Neurosurgery
LEARNING OBJECTIVES
After studying this chapter the reader will be able to:
• Correlate the pathologic morphology of central nervous system tumors to specific cellular components
• Identify tissue layers involved in opening and closing sequences of cranial procedures
• Locate areas of the brain responsible for specific motor and sensory functions of the body
• Identify internal and external anatomical landmarks of the cranium
• Discuss the anatomical mechanism of collateral cerebral blood flow
• Identify types of vascular abnormalities within the CNS, their origin, and impact
• List the names, numbers, and specific functions of the cranial nerves
• Identify the anatomical characteristics of the spinal cord of an adult
• Examine the structural configuration of the spinal column and its individual vertebral bodies
• Discuss the impact of spinal pathological conditions on patient mobility and quality of life
• Compare diagnostic methods for determination of surgical interventions and approaches
• Identify specialized instrumentation, equipment, and supplies utilized for neurosurgical procedures
• List the pharmacologic and hemostatic agents used during neurosurgical procedures
• Review specific procedural steps as a guide for clinical considerations
Overview
Neurosurgery is a word that evokes a certain sense of fear, awe, or mystery among both lay people and medical providers. Neurosurgeons have some of the most extensive training and lengthiest residency requirements of all surgical specialties. Surgical technologists who participate in this specialty must be able to apply all of their skill and expertise to these highly challenging surgical interventions. Soft tissue or bone, neonates to geriatrics, anomalies to accidental injuries, superior sagittal sinus to tarsal tunnel and everything in between, the perioperative neurosurgical team must be prepared to provide the highest level of care possible. To reference an old adage, it may not be rocket science, but it is brain surgery.
A working knowledge of neuroanatomy and physiology is crucial for optimal patient care. The neurosurgeon, anesthesia provider, perioperative nurse, surgical first assistant or physician assistant, and surgical technologist are all responsible for protecting the patient from injury secondary to factors including: prolonged anesthesia, positioning, exposure and manipulation of vital structures, and other potential risks within the preoperative, intraoperative, and postoperative phases of care. Surgical interventions, or lack thereof, in cases involving neurosurgical diagnoses constitute an inherent risk of altering an individual patient in profound ways, unlike any other specialty or disease process. Patients may suffer neurological loss of function or sensation, whether transient or permanent. They may have personality changes following cranial injury or surgery that leaves family and friends bewildered. Chronic conditions rob patients of independence and society of human resources. But, in many cases, neurosurgical interventions restore function, relieve pain, maintain, and prolong the quality of life for those who put their trust in the hands of skilled perioperative personnel.
A knowledgeable surgical technologist will be able to correlate the diagnosis obtained from an intraoperative frozen section with the cellular level of neuroanatomy. An experienced surgical technologist will be able to anticipate the need for specialized equipment based on the surgical procedure scheduled. An expert and attentive surgical technologist will hand the neurosurgeon the appropriate instrumentation without being asked, based on an understanding of the procedural steps and tissue layers involved. Practitioners must have the capacity to remain focused on the surgical procedure for long periods, organize multiple setups and instrument trays, operate complex imaging and magnification systems, and be vigilant about the integrity of the sterile field in a room potentially filled with many other additional health professionals including: radiology technologists, industry representatives, neuromonitoring personnel, and autologous blood salvage unit operators. With so much at stake, it is no wonder the mention of neurosurgery can strike a nerve with the inexperienced surgical technologist and send shivers up the spine.
This chapter will provide a comprehensive blueprint of anatomy and physiology, neuropathology, procedural considerations, equipment and instrumentation, diagnostic methods, research, and history to assist the surgical technologist to build a solid foundation of knowledge. Putting the specialty of neurosurgery under the microscope will demystify its complexity and provide greater accessibility to any perioperative practitioner willing to join the surgical team.
Surgical Anatomy
The nervous system is the most complex and least understood of body systems. It is divided structurally into the central nervous system (CNS) consisting of the brain and spinal cord and the peripheral nervous system (PNS), which encompasses every neurologic structure outside the CNS, including the cranial and spinal nerves. The brain and spinal cord are protected by the skull and vertebral columns, respectively. The cranial nerves originate within the brain and emerge through openings in the skull to run peripherally. The spinal nerves that emerge from the spinal cord through the vertebral foramina also run peripherally.
The nervous system is divided functionally into a voluntary system and an autonomic, or involuntary, system. It provides a means of communication for the rest of the body. The functions of all body systems depend, in part, on nervous system function. In turn, the nervous system depends directly on circulatory system function to obtain life-sustaining glucose and oxygen. Nervous system functions include motor and sensory functions, orientation, coordination, conceptual thought, emotion, memory, and reflex response.
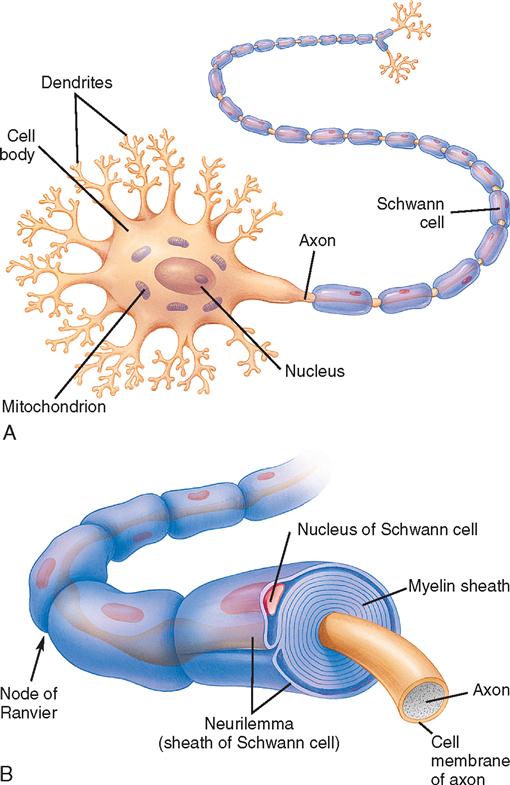
Nervous system tissue is composed of vast amounts of neurons and far more neuroglial cells. Neurons are intercommunicating nerve cells that encode, conduct, and transmit information to other neurons, muscle, and glandular tissue (Figure 12-1). They are composed of a body or soma with branches or extensions, called dendrites and axons, that communicate with other cells at synapses. Dendrites are short branches that conduct impulses toward the soma. Cell bodies and dendrites are mostly confined to areas of gray matter in the CNS. Axons are long branches, often encased in a white myelin sheath, that conduct impulses away from the soma. Axons pass into bundles of nerve fibers that tend to form tracts or pathways and are referred to as white matter. Tracts that cross midline to create a communication pathway from each side of the body to the opposite side of the brain are called commissures. Neuroglial cells support the neurons by creating and maintaining an appropriate environment in which neurons can operate efficiently. Glial cells include astrocytes, oligodendrocytes, ependymal cells, and microglia. The mutation of these cells can form a glioma, one of the more common brain tumors.
This chapter divides the nervous system into logical divisions within the framework of neurosurgical techniques. The brain and adjacent structures include the cranial nerves of the PNS, which are commonly encountered during brain surgery. Discussion of the spine and spinal cord includes the adjacent spinal nerves and the disks and ligaments that support the spine. Surgically significant pathology is incorporated with the normal anatomy of structures.
BRAIN AND ADJACENT STRUCTURES
Scalp
Scalp layers (Figure 12-2) include skin, subcutaneous tissue, galea, and periosteum. Scalp skin is thick. The subcutaneous tissue, which is exceptionally dense, tough, and vascular, is firmly attached to the galea. Most of the blood vessels lie superficial to the galea. The subgaleal space contains loose areolar tissue that permits mobility of the scalp. It is in this bloodless plane that the standard craniotomy scalp flap is created. The pericranium, or outer periosteum of the skull, separates the galea from the cranium.
The arterial supply of the scalp comes from the external carotid artery through the superficial temporal, posterior auricular, occipital, frontal, and supraorbital branches. Most veins roughly follow the course of their corresponding arteries, except the emissary veins, which drain directly through the skull into the intracranial venous sinuses. The scalp, the extracranial arteries, and portions of the dura mater are the only pain-sensitive structures that cover the brain. The brain itself is insensate.
Skull
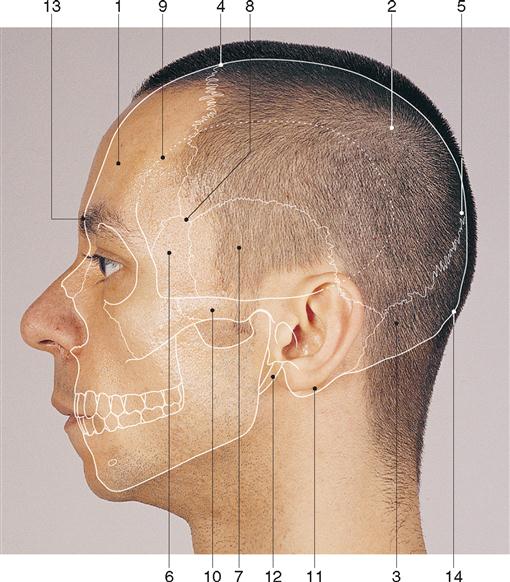
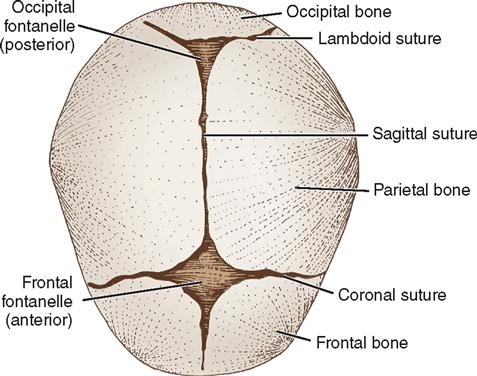
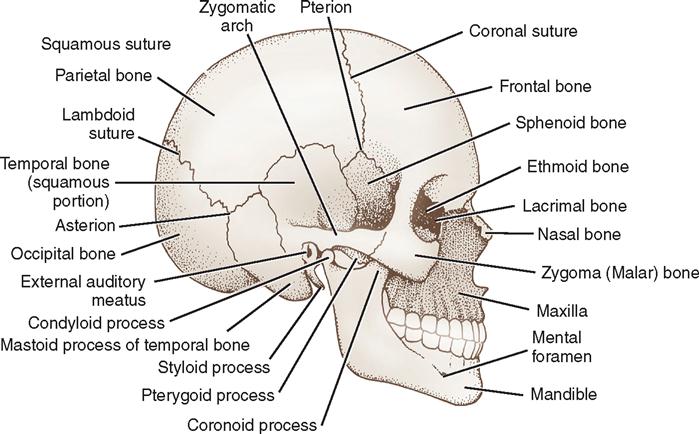
The skull provides protection for the brain. It is formed by 28 bones, most of which are paired although some in the median plane are single. Many of the bones are flat bones, consisting of two thin plates of compact bone encasing a spongy layer of cancellous bone containing bone marrow (see Figure 12-2). Infants are born with two fontanelles. These are openings in the skull that are located both anterior and posterior to the parietal bones (Figure 12-3). The posterior fontanelle is generally closed by 2 months and the anterior by about 18 months after birth. The bones of the skull are joined by bony seams called sutures. Eight bones form the walls of the cranial cavity, which houses the brain. There are four single bones (frontal, occipital, ethmoid, and sphenoid) and four paired bones (temporal and parietal) (Figure 12-4). The sagittal suture lies in the medial plane and joins the two parietal bones. The coronal suture joins the frontal and parietal bones. The squamous sutures border the squamous part of the temporal bones. The lambdoid suture joins the occipital and parietal bones. Skull bones vary in thickness and tend to be thinner where they are covered in muscles, for example, in the temporal and posterior fossae. The skull articulates with the first cervical vertebra to allow for flexion and extension of the skull. The skeletal surface landmarks of the head can be palpated and are commonly used to plan surgical approaches (Figure 12-5).
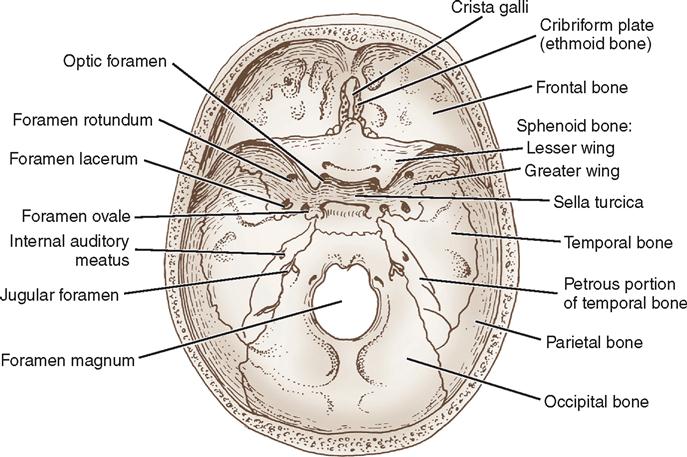
The interior of the skull is anatomically divided into three cranial fossae: anterior, middle, and posterior (Figure 12-6). The anterior fossa is limited posteriorly by the sphenoid ridge, along which pituitary tumors and aneurysms of the circle of Willis are generally approached. The frontal lobes and olfactory bulbs and tracts lie in the anterior fossa. The temporal lobes lie in the middle fossa, which is shaped like a butterfly. The sella turcica, formed by the sphenoid bone, is the most central part of the middle fossa and houses the pituitary gland. The floor and lateral walls of the middle fossa are shaped from the greater wings of the sphenoid bone and parts of the temporal bone, which house the internal and middle ear structures. The posterior fossa, the largest and deepest fossa, is formed by the occipital, sphenoid, and petrous portions of the temporal bones; the cerebellum and brainstem lie here, as do many cranial nerves. The foramen magnum, the largest opening in the skull, provides passage for the spinal cord to join the brainstem in the posterior fossa. Numerous other openings exist in the base of the skull for passage of arteries, veins, and cranial nerves.
Skull Fractures.
The severity of skull fractures depends on the degree of resulting brain injury. Simple skull fractures can be serious if they cross major vascular channels in the skull. If vessels are torn, epidural or subdural hematomas may form. Depressed skull fractures require a surgical procedure to elevate the depressed bone. Open skull fractures should be irrigated copiously and closed to prevent infection. Basilar skull fractures may cause cerebrospinal fluid (CSF) rhinorrhea or otorrhea. A few patients with these CSF leaks require surgical repair if they do not resolve after 2 weeks.
Deformities of the Cranium.
Craniosynostosis is the most common pediatric skull deformity seen and treated by the neurosurgeon. The phenomenon is a premature closure or lack of formation of cranial sutures, leading to cosmetic abnormalities, eventual life-threatening intracranial pressure (ICP) increases, and arrested brain development unless diagnostic and surgical interventions ensue. Cranial remodeling is most often undertaken during the first year of life, when brain capacity triples (see Chapter 17).
Meninges
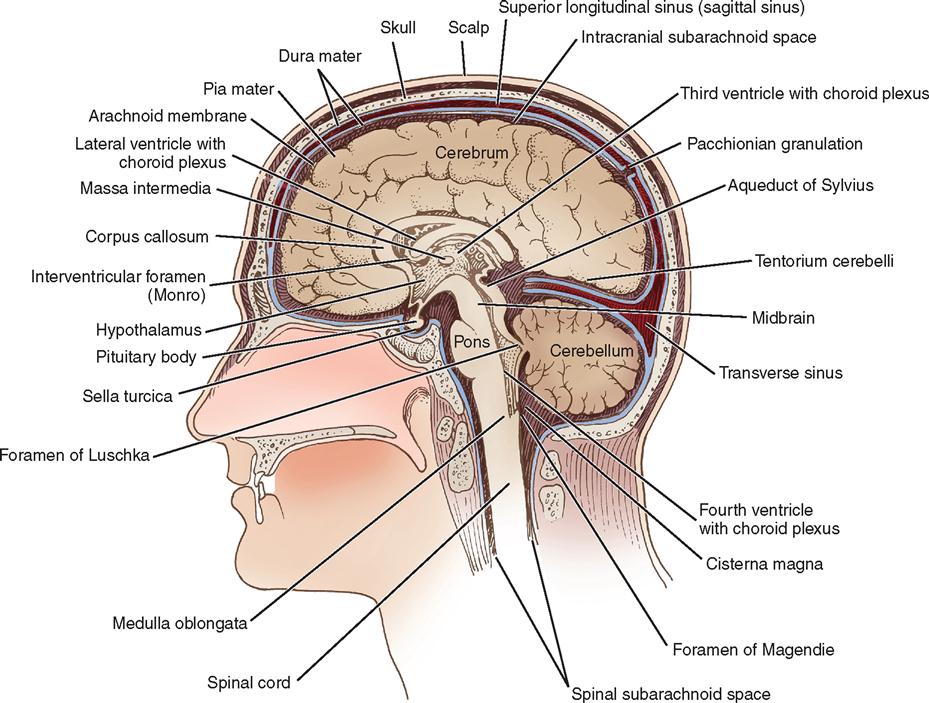
The brain and spinal cord are completely enveloped by the meninges, which are three membranes that provide support and protection. The meningeal layers from superficial to deep are the dura mater, arachnoid mater, and pia mater (see Figure 12-2). The space superficial to the dura is known as the epidural space. The cranial meninges are located between the skull and the brain.
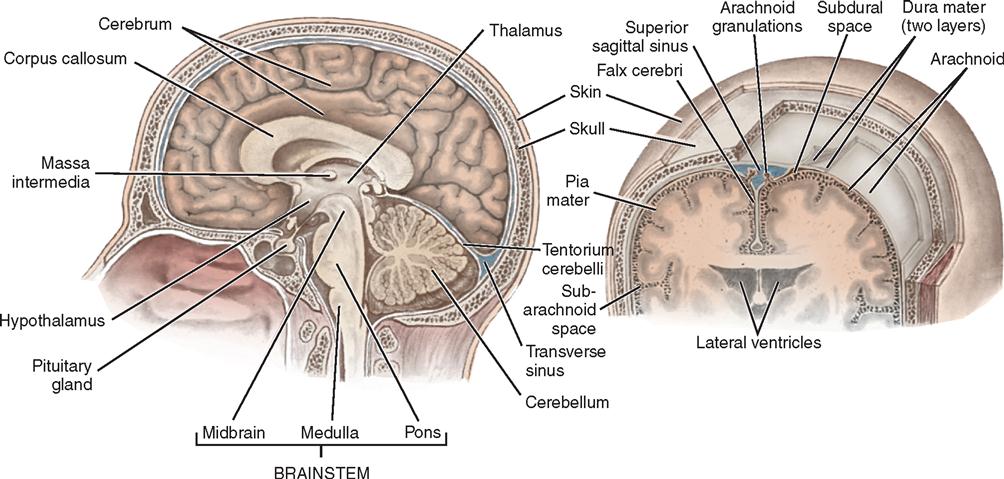
The dura mater is a tough, shiny, fibrous membrane that is close to the inner surface of the skull and folds to separate the cranial cavity into compartments. The largest fold is the falx cerebri—an arch-shaped, vertically placed, midline structure separating the right and left cerebral hemispheres (see Figure 12-2). A smaller fold of dura mater, the falx cerebelli, separates the cerebellar hemispheres vertically. A transverse fold, the tentorium cerebelli, forms the roof of the posterior fossa. The tentorium supports the temporal lobe and occipital lobes of the cerebral hemispheres. Below the tentorium lie the cerebellum and brainstem. Structures above the tentorium are referred to as supratentorial and those below as infratentorial (Figure 12-7). At margins of these dural folds lie large venous sinuses that drain blood from the intracranial structures into the jugular veins. Accidental breaching of a sinus during surgery can cause severe bleeding that is difficult to control and may put the patient at risk for a venous air embolism. Several arteries also lie within the layers of the dura. The largest is the middle meningeal, a source of serious epidural hemorrhage if torn by an overlying skull fracture. The rigid skull makes hemorrhage and swelling in the brain critical events. The volume of the intracranial cavity is fixed. Increasing the intracranial contents by a hemorrhage, tumor, or edema may lead to serious ICP problems. Pressure on brain tissue may cause irreparable damage.
Beneath the dura mater is a transparent membrane called the arachnoid. Although the outer layer of arachnoid closely approximates the dura mater, the space between is considered the subdural space. The inner arachnoid layer forms innumerable weblike filaments that bridge to the surface of the brain (see Figure 12-2). The arachnoid passes over the sulci and fissures of the brain, without dipping into them. The arachnoid is separated from the pia mater beneath it by the subarachnoid space, which is filled with CSF that bathes the brain. Around the base of the brain, particularly, this space becomes enlarged to form cisterns. The major intracranial nerves and blood vessels pass through these compartments. Intracranial approaches can be charted in terms of the basal cisterns.
The pia mater, the innermost membrane, closely follows the contours of the surface of the brain into the sulci and fissures. Only the microscopic subpial space separates the pia from the brain. The pia mater has a rich vascular network. Vascular fringes of pia mater project into the ventricles to form the choroid plexus of the ventricles, which produce CSF.
Brain
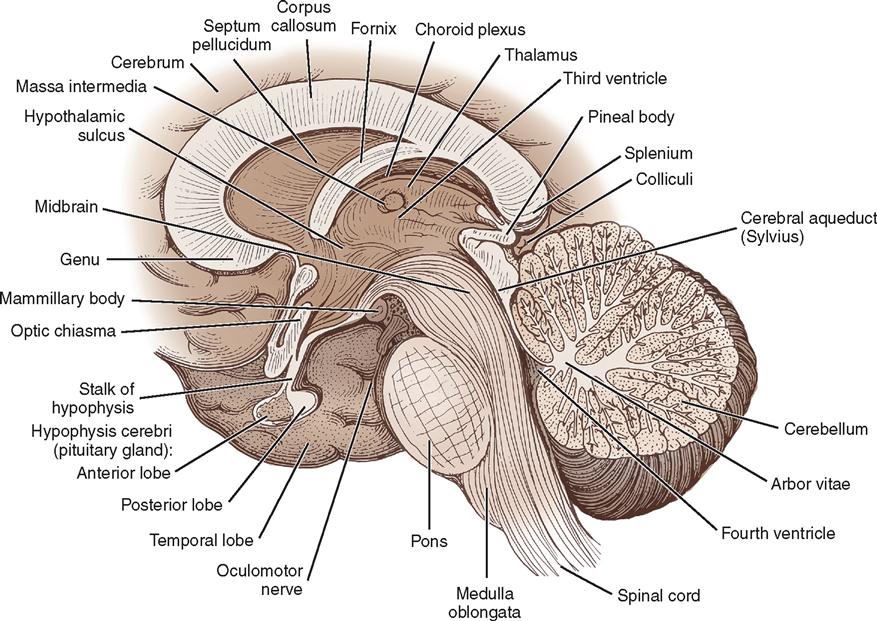
The anatomy of the brain, formally known as the encephalon, can be considered in multiple ways. Based on prenatal development, the principal divisions from rostral (head) to caudal (tail), descending toward the spinal cord, are the forebrain or prosencephalon, the midbrain or mesencephalon, and the hindbrain or rhombencephalon. The rhombencephalon is subdivided into the cerebellum, the medulla oblongata, and the pons. The prosencephalon includes the diencephalon and the telencephalon, or cerebrum. The medulla oblongata, pons, and midbrain are collectively referred to as the brainstem (Figures 12-7 and 12-8).
Cerebrum.
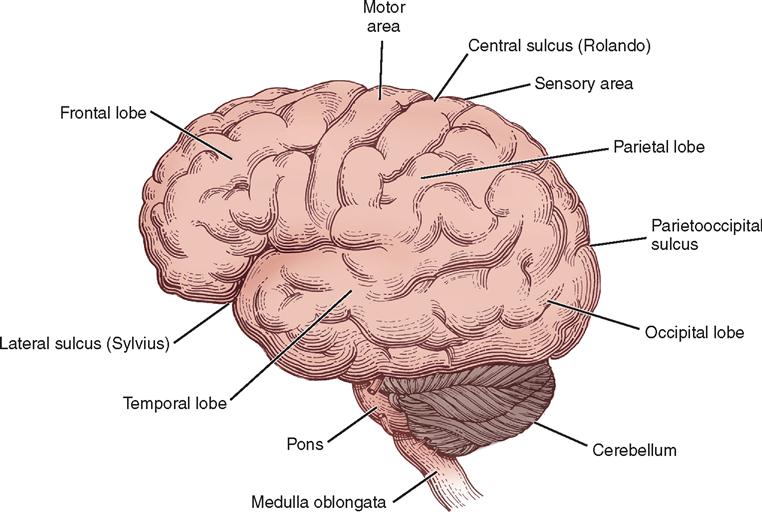
The right and the left cerebral hemispheres are the largest parts of the brain and occupy the anterior and middle fossae. Each hemisphere is divided into frontal, parietal, occipital, and temporal lobes. The two hemispheres are separated by the longitudinal fissure and the falx cerebri but remain connected underneath the falx by a large transverse bundle of nerve fibers called the corpus callosum (see Figure 12-8). Each of the cerebral hemispheres controls sensation and motor activity to and receives sensory stimuli from the opposite half of the body.
The convoluted surface of the cerebrum consists of gray matter, called the cerebral cortex, which contains the cell bodies of the many nerve pathways of the brain. The underlying white matter contains millions of myelinated nerve axons and is relatively avascular compared with the cortex. The nerve pathways, or fiber tracts, are of three types: (1) commissural fibers, which pass from one cerebral hemisphere to the other; (2) association fibers, which connect regions of gyri and lobes longitudinally within a cerebral hemisphere; and (3) projection fibers, including the great motor and sensory systems, which run vertically to connect the cortical regions with other portions of the CNS.
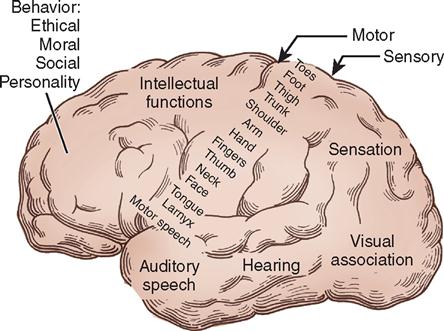
The surfaces of the hemispheres form convolutions called gyri and intervening furrows called sulci, which serve as anatomic landmarks. Two sulci of particular anatomic importance during surgery are (1) the lateral sulcus, or sylvian fissure, which divides the temporal lobe from the frontal and parietal lobes, and (2) the central sulcus, or fissure of Rolando, which separates the frontal from the parietal lobe. The central sulcus also separates the motor cortex (precentral gyrus) from the sensory cortex (postcentral gyrus). The motor cortex lies anterior to the central sulcus, and the sensory cortex lies posterior to the central sulcus. Both the motor cortex and the sensory cortex can be represented by a topographically organized map called a homunculus that proportionately represents each body part at the area of the gyri that controls it. The diagrams illustrate how the number of neurons corresponds to the degree of motor and sensory control required. For example, areas that need more fine motor control, such as the fingers and face, have a higher concentration of neurons than other areas. Keep in mind that the left motor and sensory cortices control the right side of the body and vice versa (Figure 12-9). Destruction of an area of motor cortex results in loss of voluntary motor function on the corresponding area of the opposite side of the body (Figure 12-10).
The frontal lobe is anterior to the central sulcus and controls the higher functions of intellect and abstract reasoning, along with movement, language, and personality. Posterior to the central sulcus is the parietal lobe, extending back to the parietooccipital fissure. This area contains the final receiving and integrating station for sensory impulses, such as pain and touch, from the contralateral side of the body. It is also involved with special relationships and object identification. The occipital lobe lies posterior to the parietooccipital fissure. It receives and integrates visual impulses and registers them as meaningful images (see Figures 12-9 and 12-10).
Inferior to the lateral sulcus, in the middle fossa, is the temporal lobe, which is involved with memory, speech, and smell. Lesions of the left temporal lobe in right-handed persons and in many left-handed persons may affect the comprehension and verbalization of words, resulting in aphasia. The insula (island of Reil) is an area of cortex that lies deep within the lateral sulcus and can be exposed when the upper and lower lips of the fissure are separated. The insula is believed to be involved with smell, taste, touch, and possibly language.
The limbic system consists of large parts of the cortex near the medial wall of the cerebral hemisphere (cingulate and parahippocampal gyri) along with the hippocampus, amygdala, and septum. It is closely and significantly connected with the hypothalamus. It has a diffuse distribution in the brain, and many components of the limbic system have overlapping functions. The hippocampus is critical for learning and memory. The amygdala regulates the perceptive and expressive aspects of emotional and social behavior. The limbic system affects endocrine and autonomic functions of the body, recent memory, emotions, behaviors, and motivational and mood states. Restlessness and hyperactivity may result from lesions of this area.
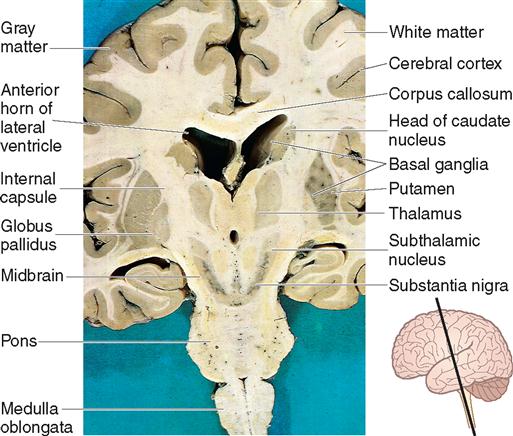
The basal ganglia are subcortical collections of nuclei (gray matter) that include the caudate nucleus, putamen, and globus pallidus (collectively referred to as the corpus striatum); the substantia nigra (which is located in the midbrain); and the subthalamic nucleus (part of the diencephalon). The basal ganglia influence movement and behavior through projections to the thalamus and brainstem and subsequently the cortex (Figure 12-11). The basal ganglia function to promote and support patterns of behavior and movement that are appropriate in a given situation and to inhibit unwanted or inappropriate behavior and movements. Disorders of the basal ganglia are principally characterized by abnormalities of movement, muscle tone, and posture. Damage to these neural components may cause rigidity of the skeletal muscles and various types of spontaneous tremors.
Diencephalon.
The diencephalon is composed of the thalamus, hypothalamus, epithalamus, and subthalamus and surrounds the third ventricle. The thalamus is the major relay station for incoming sensory stimuli. Except for some olfactory impulse transmission, all sensory information transmitted to the cerebral hemispheres is relayed through the thalamus. This is also true for motor pathways from the cerebellum and basal ganglia. Because of the central role of the thalamus in perception of body sensations, surgical lesions can be made in this area in an attempt to alleviate pain.
Along the floor of the third ventricle is the hypothalamus (see Figure 12-8), which is concerned principally with the autonomic regulation of the body’s internal environment and is intimately connected with the pituitary gland. It controls fluid and electrolyte balance, appetite, reproduction, thermoregulation, immune response, and many emotional responses. It influences levels of attention and consciousness. The pituitary gland is suspended from the base of the hypothalamus by the pituitary stalk. It secretes multiple hormones that are regulated by the hypothalamus. A pituitary tumor can result in a hormonal imbalance. It can also encroach on the optic chiasma, causing vision changes.
The subthalamus is a complex region of nuclear groups and fiber tracts, including the subthalamic nucleus, which is considered with the basal ganglia. The epithalamus consists of multiple nuclei and the pineal gland, an endocrine gland that regulates the circadian rhythm.
Brainstem.
The brainstem consists of the midbrain, pons, and medulla oblongata. It is located in the posterior fossa and forms the floor of the fourth ventricle. It is the site of many ascending and descending fiber tracts that allow for communication among the structures of the brain and between the brain and spinal cord. All but 2 of the 12 cranial nerves attach to the brainstem. The short, stocky portion of the brain, between the cerebral hemispheres and pons, is the midbrain (see Figure 12-7), also referred to as the mesencephalon. It is composed of the cerebral peduncles, the substantia nigra, numerous nerve tracts and nuclei, and association centers that control the majority of eye movements. Immediately below the midbrain is the pons, which contains control areas for horizontal eye movement and face movement. The medulla oblongata is continuous with the spinal cord at the foramen magnum. It contains the vital cardiovascular and respiratory regulatory centers (see Figure 12-9). Damage to the brainstem is often devastating and life-threatening, because it can affect movement, senses, consciousness, perception, and cognition.
Cerebellum.
The cerebellum, which occupies most of the posterior fossa, forms the roof of the fourth ventricle (Figures 12-8 and 12-12). It has two lateral lobes, or hemispheres, and a medial portion, the vermis. The fissures of the cerebellum are small and run transversely. The cerebellum is concerned principally with balance and coordination of movement. It has many complex connections with higher and lower centers and exerts its influence unilaterally—in contrast to the cerebral hemispheres, which act contralaterally. By splitting the vermis in the exact midline, a satisfactory exposure of tumors that lie in the fourth ventricle is obtained without sacrificing the important cerebellar functions.
Pathologic Lesions of the Brain.
An estimated new 22,070 brain and other nervous system cancers were estimated to occur in 2009 (American Cancer Society [ACS], 2009). Brain metastases outnumber primary neoplasms by at least 10 to 1, and they occur in 20% to 40% of cancer patients. Because no national cancer registry documents brain metastases, the exact incidence is unknown, but it has been estimated that 98,000 to 170,000 new cases are diagnosed in the United States each year (National Cancer Institute [NCI], 2009).
Multiple factors are suspected of playing a role in the pathogenesis of intracranial neoplasms. Early diagnosis simplifies surgical treatment because increased ICP and severe neurologic changes are not usually present. Brain tumors are either malignant or benign, depending on the cell type. Primary tumors generally do not resemble the carcinomas and sarcomas found elsewhere in the body and rarely metastasize outside the CNS. Both primary and metastatic tumors of the brain and its membranes are included in the term intracranial tumors.
Traditionally, tumors are classified by cell type; however, classification of brain tumors is an evolving process. The widely used World Health Organization (WHO) system lists more than 120 types of brain tumors (NCI, 2009). Table 12-1 gives the approximate percentage of CNS tumors by histologic type. A brief description of a select list of brain tumors follows:
1. Tumors of intraepithelial tissue encompass gliomas, tumors believed to originate from neuroglial cells.
a. Astrocytomas are the most common of all primary brain tumors. They usually occur in the cerebellum of children and the cerebrum of adults. They are often cystic and discrete in children and infiltrating and ill-defined in adults. Astrocytomas are classified in the WHO system based on the principal cell type and on the degree of anaplasia as grade I to IV, with grade I being the more favorable type of tumor and grade IV being the most malignant. Glioblastoma multiforme (GBM), a grade IV astrocytoma, is an infiltrative, fast-growing, rapidly recurring cerebral tumor that occurs most frequently in the sixth and seventh decades of life. It is the most common type of primary brain tumor, accounting for about 50% of gliomas (Sloan et al, 2005). It is one of the few tumors that is capable of invading both cerebral hemispheres by crossing the midline. Areas of necrosis are characteristic. Recent studies have consistently demonstrated the benefits of radical surgical resection. Postoperative radiation therapy significantly improves survival. Even with aggressive multimodality therapy, median survival is less than 1 year and 5-year survival is less than 5% (Sloan et al, 2005).
b. Oligodendroglioma, typically found in the cerebral hemispheres, is usually infiltrating but occasionally moderately well-defined. It frequently presents in middle age with seizure. It is now believed that the true incidence of oligodendrogliomas is 5% to 15% of gliomas, much higher than previously thought. Therapy usually consists of surgery followed by radiation therapy and chemotherapy (Sloan et al, 2005).
c. Ependymoma occurs most frequently in children and is likely to arise in or near the ventricular walls. It commonly occurs in the fourth ventricle, where it abuts or involves vital medullary centers (Figure 12-13). It also frequently metastasizes into the subarachnoid spaces. This tumor accounts for 3% to 4% of gliomas. Surgical resection followed by radiation therapy is the usual treatment. The 5-year survival rate is 73% (Central Brain Tumor Registry of the United States [CBTRUS], 2007-2008).
d. Medulloblastoma is a fast-growing, rapidly recurring tumor of the vermis of the cerebellum and fourth ventricle that usually occurs in young children. It characteristically metastasizes into the subarachnoid spaces, usually spreading to the base of the brain by this route. It accounts for 15% to 20% of childhood intracranial brain tumors and is the most common malignant pediatric brain tumor (Figure 12-14).
2. Tumors of the meninges (meningiomas) commonly occur in people in the fourth to sixth decades of life (Al-Mefty and Heth, 2005). They are usually benign, circumscribed, slow-growing tumors, arising from arachnoid cells with secondary attachment to the dura. Various factors have been implicated in the development of meningiomas. They typically involve the cortex and bone of the skull with growth. They can be very vascular and may adhere to the dural venous sinuses or major arteries, making their complete removal challenging. However, meningiomas often can be totally surgically removed.
4. Hemopoietic neoplasms and lymphomas: CNS involvement with lymphoma may occur secondarily from a systemic lymphoma or may arise primarily in the CNS (CBTRUS, 2007-2008). The main role for surgery is for tumor biopsy. Stereotactic techniques are well suited for these often-deep tumors. The standard treatment after biopsy is radiation therapy, allowing for a median survival of 10 months. Adding chemotherapy can prolong survival (Greenberg, 2005).
5. Germ cell tumors occur in the midline (suprasellar and pineal region). Other than benign teratomas, all intracranial germ cell tumors are malignant and may metastasize by way of CSF and systemically. Tumors of the pineal region are very challenging to the neurosurgeon. Open microsurgery, endoscopy, and stereotactic biopsy are surgical options. Pineal region tumors often cause hydrocephalus. An endoscopic third ventriculostomy or a shunting procedure is routinely performed to alleviate the symptoms of hydrocephalus. Radiation therapy, chemotherapy, and radiosurgery are also treatment considerations.
b. Teratoma is a congenital tumor containing embryonic elements.
d. Choriocarcinoma is an extremely rare, very malignant neoplasm.
6. Cysts and tumorlike lesions include the following types:
7. Tumors of the sellar region include the following types:
a. Pituitary adenomas can be classified as nonfunctioning or functioning. Nonfunctioning pituitary adenomas account for approximately 30% of pituitary tumors, usually occur in people in the fourth and fifth decades of life, and do not cause clinical hormone hypersecretion. They are typically large and cause hypopituitarism or blindness from regional compression. The usual treatment is endoscopic or microscopic transsphenoidal removal of the tumor. Following operative decompression, vision improves in approximately 80% of patients (Oyesiku, 2005). Radiation therapy or stereotactic radiosurgery may also be used. Functioning pituitary adenomas secrete excess quantities of pituitary hormones. The question of medical versus surgical treatment is ever present in the management of this group of patients. Adenomas may be further subdivided into microadenomas, which are less than 1 cm in diameter and usually discovered because of an endocrinopathy, and macroadenomas, which are larger than 1 cm and usually present with compressive effects of the tumor (Oyesiku, 2005).
(2) Eosinophilic adenomas are secretory, causing an excessive amount of growth hormone in the serum.
b. Craniopharyngiomas account for 2.5% to 4% of intracranial tumors, with 50% occurring in childhood (Greenberg, 2005). They arise from the region of the pituitary stalk and typically contain both solid and cystic components. Calcification above the sella turcica is often seen radiographically. In addition to headache, vertigo, vomiting, and papilledema, diabetes insipidus and visual field changes are common. Although complete surgical removal is often impossible if it adheres to the carotid artery or hypothalamus, a subtotal resection with radiation offers favorable results.
8. Metastatic tumors are the most common brain tumor seen clinically, making up about half of brain tumors. They usually arise from carcinomas, more rarely from sarcomas, and occasionally from melanomas and retinal tumors. The most common sources are lung and breast cancer. The management of brain metastasis is complex and controversial. The current principal options for treatment include whole-brain radiation therapy, surgery, and stereotactic radiosurgery. The most important prognostic variables are the extent of systemic disease and the patient’s functional status and age. These factors, along with the size, number, and location of tumors, guide treatment decisions. Median survival only increases from 3 to 6 months with radiation therapy and steroids to 9 to 12 months with surgery and stereotactic radiosurgery (McCutcheon, 2005).
TABLE 12-1
Frequency of Primary CNS Tumors in Adults
| Tumor Type | Percentage |
| Glioblastoma | 50 |
| Meningioma | 17 |
| Astrocytoma | 10 |
| Pituitary adenoma | 4 |
| Oligodendroglioma | 3 |
| Ependymoma | 2 |
| Medulloblastoma | 2 |
| Neurilemmoma | 2 |
| Hemangioma | 2 |
| Craniopharyngioma | 1 |
| Pinealoma | 1 |
| Sarcoma | 1 |
| Others | 5 |
Modified from Janus TJ, Yung WKA: Primary neurological tumors. In Goetz CG: Clinical neurology, ed 3, Philadelphia, 2007, Saunders.
A brain lesion is diagnosed by history, neurologic examination, diagnostic studies (especially computed tomography [CT] scan and magnetic resonance imaging [MRI]), and biopsy. The manifestations of an intracranial tumor fall into two classes: those resulting from irritation or impairment of function in specific areas of the brain directly affected by the tumor and those resulting from diffuse increased ICP. The most common presentation of brain tumors is progressive neurologic deficit, usually motor weakness. Headache and seizures are also common presenting symptoms (Greenburg, 2005).
Large left or bifrontal lobe tumors may cause striking personality changes and depressive symptoms. Lesions in the left frontotemporal region, where motor speech originates, lead to aphasia. Parietal lobe lesions may result in contralateral weakness and sensory changes, along with defects in the perception of objects. Occipital tumors produce hemianoptic visual defects.
Cortical tumors frequently produce focal seizures of diagnostic value. The onset of epileptiform seizures in an adult is often associated with an intracranial neoplasm. Posterior fossa tumors often manifest their presence by blocking the CSF circulation, but they may also destroy cerebellar function, resulting in incoordination, ataxia, scanning speech, and deafness.
Treatment of brain tumors, although based on the characteristics of the tumor, can involve administration of steroids or antiepileptic medications, management of hydrocephalus, surgery, radiosurgery, radiation, and chemotherapy.
OTHER ENCEPHALOPATHIES.
Pathological conditions other than brain tumors may require surgical intervention either for definitive diagnosis or treatment of increased intracranial pressure or seizures. Signs and symptoms may be related to increased ICP and are similar to intracranial tumors. Diagnostic studies are usually the same. A brief description of several nontumor lesions follows.
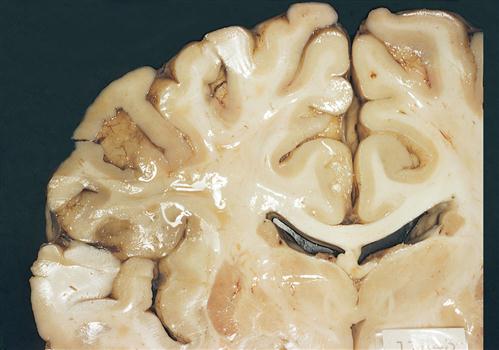
1. Brain abscess is an intracerebral collection of pus. Typically, anaerobic bacteria are involved and induce an inflammatory response that encapsulates necrotic brain tissue. Etiology may remain unknown, but is usually related to history of mastoiditis, sinusitis, dental abscess, osteomyelitis, cranial trauma, neurosurgical procedures, or endocarditis (Figure 12-15).
2. Neurocysticercosis (NCC) is a parasitic infection caused by the pork tapeworm Taenia solium in its larval form. It is the most common infestation of the central nervous system (Rajshekhar, 2010) and mainly seen in developing countries. Intracranial cysticercus lesions are diagnosed following imaging studies and confirmed by antibodies in the serum. Cysts develop to encapsulate the larvae and produce symptoms based on their location within the central nervous system. Surgical management for CNS cysticercal lesions is indicated in cases of cysts located in the ventricles, cisterns, or parenchyma when imaging studies are equivocal.
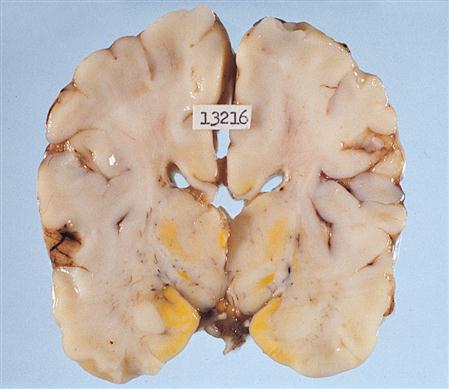
3. Encephalomalacia is a condition of softening of brain matter or loss of brain tissue. Cerebral infarct, ischemia, and trauma are common causes. Though less a cause of increased ICP, encephalomalacia may be observed during craniotomy, noted on diagnostic imaging studies, or as a pathologic finding at autopsy (Figure 12-16).
4. Kernicterus, also known as bilirubin encephalopathy, is a rare neurological condition in neonates. Severe jaundice in the first 1 to 3 weeks of infancy may result in the excess bilirubin moving from the circulatory system and collecting in brain tissue (Figure 12-17). Swelling of brain tissues may progress to late-stage full neurological syndrome and result in permanent brain damage or death. Early detection is critical to prevent this complication. Treatment may include phototherapy or exchange transfusion.
The presentation of multiple brain lesions in a patient is of grave concern, and an infective process should be considered along with the possibility of multiple tumors. Stereotactic biopsy of the lesion is most likely to provide a diagnosis. The operative team may be required to employ precautions to prevent the spread of an unknown infective process. Identification of the infective agent and process determines proper treatment.
Neural Tube Defects
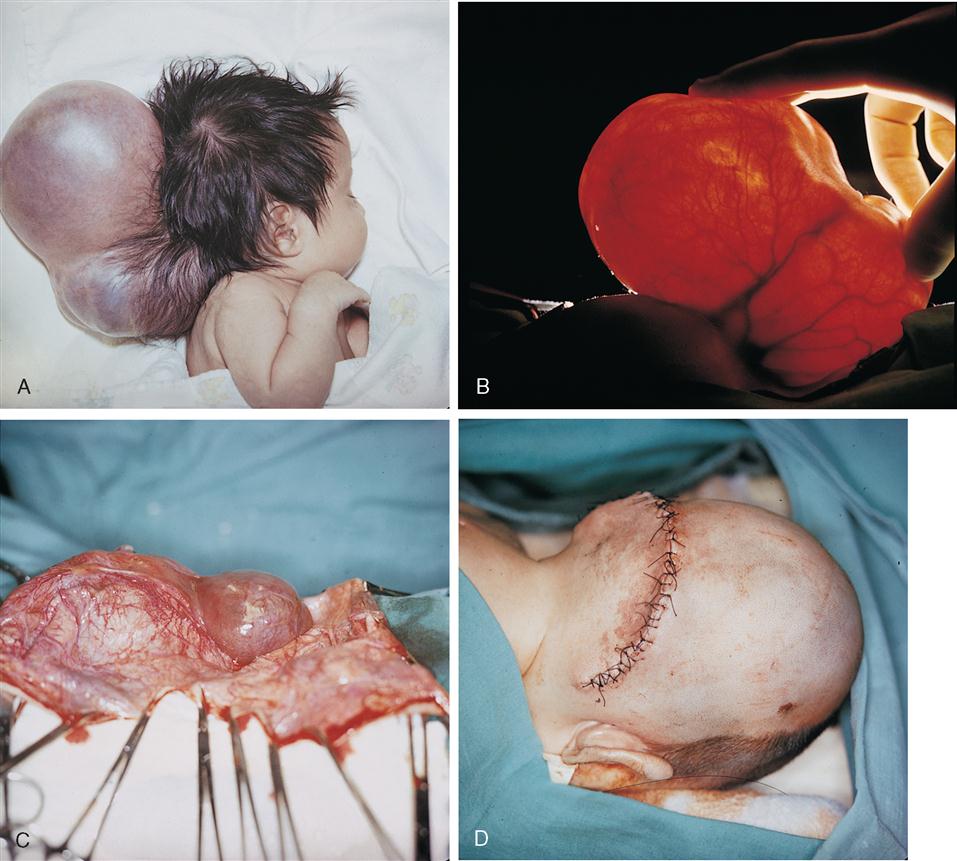
1. Encephaloceles are sac-like protrusions through skull defects in the midline. They can project anteriorly between the forehead and nose or, more commonly, posteriorly in the occipital region of the head (Figure 12-18). Meningeal membranes, CSF, and some neural tissue may be found in the sac. Posterior encephaloceles frequently present in combination with hydrocephalus, spastic quadriplegia (paralysis of all extremities), microcephaly (unusually small brain and head), ataxia (uncoordinated muscle movement affecting gait), visual difficulties, retardation, and seizures. Surgery is performed to excise the sac and close the defect. An encephalocele is a congenital anomaly that results from failure of the neural tube to close by the 28th day following conception, before most women realize they are pregnant. It is estimated that about 375, or 1 of every 10,000, babies are born yearly in the United States with encephaloceles (Centers for Disease Control and Prevention [CDC], 2009). Treatment of hydrocephalus secondary to neural tube defects is accom plished with placement of a shunt to reduce intracranial pressure.
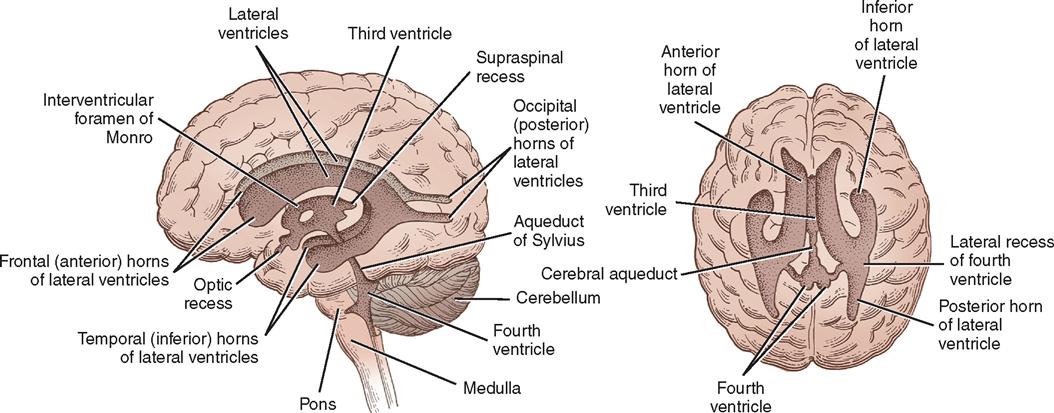
Ventricular System and Cerebrospinal Fluid
Within the brain are four communicating cavities, or ventricles, filled with CSF. In the lower medial portion of each cerebral hemisphere lies a large lateral ventricle that resembles a wishbone and is separated anteriorly from its counterpart by a thin septum (see Figure 12-12). Each lateral ventricle has a body and three horns: frontal, occipital, and temporal. Below the bodies of the lateral ventricles is a central cleft, or third ventricle. It communicates anteriorly with the lateral ventricles through the foramen of Monro and posteriorly with the fourth ventricle through the aqueduct of Sylvius—a long, narrow channel passing through the midbrain. The fourth ventricle is a cavity in the posterior fossa, between the cerebellum and the brainstem. In the roof of the fourth ventricle is the foramen of Magendie, an opening into the cisterna magna; at the lateral margins are the two foramina of Luschka, which open into the cisterna pontis. These cisterns are cavities that serve as reservoirs for CSF.
Much of the CSF originates in the choroid plexuses of the ventricles. These are tufted, vascular structures that allow certain fluid elements of the blood to pass through their ependymal linings. The choroid plexus is found along the floor in each lateral ventricle, on the roof of the third ventricle, and in the posterior portion of the fourth ventricle. Most of the fluid is formed in the lateral ventricles and flows through the interventricular foramen of Monro to the third ventricle and through the aqueduct of Sylvius to the fourth ventricle, where it escapes into the subarachnoid space of the basal cisterns through the foramina of Magendie and Luschka. From the basal cisterns the fluid flows around the spinal cord, over the cerebellar lobes, around the medulla and the base of the brain, and over the cerebral hemispheres in the subarachnoid space. The fluid is absorbed into the venous circulation through villi of the arachnoid (pacchionian granulations) into the great dural venous sinuses, particularly the superior sagittal sinus, and by diffusion through perivascular, perineural, and periradicular channels (see Figure 12-7).
Spinal fluid bathes the brain and spinal cord, helps support the weight of the brain, and acts as a cushion for the brain and spinal cord by absorbing some of the force of external trauma. By variation in its volume, it aids in keeping ICP relatively constant. If the brain atrophies, the amount of CSF increases to fill the dead space; if the brain swells, the amount of CSF decreases to compensate for the increase in brain mass. The fluid can carry certain drugs to diseased parts of the brain. It does not, however, play a significant role in supplying nutrition to the structures that it bathes. The total amount of circulating CSF averages 150 ml in the adult. The ventricles contain about 25 ml, and the remaining CSF circulates in the cranial and spinal subarachnoid space. CSF is secreted at a rate of between 21 and 24 ml/hr, or approximately 450 ml/24 hr. This means that in an adult, CSF is recirculated about three times each day.
Pathologic Conditions Related to Cerebrospinal Fluid.
CSF can be examined by the lab to provide diagnostic information. CSF is most commonly obtained by way of lumbar puncture (LP). Because the subarachnoid space surrounding the brain is freely connected to the subarachnoid space of the spinal cord, any abnormal increase in ICP will be directly reflected as an increase at the lumbar site. Tumors, infection, hydrocephalus, and intracranial bleeding can cause increased intracranial and spinal pressure (Pagana and Pagana, 2009). LP is contraindicated when ICP is increased from a suspected intracranial mass that is causing neurologic symptoms. In this situation, the sudden reduction in pressure from the release of CSF could cause brain herniation. The ventricular fluid normally has a protein content of 5 to 15 mg/dl, whereas the protein content of spinal fluid is 25 to 45 mg/dl. These values may be considerably elevated in pathologic conditions of the CNS. The characteristics of normal spinal fluid are as follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree