INTRODUCTION
Anesthesiology is a “team sport.” Providing the best and safest care for patients depends on all members of the team—surgeons, nurses, and anesthesia providers—communicating in a timely, efficient, and patient-focused manner. Anesthesiologists today not only provide patient care in the operating room but also have patient responsibilities in other areas, including preoperative anesthesia clinics (PACs), postanesthesia care units (PACUs), obstetrics, ambulatory surgery centers, endoscopy suites, postoperative pain management, critical care units, and chronic pain management.
Anesthesia is a term derived from the Greek meaning “without sensation” and is commonly used to indicate the condition that allows patients to undergo a variety of surgical or nonsurgical procedures without the pain or distress they would otherwise experience. More importantly, this blocking of pain and/or awareness is reversible. Anesthesiology is the medical practice of providing anesthesia to patients and is most commonly provided by a medical doctor, an anesthesiologist, either alone or in conjunction with a certified registered nurse anesthetist (CRNA), anesthesia assistant, or resident physician-in-training. Anesthesia is most often described as being a general anesthetic, that is, a drug-induced loss of consciousness during which patients are not arousable even by noxious stimulus and often require a controlled airway. Anesthesia can also be provided without inducing unconsciousness by utilizing regional blockade, local anesthesia with monitored anesthesia care (MAC), or conscious sedation.
HISTORY OF ANESTHESIA
One of modern medicine’s most important discoveries was that the application of diethyl ether (ether) could provide the classic requirements of anesthesia: analgesia, amnesia, and muscle relaxation in a reversible and safe manner. Crawford Long was the first to use ether in 1842, and William Morton’s successful 1846 public demonstration of ether as an anesthetic in the “Ether Dome” of Massachusetts General Hospital ushered in the modern day of anesthesia and surgery. Chloroform was used by Sir James Y. Simpson to provide analgesia to Queen Victoria in 1853 during the birth of Prince Leopold. This royal approval of inhalation agents led to the wide acceptance of their use as surgical anesthesia. Ether (flammability, solubility) and chloroform (liver toxicity) each had significant drawbacks, and over time, inhalation agents were developed with similar anesthetic effects but much safer physiologic and metabolic properties.
Cocaine’s ability to produce topical anesthesia for ophthalmic surgery was discovered in the late 1800s. The hypodermic needle was introduced in 1890 and facilitated the injection of cocaine to produce reversible nerve blockade and later the injection of cocaine via a lumbar puncture to produce a spinal anesthetic and the first spinal headache. The chemical properties of cocaine were soon determined and manipulated to synthesize numerous other local anesthetic agents used to achieve what became known as regional anesthesia, which lacks the unconsciousness and amnesia of the general anesthetics but does produce analgesia and lack of motor movement in the “blocked” region.
OVERALL RISK OF ANESTHESIA
Anesthesia is performed over 70 million times per year in the United States and is remarkably safe. The number of anesthesia-related deaths has decreased dramatically in the last 30 years because of intense scrutiny by the American Society of Anesthesiologists. The realization that most problems are related to airway compromise has led to advanced respiratory monitoring utilizing pulse oximetry and capnography for every patient undergoing anesthesia. The Institute of Medicine’s To Err Is Human complimented the anesthesiology community for its marked decrease in morbidity and mortality from anesthesia. Overall, the risk of anesthesia-related death in the healthy patient is estimated to be as low as 1:100,000-200,000 anesthetics.
The combination of amnesia, with or without unconsciousness, analgesia, and muscle relaxation is purposely induced by an anesthetic caregiver and is achieved either by administering inhalation agents in appropriate doses to affect the central nervous system (CNS) or by using specific intravenous pharmacologic agents to produce the same effects as inhaled vapors. These agents include amnestics, for example, benzodiazepines (midazolam or diazepam); analgesics, for example, the opioids morphine and fentanyl derivatives; the neuromuscular blocking drugs succinylcholine, pancuronium, or vecuronium; and sedative hypnotics, for example, sodium pentothal and propofol. All of the agents have adverse physiologic consequences: respiratory depression, cardiovascular depression, and loss of consciousness. Furthermore, some of these agents may induce allergic reactions. Some have also been known to trigger malignant hyperpyrexia.
The most common problems associated with adverse outcomes today still relate to airway compromise, medication errors, and central venous cannulation. Other concerns are postoperative neurologic complications (eg, nerve injury), ischemic optic neuropathy, coronary ischemia, anesthesia in remote locations (eg, interventional radiology sites), and probably most importantly, inadequate preoperative evaluation and preparation.
The anesthesia provider must be able to (1) achieve a state of anesthesia quickly and safely by choosing the appropriate techniques and agents, taking into consideration the patient’s medical condition; (2) maintain and monitor a state of anesthesia throughout the surgical procedure while compensating for the effects of varying degrees of surgical stimulation and blood and fluid losses; (3) reverse the muscle relaxation and amnesia as necessary; and (4) return the patient to physiologic homeostasis while maintaining sufficient analgesia to minimize postprocedure pain.
PREOPERATIVE EVALUATION
A preoperative evaluation is a responsibility of the anesthesiologist and is a basic element of anesthesia care (Table 11–1). This evaluation consists of information gathered from multiple sources, including the patient’s medical record, history and physical examinations, and findings from medical tests and consults or other evaluations performed prior to the patient being seen by the anesthesiologist. Improved patient outcome and satisfaction is the result of an adequate, structured, formal presurgical, or preprocedure evaluation and preparation performed on all patients.
Goals Optimize patient condition Understand and control comorbidities and drug therapy Ensure patient’s questions are answered Timing High surgical invasiveness: at least 1 day prior Medium invasiveness: day before or day of surgery Low invasiveness: day of surgery Content Review medical records Directed history and physical examination: airway, heart, and lungs Indicated laboratory or additional consultations |
The timing of the preoperative evaluation depends primarily on the degree of planned surgical invasiveness. For high surgical invasiveness, the initial assessment should be done at a minimum the day before the planned procedure by the anesthesia staff. Patients undergoing medium surgical invasive procedures can be evaluated the day before or even on the day of surgery, and for low surgical invasiveness, the initial assessment may be done the day of surgery. Time must be allotted to follow up on conditions discovered during the preoperative visit and to answer patient questions. Perioperative complications and deaths are most often a combination of patient comorbidities, surgical complexity, and anesthesia effects. The Physical Status Classification of the American Society of Anesthesiologists is the best known of many perioperative classification schemes (Table 11–2). This classification system does not assign risk but is a common language used to describe patients’ preoperative physical status. The system is an alert to the anesthesia practitioner and all members of the patient care team.
ASA 1 (PS1): A normal healthy patient ASA 2 (PS2): A patient with mild systemic disease ASA 3 (PS3): A patient with severe systemic disease ASA 4 (PS4): A patient with severe systemic disease that is a constant threat to life ASA 5 (PS5): A moribund patient who is not expected to survive without the operation ASA 6 (PS6): A declared brain-dead patient whose organs are being removed for donor purposes E: Emergency |
Patients should ideally be seen in a PAC staffed by anesthesia personnel who evaluate patients from the anesthetic perspective and who look for physical conditions (airway) and controlled, uncontrolled, or unrecognized medical conditions that can lead to perioperative morbidity and mortality. There must be adequate communication between anesthesiologist and surgeon such that any conditions that may result in patient compromise are optimally addressed. Optimally, a patient’s medical status has been adequately addressed by the patient’s primary care physician prior to being referred to the PAC. However, in some instances, only a cursory “cleared for anesthesia and surgery” may result in a necessary delay. Any patients other than healthy ASA 1 or 2 patients should be seen in a PAC. Prior to referring patients to the PAC, the surgeon should have already ordered the necessary preoperative labs and in many instances will have already detected uncontrolled medical conditions that require consultations from other specialties in order to recommend and in some instances improve a patient’s status.
The optimal preoperative evaluation has the following two elements: (1) content—readily accessible medical records, patient interview, a directed preanesthesia examination, indicated preoperative laboratory tests, and additional consultations when indicated; the minimum acceptable examination includes an assessment of the airway, heart, and lungs well in advance of the planned date of surgery; and (2) preoperative tests—only as indicated by comorbidities and never as a screen, these tests should be specifically aimed at helping the anesthesiologist formulate an anesthetic plan.
The anesthesiologist should specifically ask the patient about previous operations, anesthetic type, and any complications, for example, allergic reactions, abnormal bleeding, delayed emergence, prolonged paralysis, difficult airway management, awareness, or jaundice. Each of these describes a possible specific anesthetic morbidity that must be further investigated either by history or specific testing. Medical conditions detected as decreased exercise tolerance, shortness of breath, orthopnea, kidney or liver disease, and metabolic abnormalities, for example, diabetes or thyroid disease, should be ascertained. A comprehensive history seeks to identify serious cardiac conditions, for example, unstable coronary syndromes, angina, myocardial infarctions either recent or past, decompensated congestive heart failure, significant arrhythmias, or severe valvular disease. Any recent changes in cardiac symptoms or other associated diseases, for example, diabetes, renal disease, or cerebrovascular disease symptoms, should be identified.
Any family history of adverse responses to anesthetics (malignant hyperthermia) and social history of smoking, drug use, and alcohol consumption is important. Finally, a comprehensive review of concurrent medications including antihypertensives, insulin, bronchodilators, or any other medications that can interact with anesthetic agents should be documented. Certain medications may result in increased or decreased anesthetic requirements, prolongation of muscle relaxants, abnormal responses to sympathomimetics, delayed or enhanced metabolism of anesthetics, and/or augmentation of the depressant effects of anesthetics. The patient’s use of herbal medicines can have an adverse reaction with some anesthetics (Table 11–3) and all should be discontinued 2-3 weeks before surgery.
| Name (Other Names) | Alleged Benefits | Perioperative Effects | Recommendations |
|---|---|---|---|
| Echinacea | Stimulates immune system | Allergic reactions; hepatotoxicity; interference with immune suppressive therapy (eg, organ transplants) | Discontinue as far in advance of surgery as possible |
| Ephedra (ma huang) | Promotes weight loss; increases energy | Ephedrine-like sympathetic stimulation with increased heart rate and blood pressure, arrhythmias, myocardial infarction, stroke | Discontinue at least 24 h prior to surgery; avoid monoamine oxidase inhibitors |
| Garlic (ajo) | Reduces blood pressure and cholesterol levels | Inhibition of platelet aggregation (irreversible) | Discontinue at least 7 days prior to surgery |
| Ginkgo (duck foot, maidenhair, silver apricot) | Improves cognitive performance (eg, dementia), increases peripheral perfusion (eg, impotence, macular degeneration) | Inhibition of platelet-activating factor | Discontinue at least 36 h prior to surgery |
| Ginseng | Protects against “stress” and maintains “homeostasis” | Hypoglycemia; inhibition of platelet aggregation and coagulation cascade | Discontinue at least 7 days prior to surgery |
| Kava (kawa, awa, intoxicating pepper) | Decreases anxiety | GABA-mediated hypnotic effects my decrease MAC (see Chapter 7); possible risk of acute withdrawal | Discontinue at least 24 h prior to surgery |
| St. John’s wort (amber, goatweed, Hypericum perforatum, klamathe-weed) | Reverses mild to moderate depression | Inhibits serotonin, norepinephrine, and dopamine reuptake by neurons; increases drug metabolism by induction of cytochrome P-450 | Discontinue at least 5 days prior to surgery |
| Valerian | Decreases anxiety | GABA-mediated hypnotic effects may decrease MAC; benzodiazepinelike withdrawal syndrome | Taper dose weeks before surgery if possible; treat withdrawal symptoms with benzodiazepines |
After the vital signs are obtained, the physical examination begins with the upper airway. Ability to control the airway is mandatory. The focus of the examination is to assess those factors that would make airway control (eg, endotracheal intubation) difficult or impossible. Seven keys to the upper airway examination should be documented:
Range of motion of the cervical spine: Patients should be asked to extend and flex their neck to the full range of possible motion so the anesthesiologist may look for any limitations.
Thyroid cartilage to mentum distance: ideal is greater than 6 cm.
Mouth opening: ideal is greater than 3 cm.
Dentition: dentures, loose teeth, poor conservation.
Jaw protrusion: ability to protrude the lower incisors past the upper incisors.
Presence of a beard.
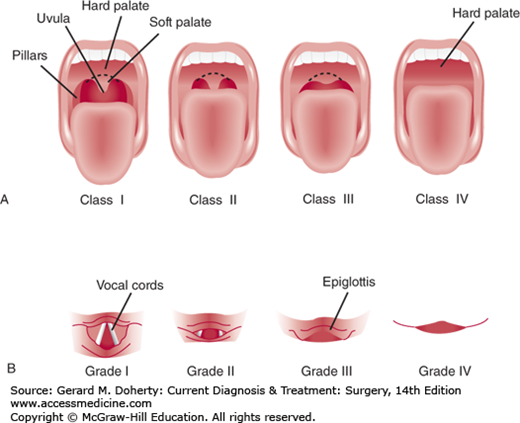
Examination and classification of the upper airway based on the size of patient’s tongue and the pharyngeal structures visible on mouth opening with the patient sitting looking forward. This visual description of the airway structures is known as the Mallampati score (Figure 11–1).
Grade I The soft palate, anterior and posterior tonsillar pillars, and uvula are visible—suggests easy airway intubation.
Grade II Tonsillar pillars and part of the uvula obscured by the tongue.
Grade III Only soft palate and hard palate visible.
Grade IV Only the hard palate is visible—suggests challenging airway.
The physical examination then focuses on heart and lungs, potential intravenous catheter sites, and potential sites for regional anesthesia. Range of motion of limbs must also be noted as this may affect positioning in the operating room. Finally, any neurologic abnormalities must be noted.
When a metabolic or physical finding or symptom is discovered during this visit, the anesthesiologist may believe that a specialty consultation is necessary to suggest ways to optimize the patient for surgery and anesthesia. If this is the case, the anesthesiologist should communicate with the surgeon in order to prevent unnecessary or unexpected delays in the surgical schedule. It is imperative that any consults ordered be completed and the results be available by the day of surgery.
The anesthesiologist can then advise the patient on appropriate options for general anesthesia versus regional techniques based on the patient’s history, physical examination, and type of surgery. Although some surgical procedures must always be performed under general anesthesia, the anesthesiologist may discuss other options with the patient. If the referring surgeon has a particular preference for a type of anesthetic, such preferences should be communicated to the anesthesiologist directly rather than through the patient. It is also best if the referring surgeon does not promise any specific agent or technique without first consulting with the anesthesia care givers.
The anesthesiologist must discuss with the patient the requirements for preprocedure fasting and the management of medications up to the time of surgery or procedure. Current guidelines for preoperative fasting are as follows: (1) No solid food should be eaten after the evening meal. At the minimum, most anesthesiologists delay an anesthetic so that the last solid food was 6-8 hours prior to nonemergent surgery or procedures involving anesthesia. (2) NPO after midnight except for sips of water to take oral medications. Water may be ingested up to 2 hours before checking in for surgery. Some institutions allow other clear liquids, for example, coffee, a few hours prior to surgery or procedure. However, because surgery schedules can change abruptly and procedure time may be moved forward, NPO after midnight is the best policy. (3) Pediatric fasting guidelines vary among institutions, so practitioners should consult with their particular pediatric anesthesia group.
In the perioperative period patients should continue taking beta-blocking agents, statin medications, antihypertensives except the angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACE) antihypertensive medications. Patients taking ARBs or ACE inhibitors can experience marked hypotension with the induction of general anesthesia and respond poorly to common vasopressors. Patients taking anticoagulants such as Coumadin, clopidogrel, and the newer anticoagulants such as dabigatran (Pradaxa) should stop these medications prior to most surgeries. The timing and bridging of cessation of these anticoagulants is the responsibility of the surgical service. Continuation of low-dose aspirin for patients with coronary stents or atrial fibrillation is recommended. Oral antihyperglycemic agents need should not be taken the day of surgery. Insulin-dependent patients should receive instructions from the anesthesiologist during the preanesthesia visit as to the management of their insulin. Other medications should be discussed with the patient and surgery team at the time of the preoperative anesthesia visit.
Comorbidities should be well controlled in the preoperative period to avoid postprocedure morbidity, and even mortality.
Hypertension is the most common preexisting medical disease identified preoperatively and is a major risk factor for renal, cerebrovascular, peripheral vascular, cardiac ischemia or infarction, and congestive heart failure. The triad of lipid disorders, diabetes, and obesity is classically found in patients with hypertension and should alert the clinician that further evaluation for these conditions is needed. Hypertension has an association with coronary artery disease, and the preoperative evaluation is a unique opportunity to identify and treat the nonessential causes of hypertension. The literature strongly supports the notion that all hypertensive patients should be treated medically to be as close to normotension as possible before any planned surgical procedure. Diastolic pressures of 110 mm Hg or higher result in a higher incidence of intraoperative hypotension and myocardial ischemia. However, the literature does not support delaying surgery if the delay would be detrimental to the patient. The introduction of perioperative selective beta-blocking drugs provides a marked benefit in reducing the incidence of significant myocardial ischemia during the perioperative period. Although somewhat controversial, starting patients on beta-blockers immediately preoperatively may have some risk, but any patient already taking beta-blockers should continue taking the drug preoperatively.
Ischemic heart disease is a leading cause of death in the United States and is the leading cause of morbidity and mortality in the perioperative period. About 25% of patients who present for surgery each year have coronary artery disease, and thus much of the preoperative evaluation focuses on detecting the presence and degree of ischemic heart disease and determining whether it is likely to impact anesthesia and surgery. A major goal of preoperative assessment of cardiac status is to determine what, if any, interventions—coronary artery bypass graft (CABG), percutaneous coronary intervention (PCI)—would benefit patients undergoing noncardiac surgery. In general, preoperative cardiac tests are recommended only if the information obtained will lead to changes in patient management. However, certain active clinical conditions (Table 11–4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree