ECM-Inspired Chemical Cues: Biomimetic Molecules and Techniques of Immobilization
The Donnelly Centre for Cellular and Biomolecular Research, Department of Chemical Engineering and Applied Chemistry, Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, Ontario, Canada
The Donnelly Centre for Cellular and Biomolecular Research, Department of Chemical Engineering and Applied Chemistry, Institute of Biomaterials and Biomedical Engineering, Department of Chemistry, University of Toronto, Toronto, Ontario, Canada
1.1 Introduction
The extracellular matrix (ECM) is a complex environment that provides chemical and physical support to cells, Figure 1.1 [1,2]. The composition of native ECM differs based on its location within the body [3–6], but it is generally comprised of proteins (fibronectin, laminin, and collagen), polysaccharides (hyaluronan and chondroitin sulfate proteoglycans [CSPGs]) and various growth factors [6]. Components of the ECM play important roles in controlling cell function. Molecules such as collagen [7] and elastin [8] function as the structural scaffold to support cell growth, whereas fibronectin, laminin, glycosaminoglycans (GAGs), and growth factors act as ligands to promote cell adhesion, proliferation, differentiation, and migration [9].
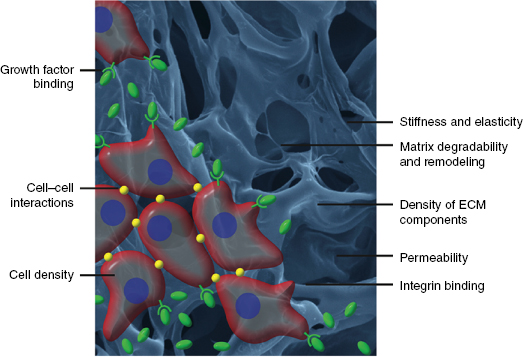
Cells can remodel the ECM in a dynamic fashion [9,10]. For example, cells can secrete proteases that can degrade the ECM to promote cell migration, which is important in tissue repair, such as neuroblast migration following traumatic brain injury (TBI) [11], as well as in disease states, such as cancer metastasis [12]. Cells can also secrete their own ECM molecules on top of the existing ECM to provide new cues affecting both self and neighboring cells [10].
The increased understanding of the role of native ECM on cellular function and interactions has resulted in extensive research into biomimetic materials for applications in tissue engineering [13,14]. Hydrogels represent a class of biomaterials that have been used for this purpose. These highly hydrated polymers provide structural scaffolds and permit diffusion of molecules throughout. Matrigel® (BD Biosciences, San Jose, CA), a decellularized ECM derived from the Engelbreth–Holm–Swarm (EHS) mouse sarcoma, is a common hydrogel used to mimic the three-dimensional (3-D) properties of the ECM [15]. This material has been shown to promote various bioactivities such as cell adhesion, differentiation, viability, and invasion in a variety of cell types; however, for studies that require a more defined 3-D environment (such as those for mechanistic elucidation studies), the use of Matrigel® is nonideal as it is ill-defined in composition and the results are often difficult to reproduce. As such, a bottom-up approach is desirable where researchers begin with a blank palette in terms of cellular interactions and then paint in desirable features, such as cell adhesion, proliferation, and migration through both chemical and physical designs. Efforts to synthesize biomimetic ECMs with defined components were significantly advanced by the discovery that short peptide sequences (e.g., RGD, YIGSR, IKVAV, etc.), derived from native ECM proteins (fibronectin and laminin, respectively), promote cell adhesion and outgrowth. It was shown that RGD interacted with extracellular integrin receptors with affinity similar to that of native fibronectin [16]. Since this discovery, a large number of studies have been conducted to immobilize this and other biomimetic sequences to various biomaterials with the intention of promoting cell adhesion on nonadherent surfaces and biomaterials, thereby increasing the posttransplantation cell viability in tissue regeneration applications, and studying cell behavior in model biomimetic systems.
This chapter is focused on recent advances in the techniques used to incorporate ECM-inspired chemical signaling molecules into different hydrogels, and their effects on cellular interactions in 3-D. While similar approaches are used in multiple areas of biology, we highlight many examples applicable to neurobiology.
1.2 Development and Immobilization of Biomimetic Cues in 3-D Biomaterials
The discovery that short peptide sequences showed comparable activity to their respective native ECM proteins from which they were derived has resulted in significant efforts to design peptide-modified biomaterials with which to study cellular interactions [17,18]. Biomaterial modification with these peptide sequences (typically 3–10 amino acids) results in inherently better-defined systems than the corresponding protein-modified systems due to the shorter sequence length and resulting 3-D structure. To take full advantage of ligand-containing biomaterials to study complex cellular interactions, it is imperative that the ligand is chemically conjugated to the biomaterial in a reproducible and specific manner in order to optimize cellular interaction. For example, conjugation at the active site of the peptide may diminish receptor binding and therefore limit bioactivity. An important consideration in designing biomimetic molecules is to include a specific functional group that has a selective chemical reactivity toward the material to which it will be conjugated.
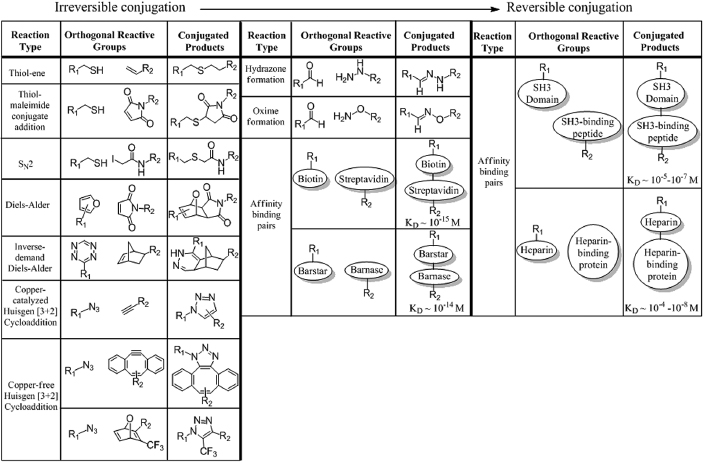
The emergence of click chemistry as a method to conjugate molecules to biomaterials has proved to be a powerful technique to allow efficient conjugation with both defined chemical reactivities and orientation [19–22]. These orthogonal reactions are specific and occur with high yield and efficiency. While detailed discussion about this topic is beyond the scope of this chapter, Figure 1.2 shows a brief summary of different conjugation reactions that have been used to immobilize various peptides and proteins to biomaterials. The following section will describe the conjugation of various peptides to different biomaterials using these techniques. While most chemical conjugations have focused on the irreversible conjugation of molecules, recent work has enabled a versatile approach to forming reversible conjugations, which has the potential to synthesize dynamic biomimetic systems [23].
1.2.1 Synthetic Peptides Derived from Fibronectin, Laminin, and Collagen
The fibronectin-derived RGD peptide sequence is among the most studied peptide sequences for cell adhesion, and has been reviewed extensively [17,24,25]. Fibronectin is a ubiquitous protein that binds to different integrin receptors and promotes cell adhesion and cell survival. Immobilizing this sequence to biomaterials using bond-forming chemistries such as 1-ethyl-3-dimethylaminopropylcarbodiimide and N-hydroxysuccinimide (EDC/NHS) is problematic because the carboxylate-containing aspartic acid (D) participates in a competing reaction with the C-terminal carboxylate, thereby complicating the orientation of the sequence immobilized [26]. Bio-orthogonal conjugation chemistries have been used to overcome this problem and have resulted in effective adhesion for a variety of cell types to different types of modified biomaterials (Table 1.1) [17,27,28].
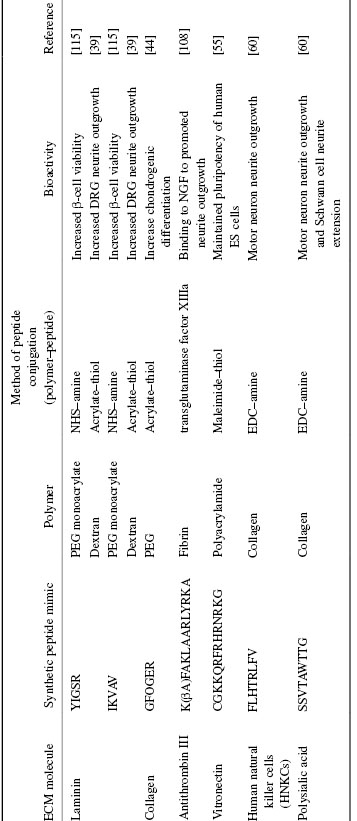
Table 1.1 Biomimetic Peptides of Common ECM Proteins and Methods of Immobilization
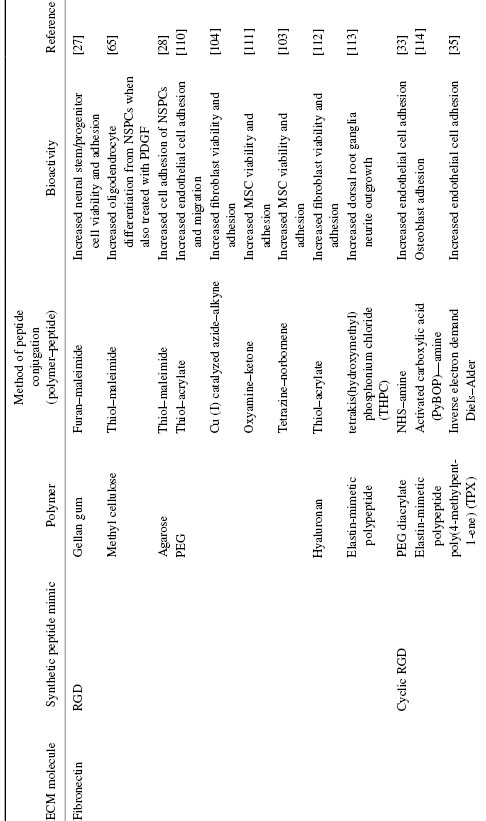
Derivatives of the linear RGD sequence have been synthesized in efforts to increase its binding to integrin receptors. Studies show that the RGD sequence in native fibronectin resides at the tip of a loop, which provides it with structural rigidity and a favorable conformation for integrin binding [29,30]. These structural characteristics have inspired the synthesis of cyclic RGD sequences [31,32]. Synthetic cyclic RGD peptides provide comparable conformational characteristics to facilitate integrin binding, and their conjugation to biomaterials have shown greater bioactivity compared with linear RGD sequences [33,34]. For example, cyclic RGD was recently conjugated to poly(4-methylpent-1-ene) (TPX) membranes for use as artificial lung supports. Endothelial cells cultured on this material showed significant cell adhesion, which is important for hemostasis use in these devices [35].
Another consideration for improved integrin interaction with immobilized RGD peptides is the distance between the peptide and the polymer backbone. A peptide that is bound too close to the polymer backbone may be hindered by steric interactions to efficiently bind with the receptors. Wilson et al. recently reported RGD peptides with a PEG linker containing greater than 27 ethylene oxide, repeat units showed significant adhesion of telomerase-immortalized human corneal epithelial cells (hTCEpi) [36].
Another ECM protein that has been extensively studied is laminin. Similar to fibronectin, laminin plays important roles in the ECM such as facilitating cell adhesion, differentiation, and migration. The most widely studied synthetic peptides for laminin are YIGSR [37] and IKVAV [38]. YIGSR has been shown to promote cell adhesion to the laminin-binding receptors, while IKVAV has been shown to promote primarily adhesion and neurite outgrowth of dorsal root ganglia (DRGs) [39], as well as differentiation of neural progenitor cells (NPCs) [40].
Collagens are another important class of proteins found in the ECM. Collagens provide structural support and also interact with receptors to mediate cell adhesion, migration, and proliferation [41,42]. The general structure of collagen consists of a triple helix, formed by three polypeptide strands, which can further assemble to supramolecular structures such as planar sheet-like networks, fibrils, and fibers [42]. There are 28 isoforms of collagen, with types I and IV being the most predominant in the ECM. Early collagen-mimetic synthetic sequences included the repeating tripeptide unit (Gly-X-Y), where X and Y were pre-dominantly conformationally rigid prolines to facilitate the formation of a triple helix. Subsequent work by Farndale and coworkers showed that the synthetic sequence (GFOGER, where O is hydroxyproline) derived from collagen I has high affinity for the α2β1 integrin. Garcia et al. have also synthesized a peptide with the GFOGER hexapeptide flanked with the triple helical sequence (GPP)5 to promote the formation of the triple helix [43]. On conjugating to various surfaces, HT1080 cells showed dose-dependent cell adhesion, and MC3T3-E1 cells showed vinculin staining, which suggests focal adhesion through integrin binding. Conjugation of a similar peptide to PEG resulted in increased chondrogenic differentiation of human mesenchymal stem cells compared with cells cultured in controls of PEG alone [44].
1.2.2 Carbohydrate-Binding Peptides
Carbohydrates play a significant role in cell recognition and binding. The chemical structures of carbohydrate complexes (glycans) are diverse and complex. They consist of numerous monosaccharide units (up to 200 total units) covalently bonded to each other linearly or as branched structures, with each structure providing a unique binding affinity to other molecules [45]. GAGs are a class of linear anionic polysaccharides that can be posttranslationally conjugated to proteins in the Golgi complex to form glycoproteins. Glycoproteins that are transported to the cell membrane function as transmembrane proteins, whereby the glycan is exposed to the extracellular space and participates in cellular recognition and protein binding [46].
Heparin is a GAG that is commonly found either in the ECM or conjugated to a transmembrane protein (proteoglycan). Heparin binds with high affinity to a variety of proteins such as antithrombin III (AT III) [47], bFGF [48], VEGF [49], and BMP-2 [50], and presents the proteins for enhanced bioactivity [51]. Thus, the conjugation of heparin to biomaterials is useful for applications that require interactions with heparin-binding proteins (HBPs). Sakiyama-Elbert and Hubbell reported that covalent conjugation of the AT III-derived sequence K(βA)FAKLAARLYRKA to fibrin matrices strongly bound to heparin [52]. A short peptide sequence (NQEQVSP) was also incorporated into the N-terminus to enable enzymatic peptide ligation to the fibrin hydrogel by transglutaminase factor XIIIa [53].
Another important role of transmembrane GAGs is to recognize chemical signals from the surrounding environment. Keissling et al. have discovered that the vitronectin-derived peptide sequence CGKKQRFRHRNRKG binds to GAGs expressed on the cell surface of human embryonic stem cells (hESCs), and can maintain their expression of pluripotent markers after 3 months [54]. Moreover, hESCs cultured on polyacrylamide hydrogels conjugated with this sequence both proliferated and maintained greater pluripotency than cells cultured on gels containing the integrin-binding sequence CRGDS [55].
1.2.3 Glycomimetic Peptides
As described earlier, carbohydrates play a significant role in cell recognition and binding. Efforts to study the interaction between glycans and cells using chemical analogs have been limited by the inability to readily and efficiently chemically synthesize complex polysaccharides, which are challenging synthetic targets due to the multiple glycosylation steps, and the need to preserve the numerous carbohydrate stereocenters. While antibodies can be used to bind to carbohydrate receptors, their size and stability have limited large-scale use.
Interestingly, synthetic peptides have been discovered that mimic the chemical structures of several complex polysaccharides. These peptides occupy a similar chemical space as the parent polysaccharides, and therefore can bind to similar polysaccharide receptors. For example, a peptide sequence (FLHTRLFV) that mimics glycans found on the cell surface of human natural killer cells (HNKCs) was discovered using phage display and antibody-binding assays [56]. Motor neurons cultured in the presence of the HNKC glycomimetic peptide showed significantly longer neurite outgrowth compared with those cultured in the absence of HNKC-peptides [56]. Masand et al. recently conjugated this peptide to collagen hydrogels using EDC chemistry, and as demonstrated, these hydrogels also increased neurite outgrowth and length of motor neurons compared with cells cultured on collagen alone.
Another important glycan group is polysialic acid (PSA), which is naturally found conjugated to a variety of different transmembrane proteins including neural cell adhesion molecules (NCAMs). The PSA is hypothesized to be involved in cell migration of neural cells and cancer cells. Novel PSA-mimetic peptides have been discovered, and delivery of these PSA-mimetic peptides into the brain and spinal cord showed improved functional recovery and tissue regeneration in various injury models [57–59]. Masand et al. have also conjugated this PSA-mimetic peptide to collagen hydrogels, and demonstrated an increase in neurite length of cultured dorsal root ganglion and motor neurons, and increased Schwann cell proliferation compared with cells cultured on collagen alone [60]. However, a mixture of both PSA and HNKC peptides to collagen hydrogels yielded neither an additive effect for neurite outgrowth nor proliferation, emphasizing the importance of understanding the underlying mechanism for synergistic effects.
1.2.4 Growth Factors
Recently, larger molecules such as proteins and growth factors have been conjugated to biomaterials in a site-specific manner. Previous methods used nonspecific conjugation of large proteins to hydrogel scaffolds through amide linkage chemistry, such as EDC coupling. This approach is problematic due to the presence of multiple amines and carboxylates found in many proteins; random amide bond formation may decrease or even block protein activity. The limitation of nonspecific amidation has been overcome by exploiting site-specific modification, including protein modification to include click moieties discussed earlier [61], or by noncovalently incorporating proteins through high-affinity binding with complementary peptides/proteins immobilized to the hydrogel [62,63].
Various genetic modifications have enabled the site-specific incorporation of sequences and functional groups that can interact with bio-orthogonal partners. Protein biotinylation is a widely studied posttranslational modification and can be selectively incorporated into a protein that has been modified with the biotin-ligase recognition sequence (GLNDIFEAQKIEWHE) [64]. Biotin ligase selectively and covalently binds a biotin moiety to the primary amine of the lysine (K) residue in this sequence. The biotinylated protein is subsequently immobilized to streptavidin-containing biomaterials through high-affinity binding (KD∼ 10−15 M). Tam et al. recently reported a thiolated derivative of methylcellulose conjugated to maleimide–streptavidin, followed by immobilization of biotin-containing platelet-derived growth factor (PDGF) [65]. This material was shown to increase the differentiation of rat neural stem/progenitor cells into oligodendrocytes in vitro, and also promote functional and tissue repair in rat models of spinal cord injury [66].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


