are sensitized by the antibody. Although many institutions perform pretransfusion testing as described above in small test tubes, some larger institutions are performing some (or most) of their testing using newer technology (gel columns and solid-phase testing).
PERFORMING A TYPE AND SCREEN
Prior to transfusion of blood, a patient’s ABO and Rh type must be determined and the patient’s plasma should be checked for expected and unexpected antibodies. The blood typing usually begins with the forward ABO grouping performed by testing a patient’s red cells for the presence of A and B antigens using commercially available anti-A and anti-B antibodies. Subsequently, a reverse grouping is performed by checking the patient’s plasma for anti-A and anti-B antibodies using reagent group A and group B red cells. With rare exception, patients who lack A or B antigens on their red cells should have an antibody to the antigen that is not present (see Table 15-1). Any discrepancy in the forward and reverse ABO grouping must be resolved prior to transfusion of blood products and if urgent transfusion is necessary, group O red cells and group AB plasma should be provided prior to the resolution of this discrepancy.
TABLE 15–1. Antigens and Antibodies Present in Each ABO Blood Group

In order to determine a patient’s blood type, the patient’s red cells must also be tested with anti-D to determine whether the patient expresses the D antigen on his or her red cells. If the patient’s red cells do not agglutinate with the anti-D reagent antibody, some institutions may perform weak D testing by adding AHG. Weak D testing will detect patients who have a quantitative or qualitative difference in the D antigen expressed on their red cells. Patients who have a weak D phenotype do not have enough D antigen on their red cells to result in direct agglutination, and agglutination is seen only after the addition of AHG. Patients who have a partial D phenotype have a qualitative difference in the D antigen that also requires AHG for agglutination. Although some institutions will perform weak D typing on patients, it is not necessary to do so as weak D and partial D patients will otherwise be considered Rh negative and will receive D-negative blood products. The same holds true for pregnant patients; while it is not necessary to perform weak D testing, some institutions choose to do so. If a pregnant patient with a weak D phenotype is typed as D negative (because the institution does not perform weak D testing on patients), she will be treated with Rh immune globulin unnecessarily (as she is not able to be immunized to the D antigen), but this will likely not result in any harm to the patient (also see discussion on prenatal testing in this Chapter). However, blood donors must be typed for the weak D antigen because red cells from a weak D donor can immunize Rh-negative patients to the D antigen.
Once the patient’s blood type (ABO and Rh) is determined, their plasma or serum must be checked for unexpected antibodies to non-ABO red cell antigens. The goal of the antibody screen is to detect clinically significant antibodies to red cell antigens that can cause hemolytic transfusion reactions (HTR) and hemolytic disease of the fetus and newborn (HDFN). Antibodies that bind to red cells at body temperature (37°C) and are detected using AHG are more likely to be clinically significant than cold reactive antibodies that do not result in agglutination at 37°C or the AHG phase of testing. Some of the more commonly encountered clinically significant antibodies include antibodies to D, C, E, c, e, S, s, K, k, Fya, Fyb, Jka, Jkb. Reagent red cells must be able to detect these antibodies as well as antibodies to M, N, P1, Lea, Leb.
CROSSMATCH
After a type and screen is completed, an appropriate blood product can be selected to transfuse the patient. Ideally, ABO identical blood products should be transfused, but due to inventory management constraints, often ABO compatible (but not ABO identical) products are issued for transfusion. Table 15-2 reviews the type of red cells and plasma that are compatible with each of the ABO blood groups.
TABLE 15–2. Blood Products That are Compatible with Each ABO Blood Group

Plasma, platelets, and cryoprecipitate can be transfused without a crossmatch. However, for RBC transfusion, each unit of red cells should be crossmatched with the recipient’s plasma prior to transfusion. The type of crossmatch necessary depends on whether the patient’s antibody screen is positive or negative.
If the patient’s antibody screen is negative, generally an immediate spin crossmatch is performed by simply mixing the red cells selected for transfusion with the patient’s plasma and checking for agglutination after centrifugation. An immediate spin crossmatch is essentially a second check of the donor and recipient’s ABO compatibility as agglutination will be seen if the patient’s plasma has ABO antibodies to cognate antigens on the red cells selected for transfusion. In some institutions, patients who have a negative antibody screen may be eligible for an “electronic crossmatch” if the institution has validated the blood bank information/computer system to not allow an incompatible blood product to be issued to the patient. If the patient had a positive antibody screen, a complete crossmatch using AHG is appropriate. Many clinically significant non-ABO alloantibodies are IgG antibodies and will not result in agglutination unless AHG is added to the suspension of red cells and plasma. Thus, patients who have a positive antibody screen should have the crossmatch performed by incubating the patient’s plasma and donor red cells at body temperature followed by addition of AHG.
The sample of blood used to perform the crossmatch (as well as the type and screen) must be recently acquired from the patient if the patient has been recently transfused or pregnant. This is necessary because the patient may produce an antibody to a RBC antigen within a few days after being exposed to allogeneic red cells. Generally, a sample should not be more than 3 days old if the patient has been recently transfused or pregnant, but a sample drawn up to 2 weeks prior to transfusion is acceptable if the patient has not had any recent exposure to blood. It is also important to review the blood bank history to prevent the transfusion of RBCs that may have antigens that the patient previously had antibodies to. In these patients, the antibody titer may decrease in strength to below detectable levels, but subsequent exposure to the antigen can result in brisk antibody production and a delayed hemolytic transfusion reaction. Unlike RBC transfusion, transfusion of other blood products does not require a recent sample because selection is based on the patient’s blood type, and a crossmatch does not need to be performed.
 TRANSFUSION OF BLOOD PRODUCTS
TRANSFUSION OF BLOOD PRODUCTS
Transfusion of blood products carries significant risks. Thus, prior to any transfusions, the risks and benefits should be carefully assessed and blood products should be provided to the patient only if the benefits outweigh the risks. Today, transfusion of whole blood is exceedingly rare in the United States. Patients are usually transfused with the specific blood component that is required (e.g., packed red cells, plasma, platelets, or cryoprecipitate).
RED CELL TRANSFUSION
The goal of red cell transfusion is to increase oxygen delivery to tissues when necessary. Red cell transfusion is appropriate for the treatment of anemia if it will ameliorate symptoms of anemia or aid in correcting or preventing the adverse physiologic consequences of anemia. Most patients will tolerate a loss of approximately 50% of their circulating hemoglobin before they start to experience significant consequences due to acute anemia. In acute blood loss, symptoms due to hypovolemia are usually seen before symptoms due to anemia. In chronically anemic patients (patients who become anemic over weeks or months), compensatory mechanisms allow patients to tolerate lower hemoglobin levels than patients who become acutely anemic. Considering the many variables involved, it is often challenging to determine whether tissue ischemia exists and whether it will be alleviated with red cell transfusion.
 Who Should Be Suspected?
Who Should Be Suspected?
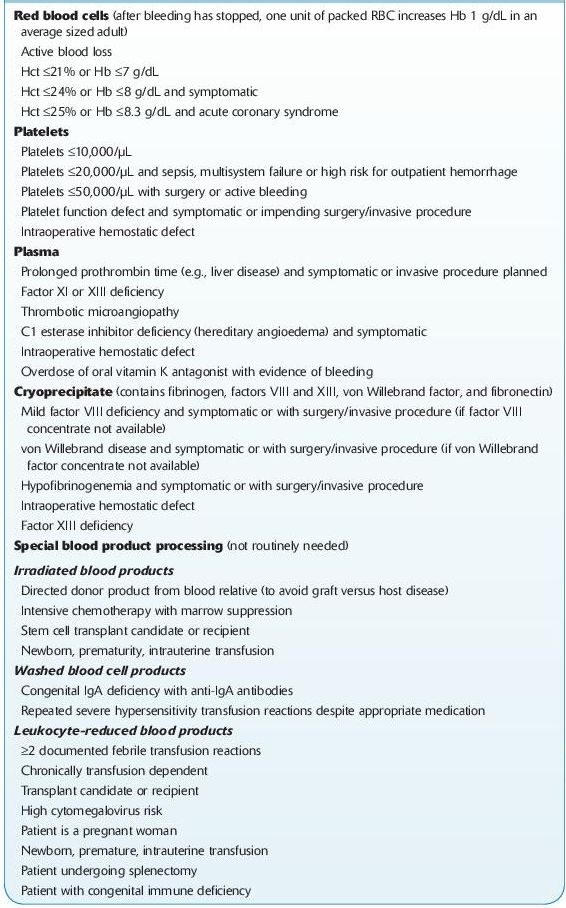
There is a significant variation in RBC transfusion practices between institutions. Most studies that have audited transfusion of blood products have reported that there was unnecessary transfusion of patients and there is a trend toward more conservative hemoglobin transfusion triggers. Authorities in TM agree that most patients with a hemoglobin of <6 g/dL will need red cell transfusion and most patients with a hemoglobin of >10 g/dL will not require red cell transfusion. There is also general agreement that within this range, the transfusion of blood products needs to be individualized to the patient. At the author’s institution, a trigger of a hemoglobin of 7 g/dL is used for most hospitalized patients with the notable exception of patients with unstable cardiac disease (see Table 15-3).
TABLE 15–3. Common Indications for Blood Product Transfusion and Special Processing
PLASMA TRANSFUSION
The previous practice of using plasma as a volume expander has largely become extinct. Today, plasma is almost always transfused to patients due to a deficiency of one or more proteins present in normal plasma. The proteins that are most commonly repleted are coagulation factors. Other deficient proteins that may be repleted with plasma transfusion include ADAMTS 13 in patients with TTP and complement factors in patients with HUS.
 Who Should Be Suspected?
Who Should Be Suspected?
Despite the most common indication for plasma transfusion being the treatment of coagulopathy, there are no clear guidelines for the appropriate use of plasma in this setting. Thus, very often plasma is transfused unnecessarily to patients who do not benefit from it.
A more proactive approach in regard to plasma transfusion is appropriate in bleeding patients who require massive transfusion of blood products. In such patients, if coagulopathy develops, it may result in excessive bleeding that can be life threatening and it may be extremely difficult to correct a severe coagulopathy. These patients have multiple factors contributing to the coagulopathy including dysfunction of the enzymes of the coagulation cascade (due to hypothermia and acidosis) and consumption of coagulation factors due to DIC.
 Considerations
Considerations
Several types of plasma products are available for transfusion. These include fresh frozen plasma (FFP), frozen plasma 24 (FP24), and thawed plasma (TP). FFP is plasma that has been separated from a whole blood collection and put into a freezer within 8 hours of collection. FP 24 is plasma that has been placed in the freezer within 24 hours of collection. Both of these products can be kept frozen up to 1 year; however, once thawed, they must be used within 24 hours. Thawed plasma is FFP or FP24 that has been relabeled as “thawed plasma” and now can be used for up to 5 days after thawing. The concentration of the majority of coagulation factors does not vary significantly among FFP, FP24, and TP with the exception of factor V and factor VIII. FP24 and TP have lower concentrations of factor V and factor VIII as these two factors have the shortest in vitro half-life. However, factor 5 deficiency is rare, and factor 8 is an acute-phase reactant that is often elevated in patients requiring plasma transfusion. Most often, the vitamin K–dependent factors (II, VII, IX, and X) are the factors that need to be replaced in order to correct coagulopathy. These factors are stable at refrigerator temperatures and not significantly decreased in FP24 or TP.
 Laboratory Findings
Laboratory Findings
When assessing a patient for coagulopathy (usually an acquired coagulopathy), the most common laboratory tests ordered are a prothrombin time (PT)/international normalized ratio (INR) and an activated partial thromboplastin time (aPTT). The PT is very sensitive and will be abnormal before coagulopathy will result in bleeding. Generally, an increase in bleeding is not usually seen until the PT is >1.3 times the upper limit of the normal range (which usually corresponds to an INR of approximately 2). In nonbleeding patients with an elevated INR due to warfarin use, vitamin K can be used to correct the coagulopathy (over 6–24 hours) without plasma transfusion. It is also important to note that plasma transfusion is significantly more effective in decreasing a patient’s INR when the INR is significantly elevated. As the patient’s INR gets closer to or below 2, a large amount of plasma will result in a much smaller decrease in the INR than the same volume of plasma given to the same patient at a higher INR. Another important consideration when transfusing plasma, especially prior to an invasive/surgical intervention, is that the plasma should be transfused within a few hours of the intervention. The in vivo half-life of some of the coagulation factors that need correction (such as factor 7) is a few hours and correcting the coagulopathy with plasma transfusion >8 hours prior to transfusion will likely be of little benefit. Additionally, the use of plasma for warfarin reversal will likely decrease significantly with the recent approval of four factor prothrombin complex concentrate (PCC) in the United States.
CRYOPRECIPITATED AHF TRANSFUSION
A unit of cryoprecipitated AHF (also known as cryoprecipitate) is prepared from a unit of plasma. When frozen plasma is placed in a refrigerator and starts to thaw, there is precipitation of some proteins. This precipitate contains a significant proportion of the factor VIII, von Willebrand factor, fibrinogen, fibronectin, and factor XIII that is present in the unit of plasma. Subsequently, the unit is centrifuged to separate the precipitate from the supernatant plasma. The cryoprecipitate is then frozen until it is necessary to transfuse.
 Who Should Be Suspected?
Who Should Be Suspected?
In the past, cryoprecipitate was used to treat patients who had hemophilia A. But due to safer alternatives, cryoprecipitate is no longer used for this purpose in the United States. The primary use for cryoprecipitate today is to replete fibrinogen in patients with hypofibrinogenemia or disfibrinogenemia (e.g., patients with DIC, patients requiring massive transfusion).
 Laboratory Findings
Laboratory Findings
Fibrinogen levels between 50 and 100 mg/dL are generally adequate in patients to achieve hemostasis. However, levels <100 mg/dL may cause in vitro laboratory testing abnormalities. The volume of a unit of cryoprecipitate is approximately 15 mL, and for transfusions in adults, generally 10 units are pooled for an adequate dose. However, it is possible to calculate the exact number of units needed to reach a specific fibrinogen level if the patient’s fibrinogen level is known. Occasionally, cryoprecipitate is also used in uremic patients and in patients with von Willebrand disease as a source of von Willebrand factor.
PLATELET TRANSFUSION
Platelet transfusion is generally performed to correct an abnormality of platelet function or platelet count in order to prevent bleeding or treat active bleeding. The most common indication for platelet transfusion is prophylaxis in thrombocytopenic patients. Although there are no well-accepted guidelines in regard to prophylactic platelet transfusion, some consensus has been achieved and many institutions follow similar practices.
 Laboratory Findings
Laboratory Findings
In patients who are severely thrombocytopenic and do not have additional risk factors for bleeding, a threshold platelet count of 10,000/μL is commonly accepted. In patients who are having an invasive CNS, ophthalmologic, or possibly a pulmonary procedure, a count of 100,000/μL is often targeted. Even though most experts agree that this count is higher than needed to achieve hemostasis, it is still considered a reasonable target because it protects against a precipitous drop in platelet count that can result in bleeding into these organs causing serious morbidity or mortality. For other invasive or surgical procedures, most authorities concur that platelet counts above 50,000/μL are generally adequate.
 Considerations
Considerations
Some of the causes of qualitative defects of platelet function include congenital diseases (e.g., Glanzmann thrombasthenia, Bernard-Soulier disease), medications (e.g., aspirin, clopidogrel), and dysfunction due to medical device interaction (e.g., cardiopulmonary bypass). In these patients, the platelet counts may be normal, but due to abnormal platelet function, transfusion of one unit of apheresis platelets (or an equivalent dose of whole blood–derived platelets) is often appropriate. In patients on antiplatelet medications who present with acute bleeding, especially bleeding into the central nervous system, two apheresis platelets (or equivalent) are often transfused.
Platelet products can be collected using apheresis technology or can be produced by separation from whole blood collections. For adult patients, 4–8 units of whole blood–derived platelets must be pooled for one transfusion dose or alternatively a single apheresis unit collected from one donor can be transfused. Both types of platelet products are commonly used, and each one has some advantages and disadvantages. One significant advantage of apheresis platelet products is that they are collected from a single donor and can be used to transfuse patients who require HLA-selected platelet products.
 Who Should Be Suspected?
Who Should Be Suspected?
Patients who get immunized to HLA antigens due to pregnancy, organ transplantation, or previous exposure to blood products may not respond adequately to platelet transfusion. Platelets have HLA class 1 (A and B) antigens on their surface, and if the recipient has antibodies to the HLA antigens of the donor, there may be no increment in platelet count posttransfusion. The most effective way to prevent immunization to HLA antigens is by leukoreduction of blood products. However, if a patient is already immunized to HLA antigens, there are several strategies that can be employed to transfuse these refractory patients. These include platelet crossmatching, HLA matching, and selection of platelet products from donors who lack the cognate HLA antigens to which the patient has antibodies. However, prior to attaining HLA-selected platelet units, it might be reasonable to transfuse fresh ABO identical apheresis platelets as ABO incompatibility and older age of transfused platelets have been reported to decrease posttransfusion platelet increments.
Platelet crossmatching is performed by mixing a sample of the patient’s plasma with platelet products that may potentially be transfused and checking for a reaction between them. If reactivity is present, the crossmatch is interpreted as positive and the platelet product is not transfused to the patient. If necessary, additional platelet products can be crossmatched with the patient until a product that does not have reactivity with the patient’s plasma is procured. One substantial benefit to HLA crossmatching is that it is possible to find compatible platelet products without determining the patient or the donors HLA type. However, HLA crossmatching can only be performed on platelet products that are ABO compatible, and the data available comparing HLA crossmatching with HLA matching show that platelet crossmatching is likely an inferior strategy.
HLA matching is performed by HLA typing patients as well as platelet donors. Once HLA typing data are available, platelet products that either completely match or are very similar for HLA class 1 (A and B) antigens are selected for transfusion. The HLA match of the recipient to the donor can be rated from A (all four antigens match) to D (two or more antigen mismatch). HLA matching of platelets is effective in achieving good count increments when the donors are very well matched (A or B level matches) to the patient. However, if a patient is widely immunized and the platelet product is not well matched (C or D level matches), the patient will likely not respond to the platelet transfusion. Additionally, HLA matching may be difficult in smaller institutions that do not have HLA laboratories or large platelet inventories.
A third strategy that is as successful as HLA matching and likely superior to crossmatching is selection of platelet products that avoids HLA antibodies present in the patient. In order to use this strategy, the patient must be tested for HLA antibodies. Once the specificity of the HLA antibodies in the patient is determined, platelet products from donors that lack cognate HLA antigens are selected for transfusion. This strategy can be very effective for patients who are refractory, even if an institution has a limited platelet inventory. However, if the patient is widely immunized to HLA antigens, donors with compatible HLA types will need to be recruited or compatible apheresis platelet products will need to be brought in from large blood centers with extensive inventories of platelets. This strategy has the additional benefit of determining whether the patient is refractory due to the presence of HLA antibodies or other causes. It is also important to reassess the patient every few weeks to check for any changes in the HLA antibodies present.
Another less common immunologic cause for platelet refractoriness is antibodies to antigens present on platelets other than HLA. Patients with these plateletspecific antibodies will require platelet products from rare donors that lack these antigens. The most commonly encountered antibody to a platelet-specific antigen is anti–HPA-1a (anti-PlA1).
In addition to these immunologic causes, there are many nonimmune causes for an inadequate response to platelet transfusion. These include fever, sepsis, disseminated intravascular coagulation (DIC), splenomegaly, bleeding, as well as treatment with certain medications. Most of these reasons for refractoriness may persist until the patient’s underlying medical condition is adequately treated.
GRANULOCYTE TRANSFUSION
Granulocyte transfusion has decreased dramatically over the last two decades. Previously, granulocytes were frequently transfused to neutropenic patients undergoing chemotherapy or to patients with congenital neutrophil abnormalities (e.g., chronic granulomatous disease) who had infections.
 Who Should Be Suspected?
Who Should Be Suspected?
Due to advances in antimicrobial therapy, granulocytes are now only transfused to patients who are temporarily neutropenic and have a life-threatening bacterial or fungal infection that is unresponsive to antimicrobial therapy. Based on published literature, it is not clear if the addition of granulocyte transfusion provides any benefit when compared to antimicrobial therapy alone. The published studies have had variable conclusions, but there appears to be a trend toward benefit in the studies that have transfused higher doses of granulocytes with each transfusion. If a decision is made to transfuse granulocytes, generally one product is transfused daily until the patient has an absolute neutrophil count above 500/μL.
Granulocytes are collected using an apheresis device from healthy volunteer donors. However, granulocyte transfusion carries significant risks. Some of the risks include CMV transmission, HLA immunization, and severe pulmonary reactions. Additionally, granulocytes must be transfused the same day that they are collected and thus are transfused prior to the completion of standard infectious disease testing performed on blood donors. Platelet donors who recently had negative infectious disease testing are often used as granulocyte donors, but there remains a risk of infectious disease transmission. As with any other blood product transfusion, the risk– benefit ratio must be carefully examined prior to the transfusion of granulocytes.
 RISKS AND ADVERSE CONSEQUENCES RELATED TO BLOOD PRODUCT TRANSFUSION
RISKS AND ADVERSE CONSEQUENCES RELATED TO BLOOD PRODUCT TRANSFUSION
Transfusion of blood products may be life saving but also carries a significant risk of adverse consequences for the recipient (Table 15-4). Some of the possible adverse consequences that have been well documented include infectious disease transmission, transfusion-associated immunomodulation, and transfusion reactions.
TABLE 15–4. Adverse Effects of Blood Transfusions
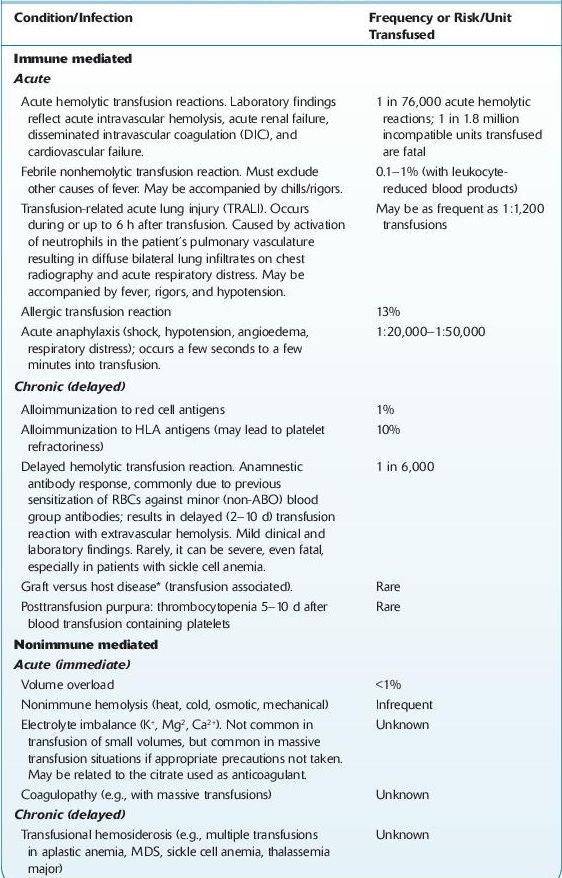
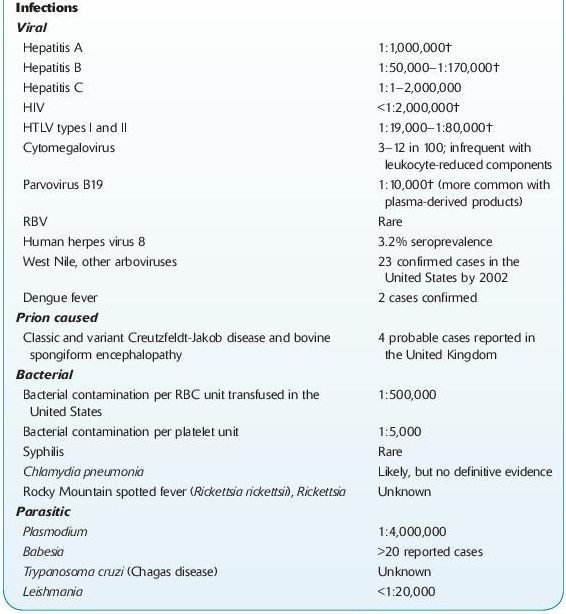
*Transfusion-associated graft versus host disease (TA-GVHD) occurs when immunocompetent T lymphocytes are transfused into a patient who cannot reject them, and they engraft in the recipient, proliferate, and mount an immune attack against the host tissues. TA-GVHD occurs 4–30 d after transfusion of any cellular blood component. It may occur in a nonimmunocompetent recipient or in an immunocompetent recipient who receives histocompatible donor lymphocytes, especially from blood relatives, which can recognize a different HLA haplotype in the recipient. There are molecular techniques to diagnosis TA-GVHD. Mortality is nearly 90% in the full-blown syndrome. Irradiation of blood products virtually eliminates the risk of this complication. †Estimates published by the British Committee for Standards in Haematology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


