WHAT CAUSES ABNORMAL TEST RESULTS (BESIDES DISEASE)?
The total testing process defines the preanalytic, analytic, and postanalytic phases of laboratory testing and serves as the basis for designing and implementing interventions, restrictions, or limits that can reduce or remove the likelihood of errors. Over the last several years, there has been a remarkable decrease in error rates, especially analytic errors. Evidence from recent studies demonstrates that a large percentage of laboratory errors occur in preanalytic and postanalytic steps. Errors in the preanalytic (61.9%) and postanalytic (23.1%) processes occurred much more frequently than occurrences of analytic errors (15%). About one fourth of these can have consequences to the patient either in delay of the test result or life threatening.
PREANALYTIC ERRORS
Preanalytic factors act on both the patient and the specimen before analyses. These factors may be divided further into those acting in vivo (biologic or physiologic) and those acting in vitro (specimen handling and interference factors).
PHYSIOLOGIC FACTORS
Some physiologic factors are beyond our control. They include age, sex, and race, and so on, and can be managed by placing appropriate reference limits. Others factors such as diet, starvation, exercise, posture, diurnal and seasonal variations, menstrual cycle, and pregnancy must be considered in the interpretation of the test results. Age has noticeable effect on some test results and the need for establishing appropriate reference intervals. In newborns, the composition of blood is affected by the maturity of the infant at birth. At birth, RBC and hemoglobin values are higher than adults due to low levels of oxygen in the uterus. They continue to decrease and level out to adult values about the age of 15. Adult values are usually taken as the reference for comparison with those of the young and the elderly. The concentration of most test constituents remains constant between puberty and menopause in women and between puberty and middle age in men. The plasma concentrations of many constituents increase in women after menopause. Hormone levels are affected by aging. However, changes in concentrations are much less pronounced than an endocrine organ’s response to stimuli. Until puberty, there are few differences in laboratory data between boys and girls. After puberty, the characteristic changes in the levels of sex hormones become apparent.
In addition to the commonly known hormonal changes during the menstrual cycle, there is a preovulatory increase in the concentrations of aldosterone and renin. Coincident with the ovulation, serum cholesterol levels are lower than at any other phase of the menstrual cycle. In pregnancy, a dilutional effect is observed due to the increase in mean plasma volume, which in turn causes hemodilution. Normal pregnancy is characterized by major physiologic adaptations that alter maternal blood chemistry and hematology laboratory values. In addition, there are time-related fluctuations in the levels of certain analytes. Many analytes—such as cortisol, thyrotropin (TSH), growth hormone, potassium, glucose, iron, and proinflammatory cytokines—exhibit diurnal variation. Hormones such as luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone released in short bursts lasting barely 2 minutes make accurate measurements problematic. Seasonal changes also affect certain analytes like vitamin D (higher during summer), cholesterol, and thyroid hormones (higher during winter). Changes in the levels of some of the constituents in blood occur when measured at sea level as opposed to measurement at a higher altitude. Hematocrit, hemoglobin, and C-reactive protein (CRP) can be higher at high altitude. Levels of plasma renin, transferrin, urinary creatinine, creatinine clearance, and estriol decrease with increasing altitude.
The dietary effect of laboratory test results is complex and simply cannot be separated into the categories of “fasting” and “nonfasting” status of the patient. A significant variation in some routine tests after a regular meal showing that fasting time needs to be carefully considered when performing tests to prevent spurious results. Clinically significant differences are observed within 1–4 hours for triglycerides, albumin, ALT, calcium, iron, LDH, phosphorus, magnesium, lymphocytes, RBC, hemoglobin, hematocrit, etc. The type of diet (high fat, low fat, vegetarian, malnutrition), length of time since last meal, and test-specific dietary concerns can affect some tests. Consumption of caffeine, bran, serotonin (consumption of fruits and vegetables such as bananas, avocados, and onions), herbal preparations (e.g., aloe vera, Chinese rhubarb, senna, quinine, and quinidine), recreational drug use, ethanol, and smoking can induce both short- and long-term effects that alter the results of several analytes. Differentiations of the effects of race from those of socioeconomic conditions are difficult. Carbohydrate and lipid metabolism differ in blacks and whites. Glucose tolerance is less in blacks, Polynesians, and Native Americans in comparison to whites.
Physical stress and mental stress influence the concentrations of many plasma constituents, including cortisol, aldosterone, prolactin, TSH, aldosterone, cholesterol, glucose, insulin, and lactate. With blindness, the normal stimulation of the hypothalamic–pituitary axis is reduced. Consequently, certain features of hypopituitarism and hypoadrenalism may be observed. In some blind individuals, the normal diurnal variations of cortisol may persist; in others, it does not. Fever provokes many hormonal responses as does shock and trauma. The stress of surgery has been shown to reduce the serum triiodothyronine (T3) levels by 50% in patients without thyroid disease.
Transfusions and infusions can also significantly affect the concentration of certain laboratory values. For persons receiving an infusion, blood should not be obtained proximal to the infusion site. Blood should be obtained from the opposite arm. A minimum of 8 hours must elapse before blood is obtained from a subject who has received fat emulsion. For patients receiving blood transfusions, the extent of hemolysis and with it increased levels of potassium, lactate dehydrogenase (LDH), and free hemoglobin are released progressively to the age of the transfused blood.
Exercise such as running up and down several flights of stairs or strenuous activity such as working out in a gymnasium or marathon running the night before the specimen collection can affect the results obtained for several analytes. To minimize preanalytic variables introduced by exercise, subjects should be instructed to refrain from strenuous activity on the night before testing and not to exert themselves by walking a long distance, running, or climbing stairs before blood specimen collection. In addition, the muscle damage associated with trauma of surgery will increase the serum activity of enzymes originating in skeletal muscles, and this activity may persist for several days.
The plasma and extracellular volumes decrease within a few days of the start of bed rest. With prolonged bed rest, fluid retention occurs and plasma protein and albumin levels may be decreased by an average of 0.5 and 0.3 g/dL, respectively. As a result, concentrations of bound protein are also reduced. Changes in posture during blood sampling can affect the concentrations of several analytes measured in serum or plasma. Change in posture from a supine to an erect or sitting position can result in a shift in body water from intravascular to interstitial compartments. As a result, the concentrations of larger molecules that are not filterable are increased. These effects are accentuated in patients with a tendency for edema, such as in cardiovascular insufficiency and cirrhosis of the liver.
SPECIMEN HANDLING FACTORS
Among controllable preanalytic variables, specimen collection is most critical. Unacceptable specimen collection due to misidentification, insufficient volume to perform test, incorrect whole blood-to-anticoagulant ratio, and specimen quality (hemolysis, clots, contaminated, collected in wrong container) accounts for the majority of preanalytic errors. Hemolysis, lipemia, and icteric samples have variable effects on assays and depend upon testing method and analyte. The time and temperature for storage of the specimen and the processing steps in the preparation of serum or plasma or cell separation can introduce preanalytic variables.
Pneumatic tube systems of various lengths are routinely used in many hospitals to transport blood collection tubes to the testing laboratory. Significant differences between plasma but not serum levels of LDH are observed when blood collection tubes are transported through pneumatic tube systems. The application of a tourniquet, by reducing the pressure below the systolic pressure, maintains the effective filtration pressure within the capillaries. As a result, small molecules and fluid are transferred from the intravasal space to the interstitium. Application of tourniquet for longer than 1 minute can result in hemoconcentration of large molecules that are unable to penetrate the capillary wall. To minimize the preanalytic effects of tourniquet application time, the tourniquet should be released as soon as the needle enters the vein. Avoidance of excessive fist clenching during phlebotomy and maintaining tourniquet application time to no more than 1 minute can minimize preanalytic errors.
Various salts of heparin, ethylenediaminetetraacetic acid (EDTA), and sodium citrate are used widely in the clinical laboratory. Heparin is the preferred anticoagulant for blood specimens for electrolyte levels and other routine chemistry tests. Obvious differences in the results of certain analytes between serum and heparinized plasma are related to the consumption of fibrinogen and the lysis of cellular elements during the process of clotting. EDTA is the commonly used anticoagulant for routine hematologic determinations. It functions as anticoagulant by chelating calcium ions, which are required for the clotting process. Citrate has been used as an anticoagulant for collection of blood specimens intended for global coagulation tests such as prothrombin time (PT) and partial thromboplastin time (PTT). A laboratory that has been using one of the concentrations (3.2% or 3.8%) to perform PT determination for patients receiving oral anticoagulant therapy should not interchange the formulations. Doing so will affect the international normalized ratios (INRs) that are used to report the results of PT. Sodium fluoride and lithium iodoacetate have been used alone or in combination with anticoagulants such as potassium oxalate, EDTA, citrate, or lithium heparin for blood collection. In the absence of glycolytic inhibitors, a decrease in the glucose level of as much as 24% can occur in 1 hour after blood collection in neonates, in contrast to a 5% decrease in healthy individuals when specimen is stored at room temperature. The anticoagulant-to-blood ratio is critical for some laboratory tests. In general, collecting of blood specimens to less than nominal volume increases the effective molarity of the anticoagulant and induces osmotic changes affecting cell morphologic features. Furthermore, the binding of analytes such as ionic calcium or magnesium to heparin can be enhanced when the effective concentration of unfractionated heparin increased beyond the normal 14.3 U/mL of blood. In addition, stability of various analytes are significantly reduced in plasma (Lithium Heparin) compared to serum tubes when plasma is stored, but not separated from the gel after centrifugation.
Virtually, all drugs may affect the results of clinical laboratory tests that includes both in vivo (pharmacologic) and in vitro (interferences and methodologic) effects. The problem of drug interference is complex, and physicians are generally aware of main therapeutic effects of drug, but many secondary effects are ignored. Some of the examples are increase in liver enzymes with Dilantin and barbiturates, increase in fibrinogen, transferrin, amylase, etc. following oral contraceptives and contrast agents (gadolinium) decreasing total calcium levels.
In coagulation, testing knowledge or access to the patient history may be necessary, as many medications such as anticoagulant therapies (warfarin, heparin, and direct thrombin inhibitors), blood product, and component transfusion and coagulation factor replacement therapies all impact coagulation test results. Over-the-counter drugs (aspirin) have prolonged effect on platelet function studies. In addition, the patient’s physiologic state plays a role.
The quality of the specimens submitted to the microbiology laboratory is critical for optimal specimen evaluation. The general techniques of specimen collection and handling that have been established both to maximize the yield of organisms and isolate relevant pathogens from specimens obtained from different body sites should be reviewed with clinical laboratory prior to obtaining the specimen. In addition, valid interpretation of the results of culture can be achieved only if the specimen obtained is appropriate for processing. As a result, care must be taken to collect only those specimens that may yield pathogens, rather than colonizing flora or contaminants. Specific rules for the collection of material vary, depending on the source of the specimen, but several general principles apply. Prompt transport of specimens to the microbiology laboratory is essential to optimize the yield of cultures and the interpretation of results. Delays in processing may result in the overgrowth of some microorganisms or the death of more fastidious ones. Samples for bacterial culture should ideally arrive in the microbiology laboratory within 1 to 2 hours of collection. If a delay is unavoidable, most specimens (with the exception of blood, cerebrospinal fluid, joint fluid, and cultures for Neisseria gonorrhoeae) should be refrigerated until transported.
ANALYTIC ERRORS
Clinical laboratories have long focused their attention on quality control methods and quality assessment programs dealing with analytic aspects of testing. Total analytic error (or measurement error) refers to assay errors from all sources arising from the data collection experiment. Some error is expected, because not all components of measuring are the same. There are four major types of experimental error: random (not predictable), systematic (one direction), total (random and systematic), and idiosyncratic (nonmethodologic).
Errors due to analytic problems have been significantly reduced over time, but there is evidence that, particularly for immunoassays, interference may have a serious impact on patients. Paraproteins can interfere in chemical measurements when they form precipitates during the testing procedure. Heterophilic antibodies are human antibodies that can bind animal antibodies. They can cause problems in immunoassays, particularly immunometric assays, where they can form a bridge between the capture and detection antibodies, leading to false-positive results in the absence of analyte or if analyte is also present, a false increase in measured concentrations. Very rarely, heterophilic antibodies can also lead to false-negative or falsely low results.
Very high hormone levels can interfere with immunoassay systems, resulting in falsely low analyte determinations. This is attributable to the “hook effect,” which describes the inhibition of immune complex formation by excess antigen concentration. There are proteins that are well known to form aggregates with immunoglobulins or high molecular weight proteins. Clinically relevant proteins that can have “macro” forms—including amylase, creatinine kinase, LDH, and prolactin—can elevate the results when using certain laboratory tests, yet the patient lacks clinical disease related to elevated analyte concentration.
Immunoassay interference is not analyte specific and is variable with respect to time. In some patients, this interference can lost for a long time and in some for only a short time. This interference affects lots of assays but not all of them. In addition, a different manufacturer’s test kits have different cross-reactions with interference compounds, and the test results vary from lab to lab.
Incorrect results can also occur as a result of a large number of biologically common phenomena causing analytic variation. These include cold agglutinins, rouleaux, osmotic matrix effects, platelet agglutination, giant platelets, unlysed erythrocytes, nucleated erythrocytes, megakaryocytes, red cell inclusions, cryoproteins, circulating mucin, leukocytosis, in vitro hemolysis, extreme microcytosis, bilirubinemia, lipemia, and so on.
DIAGNOSTIC TEST VALUES
Before a method is used routinely, method evaluation protocols must ensure that the measurement procedure meets defined criteria, for example, the accuracy, precision, and stability required in meeting the laboratory’s patient population needs. Four indicators are most commonly used to determine the reliability of a clinical laboratory test. Two of these, accuracy and precision, reflect how well the test method performs day to day in a laboratory. The other two, sensitivity and specificity, deal with how well the test is able to distinguish disease from the absence of disease.
The accuracy and precision of each test method are established and are frequently monitored by the clinical laboratory. Sensitivity and specificity data are determined by research studies and clinical trials. Although each test has its own performance measures and appropriate uses, laboratory tests are designed to be as precise, accurate, specific, and sensitive as possible.
ACCURACY AND PRECISION
“Accuracy” (trueness) refers to the ability of the test to actually measure what it claims to measure and is defined as the proportion of all test results (both positive and negative) that are correct. Precision (repeatability) refers to the ability of the test to reproduce the same result when repeated on the same patient or sample. The two concepts are related, but different. For example, a test could be precise but not accurate if on three occasions it produced roughly the same result but that result differed greatly from the actual value determined by a reference standard.
Sensitivity is defined as the ability of the test to identify correctly those who have the disease. It is the number of subjects with a positive test who have the disease divided by all subjects who have the disease. A test with high sensitivity has few false-negative results. Specificity is defined as the ability of the test to identify correctly those who do not have the disease. It is the number of subjects who have a negative test and do not have the disease divided by all subjects who do not have the disease. A test with high specificity has few false-positive results. Sensitivity and specificity are most useful when assessing a test used to screen a free-living population. These test characteristics are also interdependent (Figure 1-1): an increase in sensitivity is accompanied by a decrease in specificity and vice versa.
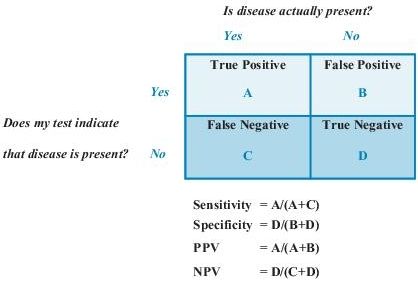
Figure 1–1 Sensitivity, specificity, and predictive values in laboratory testing. NPV, negative predictive value; PPV, positive predictive value.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


