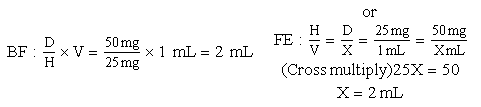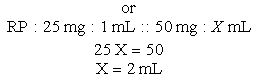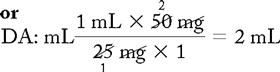PEDIATRICS
Objectives
• Use the two primary methods of determining pediatric drug dosages.
• State the reason for checking pediatric dosages before administration.
• Describe the dosage inaccuracies that can occur with pediatric drug formulas.
FACTORS INFLUENCING PEDIATRIC DRUG ADMINISTRATION
Drug dosages for children differ greatly from those for adults because of the physiological differences between the two groups. Neonates and infants have immature kidney and liver function, which delays metabolism and elimination of many drugs. Drug absorption in neonates is different as a result of slow gastric emptying. Decreased gastric acid secretion in children younger than 3 years contributes to altered drug absorption. Neonates and infants have a lower concentration of plasma proteins, which can cause toxic effects with drugs that are highly bound to proteins. They have less total body fat and more total body water. Therefore lipid-soluble drugs require smaller doses because less than normal fat is present, and water-soluble drugs can require larger doses because of a greater percentage of body water. As children grow, changes in fat, muscle, body water, and organ maturity can alter the pharmacokinetic effects of drugs. Most drugs are dosed according to weight, and doses are specifically calculated for each child. For example, a dose of cefazolin for a 34-kg, 12-year-old child is larger than a dose for a 7-kg, 8-month-old infant. It is the nurse’s responsibility to ensure that a safe drug dosage is given and to closely monitor signs and symptoms of adverse reactions to drugs. The purpose of learning how to calculate pediatric drug doses is to ensure that each child receives the correct dose within the therapeutic range.
Oral
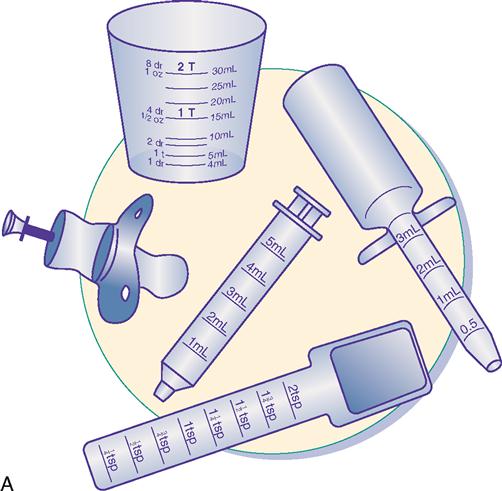
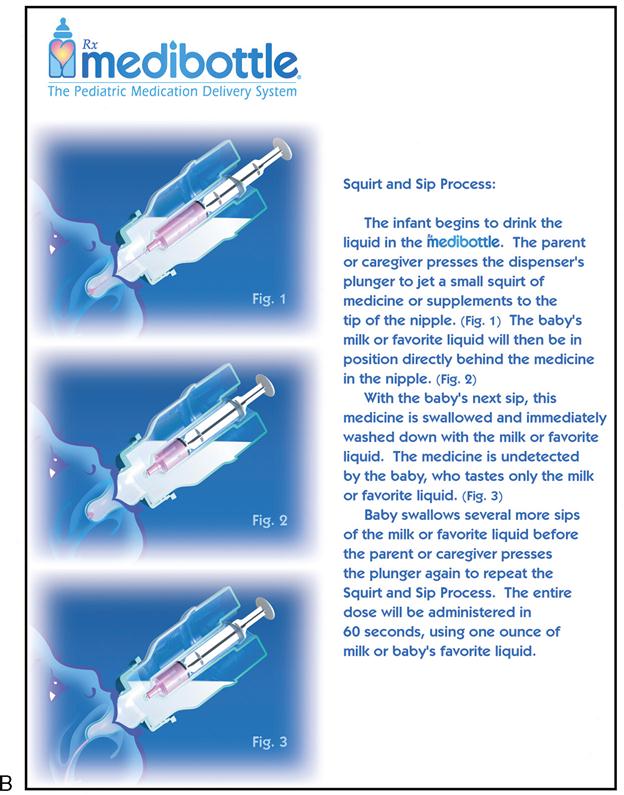
Oral pediatric drug delivery often requires the use of a calibrated measuring device because most drugs for small children are provided in liquid form. The measuring device can be a small plastic cup, an oral dropper, a measuring spoon, an oral syringe, or a specially designed pediatric medication dispenser such as the medibottle (Figure 11-1). The medibottle is a specially designed pediatric medication dispenser that provides optimum drug delivery by allowing small volumes of medication to be swallowed with oral fluids. Some liquid medications come with their own calibrated droppers. The type of measuring device chosen depends on the developmental level of the child. For infants and toddlers, the oral syringe, dropper, and medibottle, provide better drug delivery than is provided by a small cup. A young child who is cooperative is able to use a small cup or measuring spoon. All liquid medications can be drawn up with an oral syringe to ensure accuracy and then are transferred to a small cup or measuring spoon. It may be necessary to refill the cup or spoon with water or juice and to have the child drink that as well to ensure that all prescribed medication has been administered. Medicine should not be mixed in the infant’s or toddler’s bottle because the full dose will not be administered if the child doesn’t finish the bottle. Any medication with a strong taste should not be mixed in formula because the infant could begin to refuse formula. Avoid giving oral medications to a crying child or infant, who could easily aspirate the medication. Some chewable medications are available for administration to the older child. Because many drugs are enteric-coated or are provided in timed-release form, the child must be told which medications are to be swallowed and not chewed.
Intramuscular
Intramuscular sites are chosen on the basis of the age and muscle development of the child (Table 11-1). All injections should be given in a manner that minimizes physical and psychosocial trauma. The child must be adequately restrained, if necessary, and provided with a momentary distraction. The procedure must be performed quickly, with comfort measures immediately following.
TABLE 11-1
Pediatric Guidelines for Intramuscular Injections According to Muscle Group*
| AMOUNT BY MUSCLE GROUP (mL) | |||||
| Vastus Lateralis | Rectus Femoris | Ventro-gluteal | Dorsal Cluteal | Deltoid | |
| Neonates | 0.5 mL | Not safe | Not safe | Not safe | Not safe |
| Infants | |||||
| 1-12 months | 0.5-1 mL | Not safe | Not safe | Not safe | Not safe |
| Toddlers | |||||
| 1-2 years | 0.5-2 mL | 0.5-1 mL | Not safe | Not safe | 0.5-1 mL |
| Preschool | |||||
| 3-5 years | 0.5-2 mL | 0.5-1 mL | Not safe | Not safe | 0.5-1 mL |
| School age | |||||
| 6-12 years | 2 mL | 2 mL | 0.5-3 mL | 0.5-2 mL | 0.5-1 mL |
| Adolescent | |||||
| 12-18 years | 2 mL | 2 mL | 2-3 mL | 2-3 mL | 1-1.5 mL |
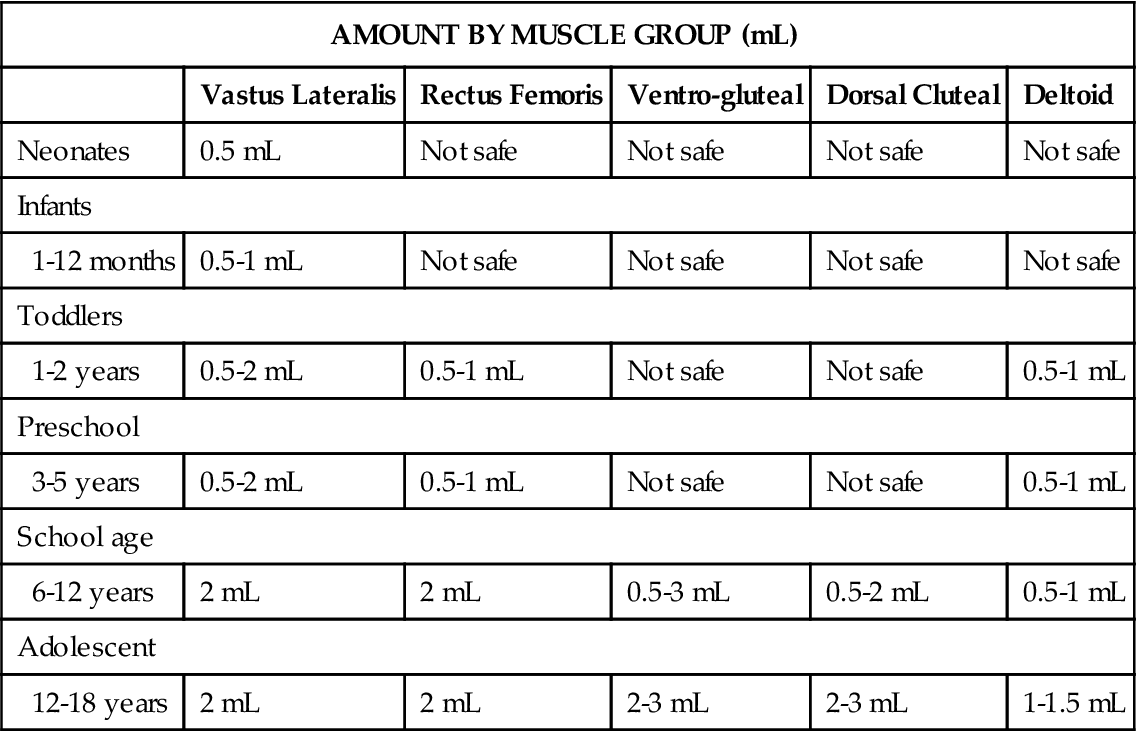
*The safe use of all sites is based on normal muscle development and size of the child. Follow institutional policies and procedures.
Intravenous
For children, the maximum amount of intravenous (IV) fluid varies with body weight. Their 24-hour fluid status must be monitored closely to prevent overhydration. The amount of fluid given with IV medication must be considered in the planning of their 24-hour intake (Table 11-2). After the correct dosage of drug is obtained, it may need further dilution and to be given over a specified time, as mentioned in Chapter 10. Usually, the drug is diluted with 5 to 60 mL of IV fluid, depending on the drug or dosage, placed in a calibrated cylinder or syringe pump, and infused over 20 to 60 minutes, depending on the type of drug. After the drug has been infused, the cylinder is flushed with 3 to 20 mL of IV fluid to ensure that the child has received all of the medication and to prevent admixture. All fluid volume is considered intake. Refer to Chapter 10 for methods of calculating IV infusion rates.
TABLE 11-2
Pediatric Guidelines for 24-Hour Intravenous Fluid Therapy
100 mL/kg for first 10 kg body weight
50 mL/kg for the next 10 kg body weight
20 mL/kg after 20 kg body weight
Example: Child’s weight 25 kg
100 mL/kg × 10 kg = 1000 mL
50 mL/kg × 10 kg = 500 mL
20 mL/kg × 5 kg = 100 mL
1600 mL for 24 hours, or 66.6 mL/hr, or 67 mL
The safety factors that must be considered when medications are administered to children are similar to those for adults. See Appendix A for more detailed information on safe nursing practice for drug administration.
PEDIATRIC DRUG CALCULATIONS
The two main methods of determining drug dosages for pediatric drug administration are body weight and body surface area (BSA). For both, a current weight is essential. The first method uses a specific number of milligrams, micrograms, or units for each kilogram of body weight (mg/kg, mcg/kg, unit/kg). Usually, drug data for pediatric dosage (mg/kg) are supplied by manufacturers in a drug information insert. BSA, measured in square meters (m2), is considered a more accurate method than body weight. BSA takes into consideration the relation between basal metabolic rate and surface area, which correlates with blood volume, cardiac output, and organ growth and development. Although BSA has been used primarily to calculate the dosage of antineoplastic agents, BSA is used when there is a narrow margin between therapeutic and toxic doses. Pharmaceutical manufacturers are including BSA parameters (mg/m2, mcg/m2, units/m2) in the drug information.
If the manufacturer does not supply data for pediatric dosing, the child’s dosage can be determined from the adult dose. The BSA formula is used to calculate the pediatric dose. The BSA formula is considered more accurate than previously used formulas, such as Clark’s, Young’s, and Fried’s rules. Drug calculations performed according to the BSA formula are safer than those done with formulas that rely solely on the child’s age or weight. Although the BSA formula has improved the accuracy of drug dosing in infants and children, calculation of drug doses for neonates and preterm infants are weight based because BSA does not guarantee complete accuracy.
Dosage per Kilogram Body Weight
The following information is needed to calculate the dosage:
a. Physician’s order with the name of the drug, the dosage, and the frequency of administration.
b. The child’s weight in kilograms:
1 kg = 2.2 lb
c. The pediatric dosage as listed by the manufacturer or hospital formulary.
EXAMPLES
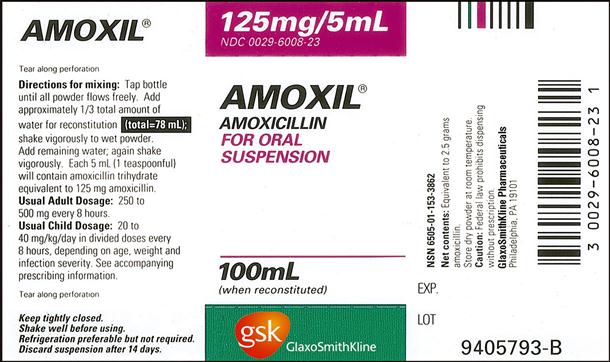
a. Order: amoxicillin (Amoxil) 60 mg, po, tid.
Child’s weight: 12½ lb.
b. Change pounds to kilograms.
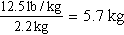
c. Pediatric dosage for children who weigh 20 kg: 20-40 mg/kg/day in three equal doses.
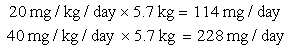
Step 2: Multiply the dosage by the frequency to determine the daily dose.
The order for amoxicillin 60 mg, po, tid means that three doses will be given per day.
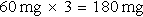
d. Drug preparation:
Use the basic formula (BF), ratio and proportion (RP), or dimensional analysis (DA).
Basic Formula
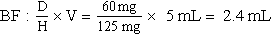
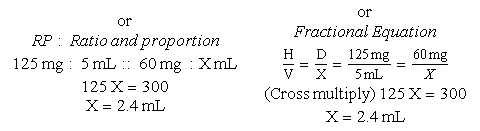
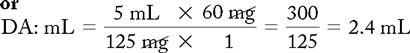
a. Order: ampicillin 350 mg, IV, q6h.
Child’s weight and age: 61.5 lb and 9 years old.
Dilution instructions: Mix with 20 mL of D5/1/4 NSS; infuse over 20 minutes.
Flush with 15 mL.
b. Change pounds to kilograms.
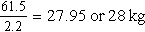
c. Pediatric dose is 25 to 50 mg/kg/day in divided doses.
Step 1: Multiply weight by minimum and maximum daily dose:
25 mg × 28 kg = 700 mg/day
50 mg × 28 kg = 1400 mg/day
Step 2: Multiply the dose by the frequency:
350 mg × 4 = 1400 mg/day
The dose is considered safe because it does not exceed the therapeutic range.
d. Drug available: When diluted, 500 mg = 2 mL. Use your selected formula to calculate the dosage.

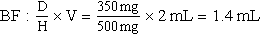
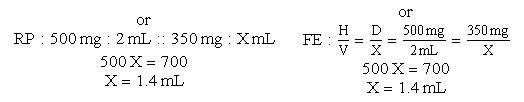
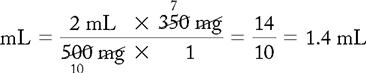
e. Amount of fluid to infuse medication:
1.4 mL + 20 mL (dilution) = 21.4 mL
f. Flow rate calculation (60 gtt/mL set):

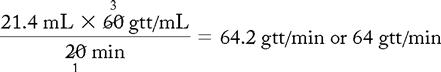
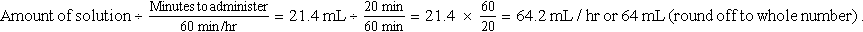
Dosage per Body Surface Area
The following information is needed to calculate the dosage:
a. Physician’s order with name of drug, dosage, and time frame or frequency.
b. Child’s height, weight in kilograms, and age.
c. Information on how the drug is supplied.
d. Pediatric dosage (in m2) as listed by manufacturer or hospital formulary.
EXAMPLES
a. Order: methotrexate 50 mg, IV, × 1.
b. Child’s height, weight, age: 134 cm, 32.5 kg, 9 yr.
c. Pediatric dose: 25-75 mg/m2 wk.
d. Drug preparation: 25 mg/mL.
e. BSA with square root (see BSA metric formula on p. 95)
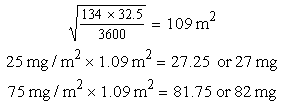
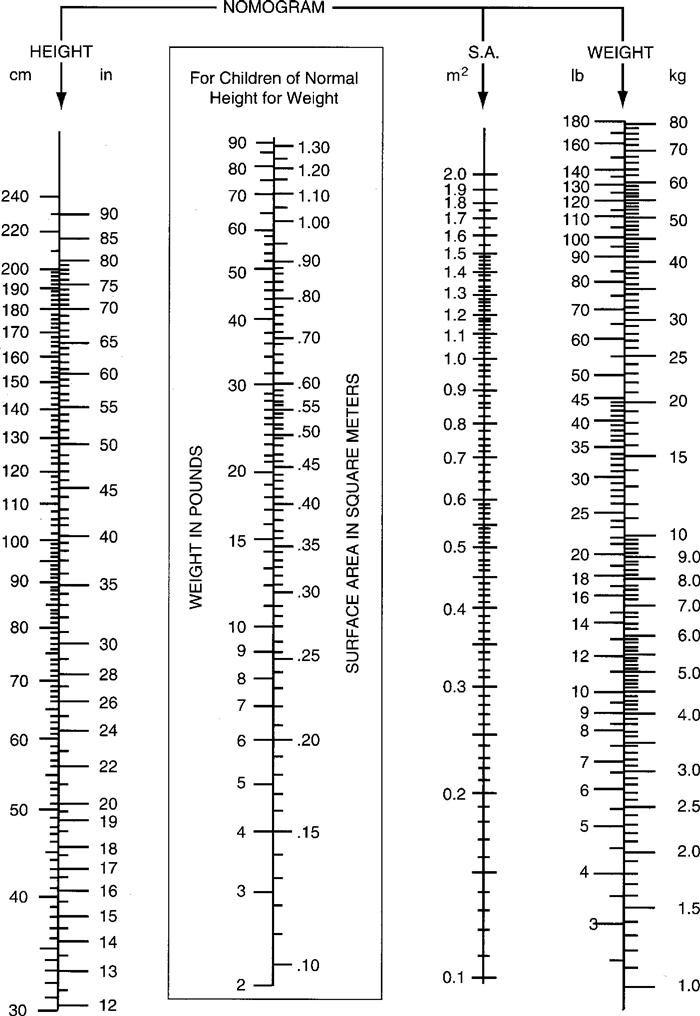
f. BSA nomogram for children: The child’s height (134 cm) and weight (32.5 kg) intersect at 1.11 m2 BSA.
Multiply the BSA, 1.11 m2, by the minimum and maximum dose. (Substitute BSA for weight.)
25 mg/m2 × 1.11 m2 = 28.0 mg
75 mg/m2 × 1.11 m2 = 83.0 mg
This dose is considered safe because it is within the therapeutic range for the child’s BSA.