Early Development of the Ovary
Follicular Growth & Development
Ovulation & Its Hormonal Regulation
EMBRYONIC IMPLANTATION, DECIDUA, & THE PLACENTA
Breast Development During Puberty
Breasts During Pregnancy & Lactation
Postlactational Regression in the Mammary Glands
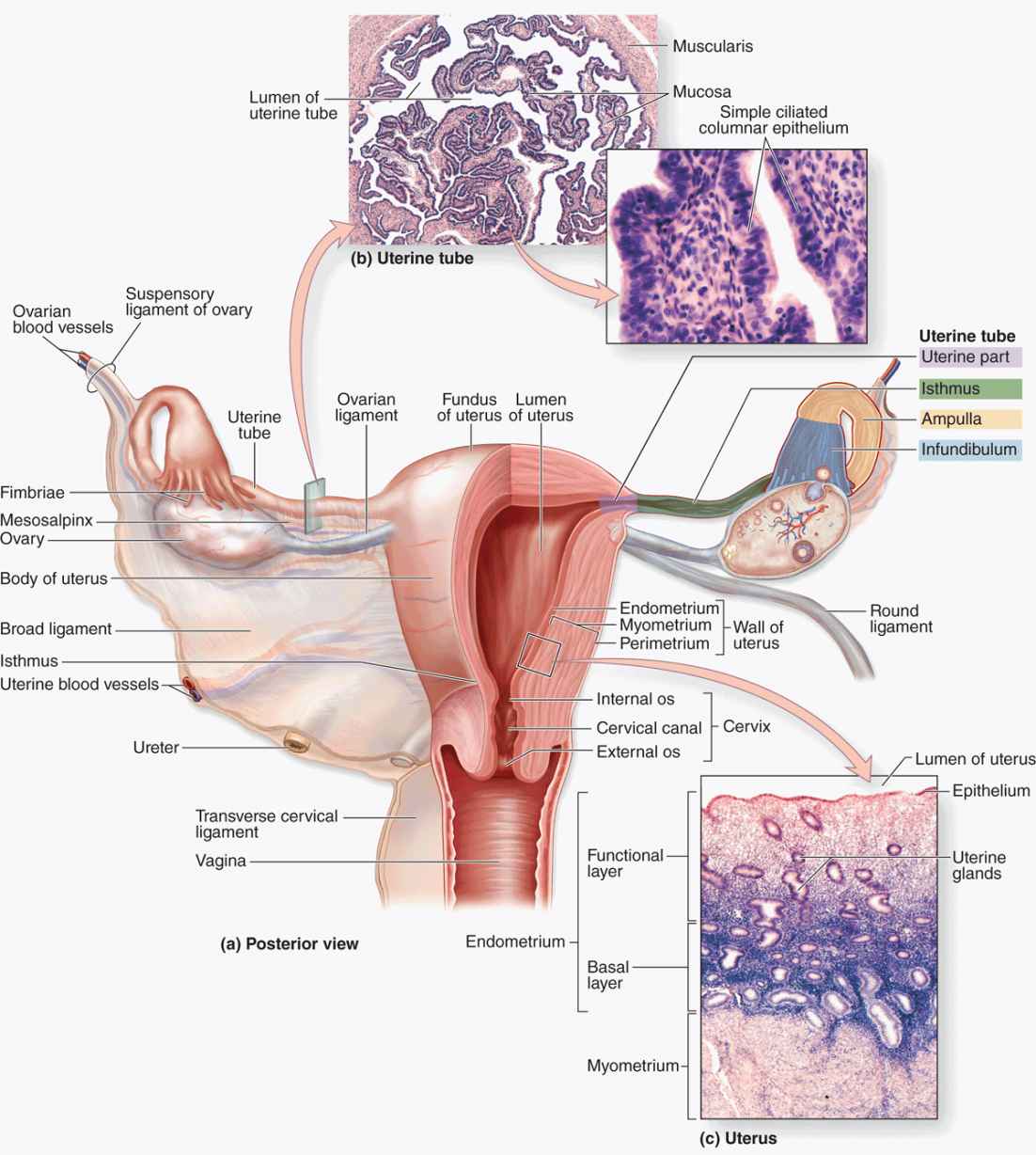
The female reproductive system consists of the paired ovaries and oviducts (or uterine tubes), the uterus, the vagina, and the external genitalia (Figure 22–1). This system produces the female gametes (oocytes), provides the environment for fertilization, and holds the embryo during its complete development through the fetal stage until birth. As with male gonads, the ovaries produce steroidal sex hormones that control organs of the reproductive system and influence other organs. Beginning at menarche, when the first menses occurs, the reproductive system undergoes monthly changes in structure and function that are controlled by neurohormonal mechanisms. Menopause is a variably timed period during which the cyclic changes become irregular and eventually disappear. In the postmenopausal period the reproductive organs slowly involute. Although the mammary glands do not belong to the genital system, they are included here because they undergo changes directly connected to the functional state of the reproductive organs.
FIGURE 22–1 The female reproductive system and overview of ovary.
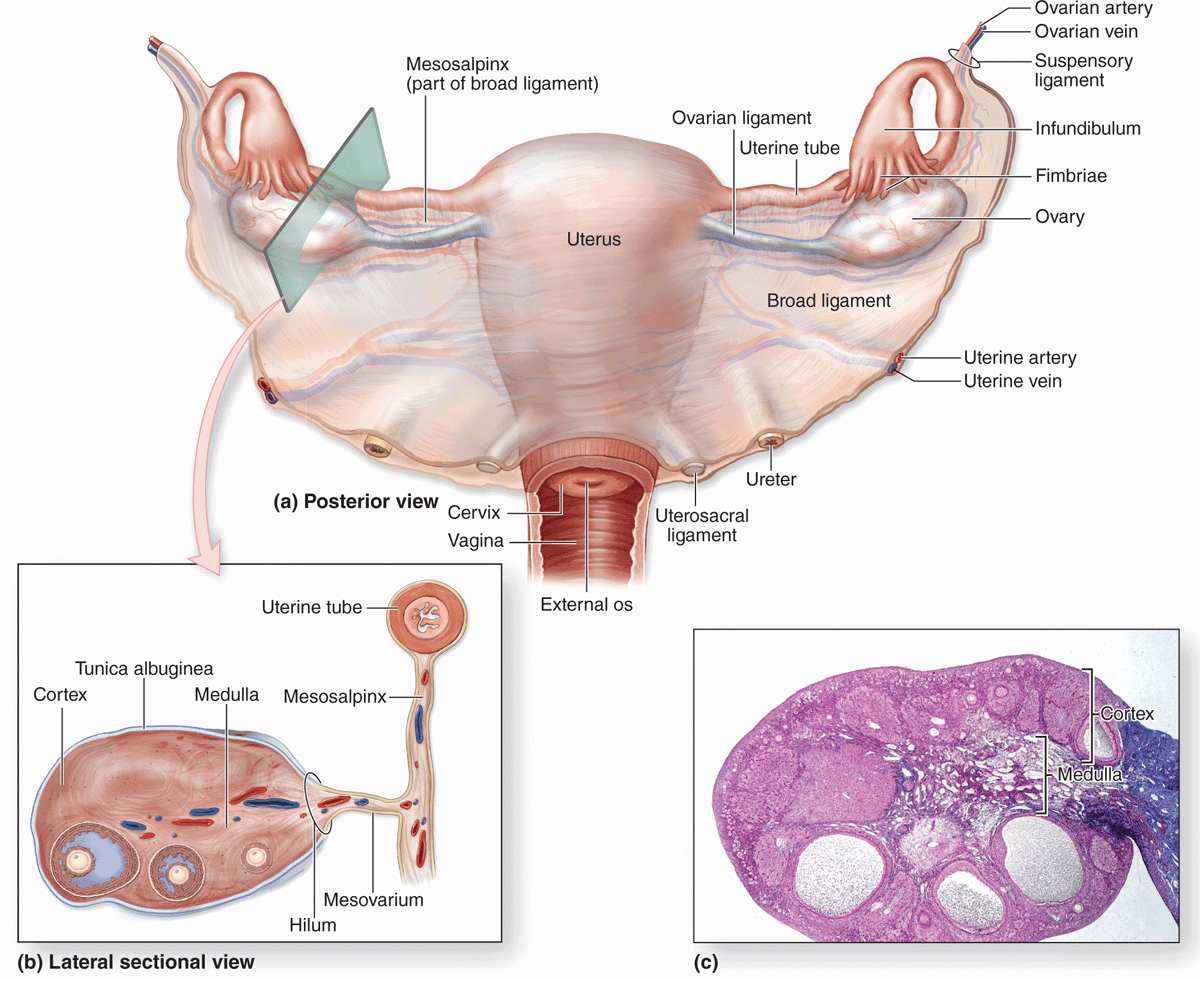
OVARIES
Ovaries are almond-shaped bodies approximately 3 cm long, 1.5 cm wide, and 1 cm thick. Each ovary is covered by a simple cuboidal epithelium, the surface (or germinal) epithelium, continuous with the mesothelium and overlying a layer of dense connective tissue capsule, the tunica albuginea, like that of the testis. Most of the ovary consists of the cortex, a region with a stroma of highly cellular connective tissue and many ovarian follicles varying greatly in size after menarche (Figure 22–1). The most internal part of the ovary, the medulla, contains loose connective tissue and blood vessels entering the organ through the hilum from mesenteries suspending the ovary (Figures 22–1 and 22–2). There is no distinct border between the ovarian cortex and medulla.
Early Development of the Ovary
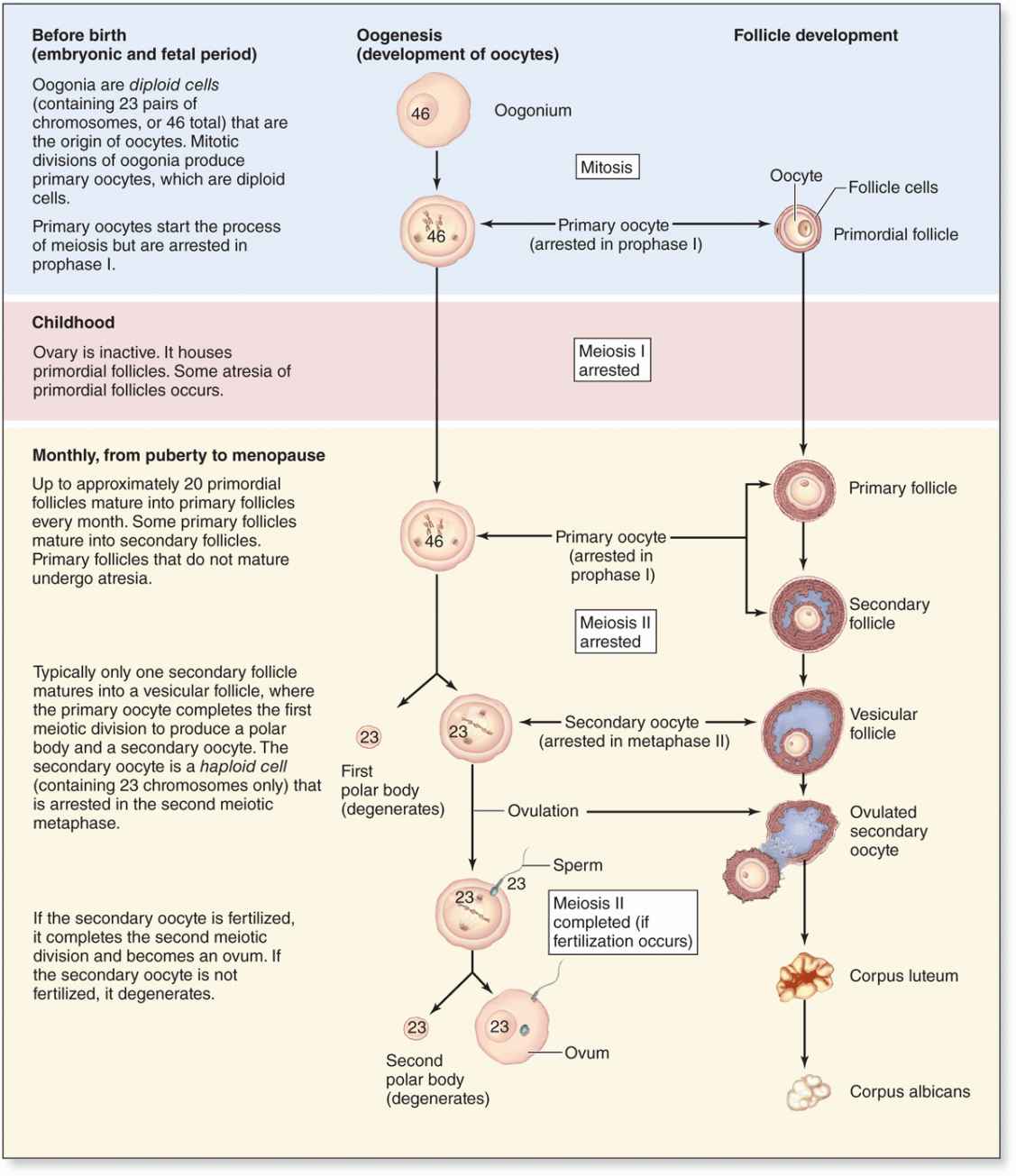
In the first month of embryonic life, a small population of primordial germ cells migrates from the yolk sac to the gonadal primordia. There the cells divide and differentiate as oogonia. In developing ovaries of a 2-month embryo, there are about 600,000 oogonia that produce more than 7 million by the fifth month. Beginning in the third month, oogonia begin to enter the prophase of the first meiotic division but arrest after completing synapsis and recombination, without progressing to later stages of meiosis (see Chapter 3). These cells arrested in meiosis are called primary oocytes (Gr. oon, egg + kytos, cell). Each primary oocyte becomes surrounded by flattened support cells called follicular cells to form an ovarian follicle. By the seventh month of development, most oogonia have transformed into primary oocytes within follicles. Many primary oocytes, however, are lost through a slow, continuous degenerative process called atresia, which continues through a woman’s reproductive life. At puberty the ovaries contain about 300,000 oocytes. Because generally only one oocyte resumes meiosis with ovulation during each menstrual cycle (average duration, 28 days) and the reproductive life of a woman lasts about 30 to 40 years, only about 450 oocytes are liberated from ovaries by ovulation. All others degenerate through atresia.
Ovarian Follicles
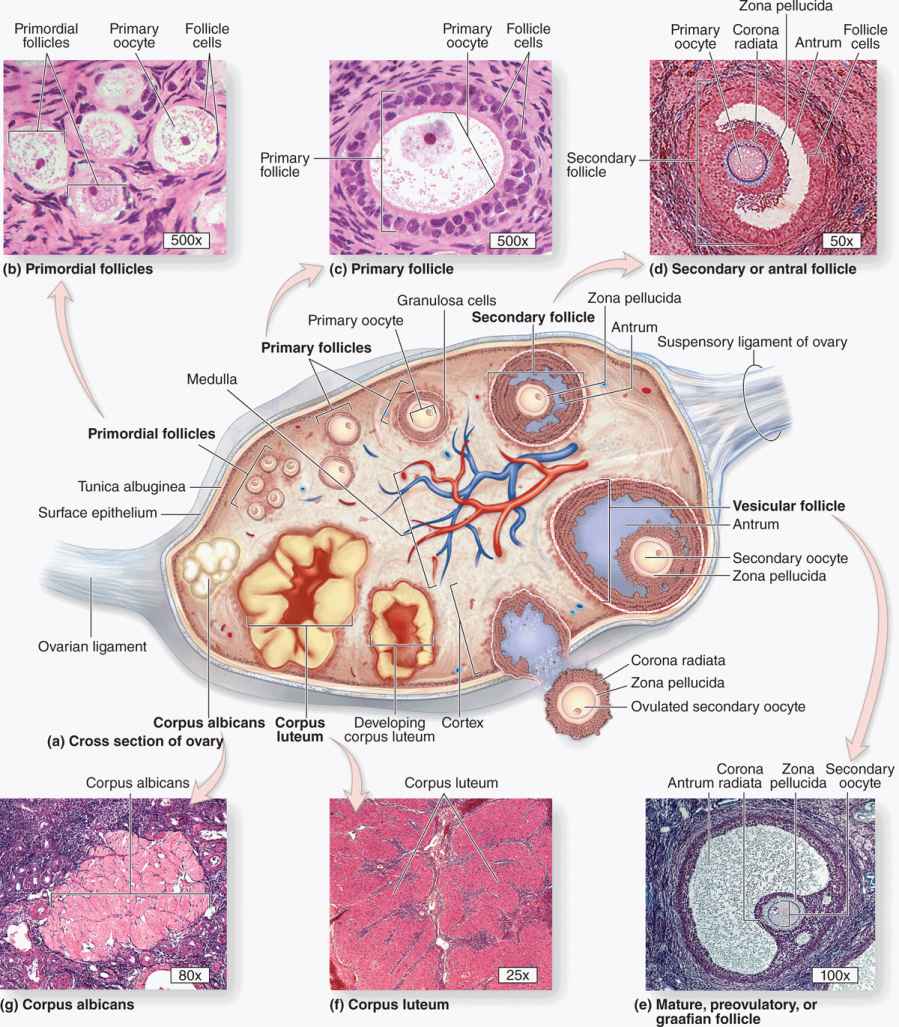
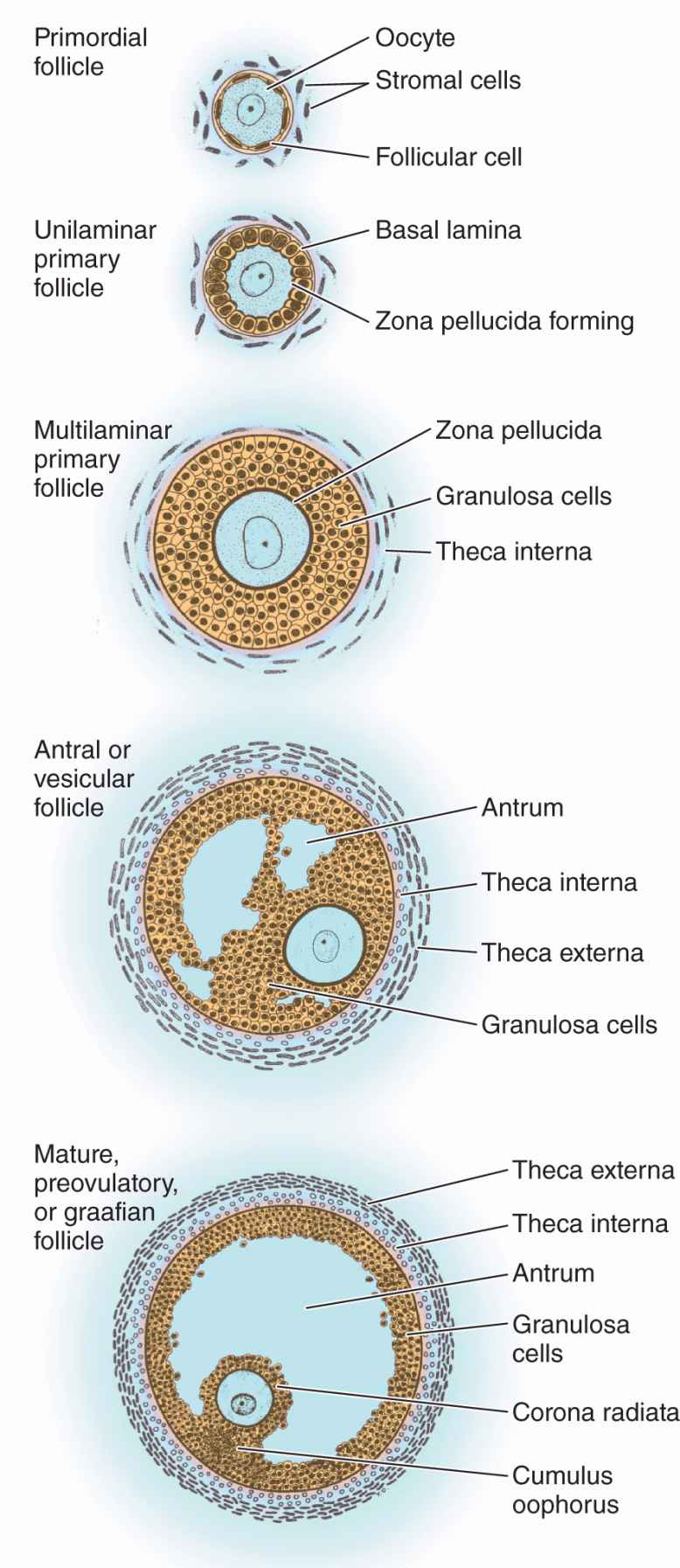
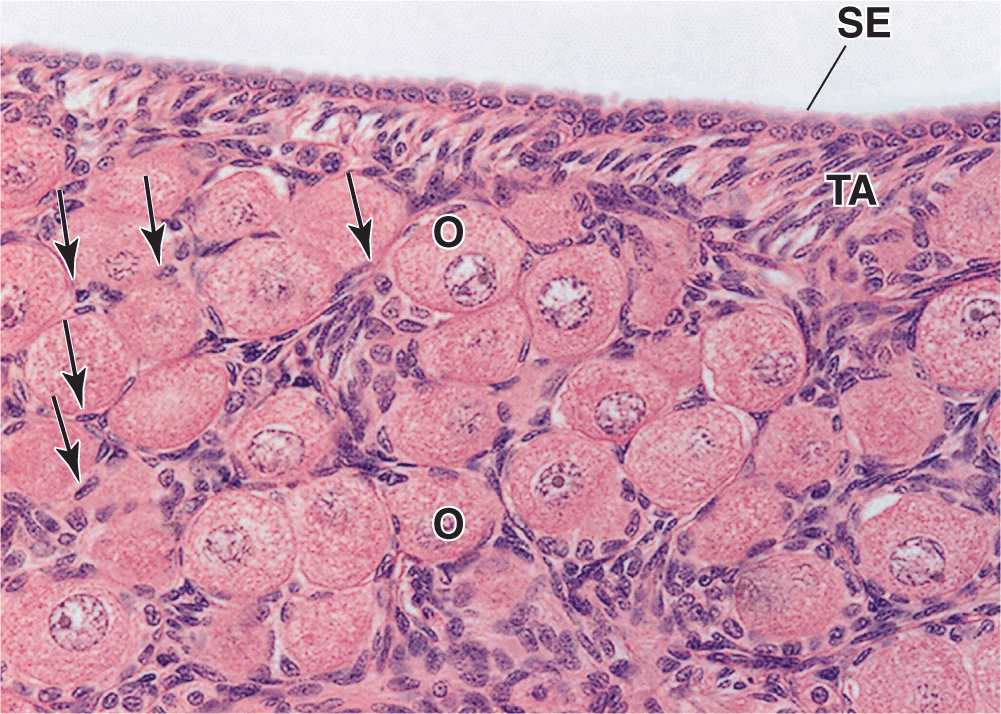
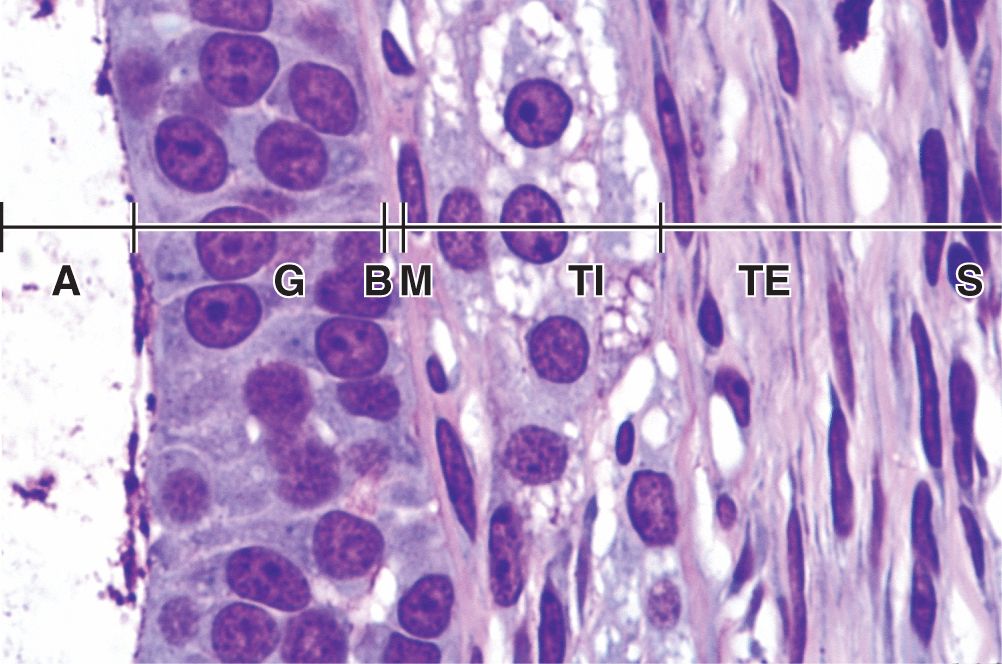
An ovarian follicle consists of an oocyte surrounded by one or more layers of epithelial cells within a basal lamina. The follicles that are formed during fetal life—primordial follicles—consist of a primary oocyte enveloped by a single layer of the flattened follicular cells (Figure 22–2b; Figures 22–3 and 22–4). These follicles occur in the superficial ovarian cortex. The oocyte in the primordial follicle is spherical and about 25 βm in diameter, with a large nucleus containing chromosomes in the first meiotic prophase. The organelles tend to be concentrated near the nucleus and include numerous mitochondria, several Golgi complexes, and extensive RER. The basal lamina surrounds the follicular cells, marking a clear boundary between the follicle and the vascularized stroma.
FIGURE 22–2 Follicle development and changes within the ovary.
FIGURE 22–3 Stages of ovarian follicles, from primordial to mature.
FIGURE 22–4 Primordial follicles.
Follicular Growth & Development
Beginning in puberty with the release of follicle-stimulating hormone (FSH) from the pituitary, a small group of primordial follicles each month begins a process of follicular growth. This involves growth of the oocyte, proliferation and changes in the follicular cells, as well as proliferation and differentiation of the stromal fibroblasts around each follicle. Selection of the primordial follicles that undergo growth and recruitment early in each cycle and of the dominant follicle destined to ovulate that month both involve complex hormonal balances and subtle differences among follicles in FSH receptor numbers, aromatase activity, estrogen synthesis, and other variables.
Prompted by FSH, an oocyte grows most rapidly during the first part of follicular development, reaching a diameter of about 120 μm. Oocyte differentiation includes the following:
 Growth of the cell and nuclear enlargement;
Growth of the cell and nuclear enlargement;
 Mitochondria becoming more numerous and uniformly distributed;
Mitochondria becoming more numerous and uniformly distributed;
 RER becoming much more extensive and Golgi complexes enlarging and moving peripherally; and
RER becoming much more extensive and Golgi complexes enlarging and moving peripherally; and
 Formation of specialized secretory granules called cortical granules containing various proteases. These lie just inside the oocyte’s plasma membrane and undergo exocytosis early in fertilization.
Formation of specialized secretory granules called cortical granules containing various proteases. These lie just inside the oocyte’s plasma membrane and undergo exocytosis early in fertilization.
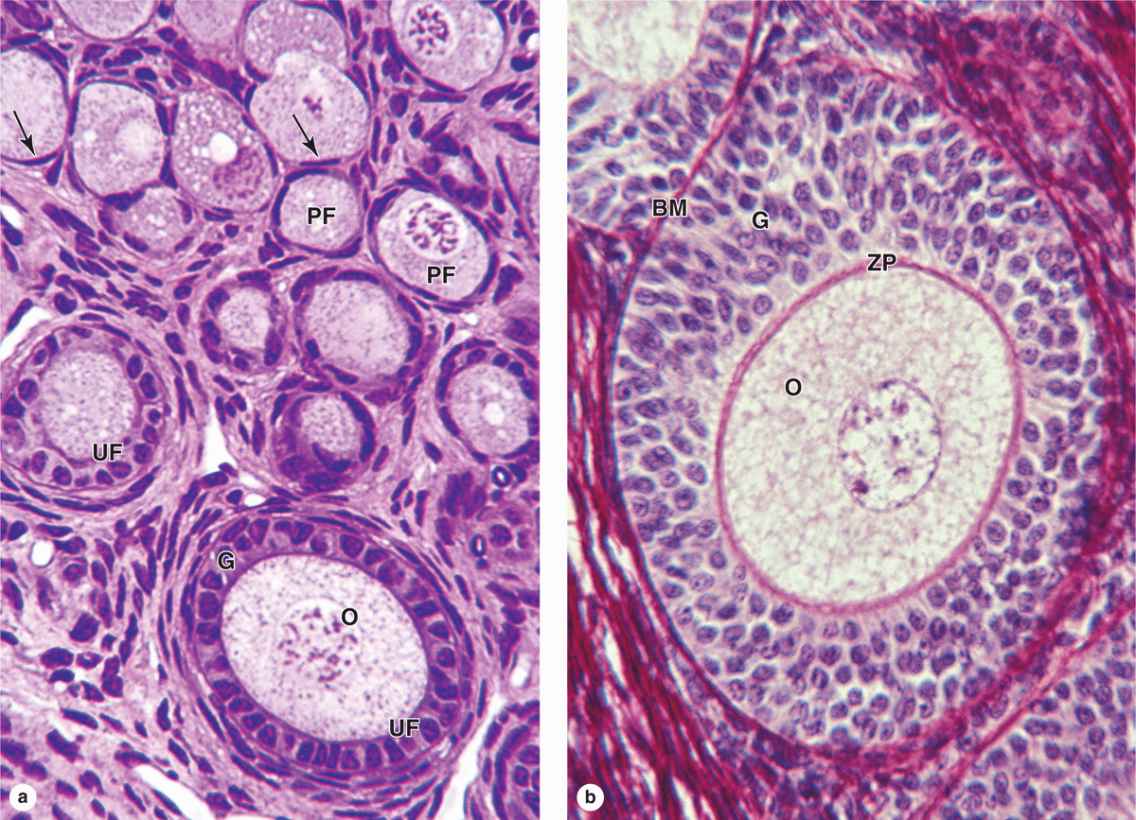
Follicular cells undergo mitosis and form a simple cuboidal epithelium around the growing oocyte. The follicle is now called a unilaminar primary follicle (Figures 22–3 and 22–5a). The follicular cells continue to proliferate, forming a stratified follicular epithelium, the granulosa, in which the cells communicate through gap junctions. Follicular cells are now termed granulosa cells and the follicle is a multilaminar primary follicle (Figures 22–3 and 22–5b) still surrounded by a basement membrane.
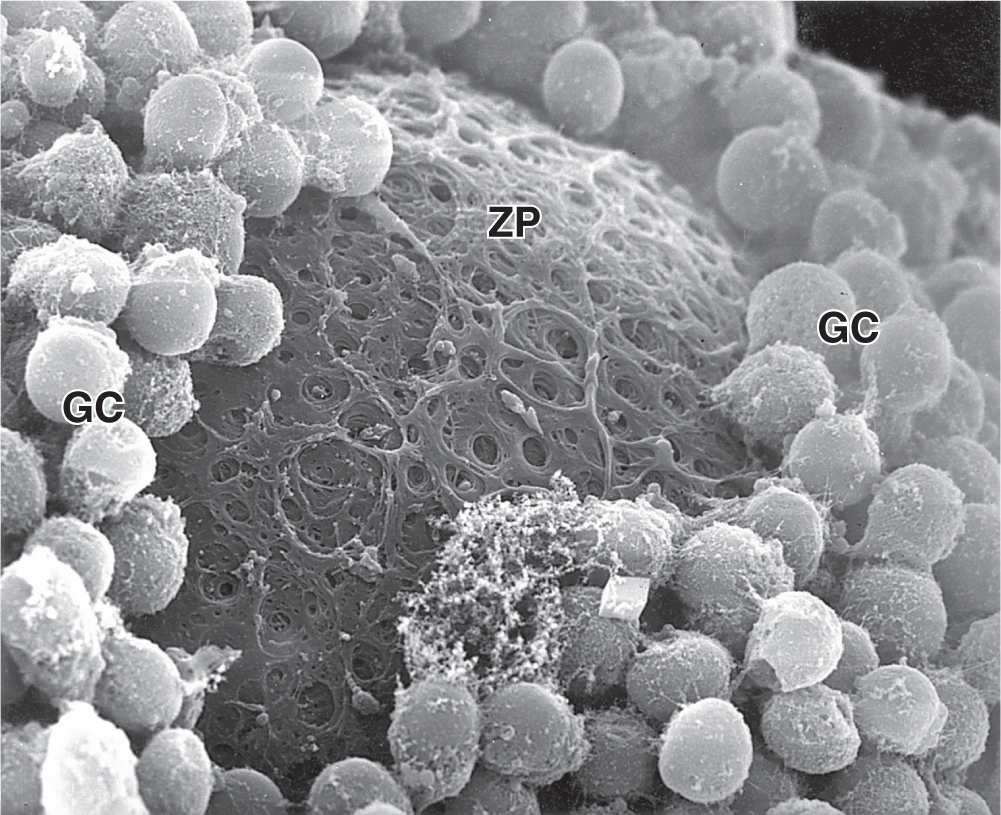
Between the oocyte and the first layer of granulosa cells of the growing primary follicle, extracellular material accumulates as the zona pellucida, 5 to 10 μm thick and containing four glycoproteins secreted by the oocyte (Figures 22–5b and 22–6). The zona pellucida components ZP3 and ZP4 are important sperm receptors, binding specific proteins on the sperm surface and inducing acrosomal activation. Filopodia of granulosa cells and microvilli of the oocyte penetrate the zona pellucida, allowing communication between these cells via gap junctions.
FIGURE 22–5 Primary follicles.
FIGURE 22–6 Ultrastructure of primary follicle and zona pellucida.
Stromal cells immediately outside each growing primary follicle differentiate to form the follicular theca (Gr. theca, outer covering). This subsequently differentiates further as two distinct tissues around the follicle (see Figure 22–3; Figures 22–7 and 22–8):
FIGURE 22–7 Antral follicle and preovulatory follicle.
FIGURE 22–8 Wall of antral follicle.
 A well-vascularized endocrine tissue, the theca interna, with typical steroid-producing cells secreting androstenedione. This precursor molecule diffuses into the follicle through the basement membrane, and in the granulosa cells the enzyme aromatase converts it to estradiol, an FSH-dependent function. This estrogen returns to the thecae and stroma around the follicle, enters capillaries, and is distributed throughout the body.
A well-vascularized endocrine tissue, the theca interna, with typical steroid-producing cells secreting androstenedione. This precursor molecule diffuses into the follicle through the basement membrane, and in the granulosa cells the enzyme aromatase converts it to estradiol, an FSH-dependent function. This estrogen returns to the thecae and stroma around the follicle, enters capillaries, and is distributed throughout the body.
 A more fibrous theca externa with fibroblasts and smooth muscle merges gradually with the surrounding stroma.
A more fibrous theca externa with fibroblasts and smooth muscle merges gradually with the surrounding stroma.
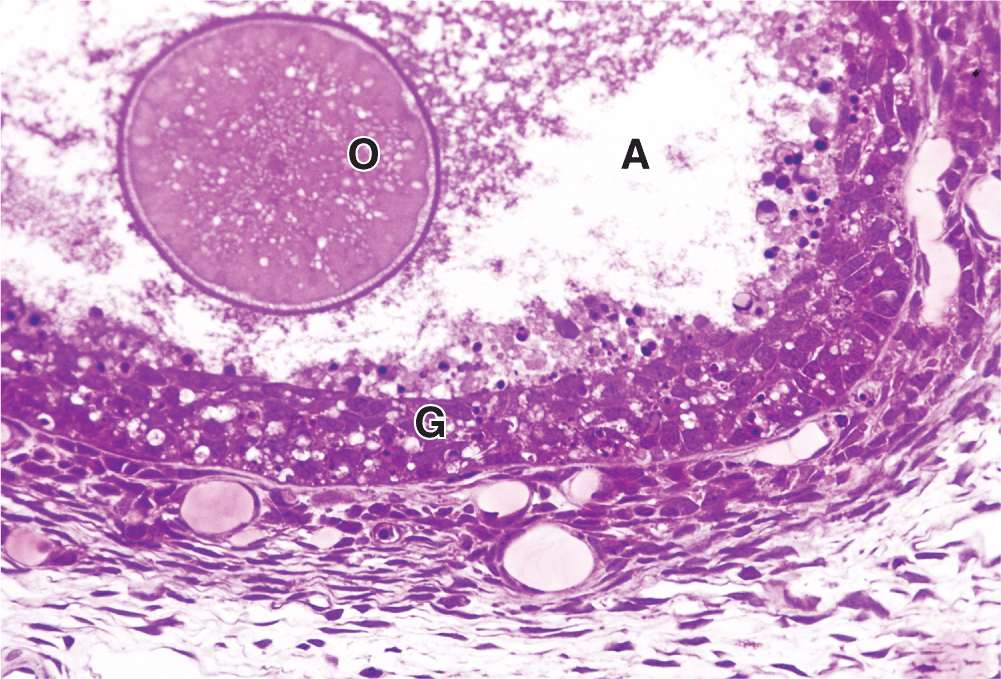
As the primary follicles grow, they move deeper in the ovarian cortex. Within such follicles small spaces appear between the granulosa layers as the cells secrete follicular fluid (or liquor folliculi). This fluid accumulates, the spaces enlarge and gradually coalesce, and the granulosa cells reorganize themselves around a larger cavity called the antrum (Figures 22–3 and 22–7a), producing follicles now called vesicular or antral follicles. Follicular fluid contains the large GAG hyaluronic acid, growth factors, plasminogen, fibrinogen, the anticoagulant heparan sulfate proteoglycan, and high concentrations of steroids (progesterone, androstenedione, and estrogens) with binding proteins.
As the antrum develops, the granulosa cells around the oocyte form a small hillock, the cumulus oophorus, which protrudes into the antrum (Figures 22–3 and 22–7b). Those granulosa cells that immediately surround the zona pellucida make up the corona radiata and accompany the oocyte when it leaves the ovary at ovulation.
The single large antrum of a mature or preovulatory follicle (or graafian follicle named after the 17th-century reproductive biologist Regnier De Graaf) accumulates follicular fluid rapidly and expands to a diameter of 2 cm or more. A preovulatory follicle forms a bulge at the ovary surface visible with ultrasound imaging. The granulosa layer becomes thinner at this stage because its cells do not multiply in proportion to the growth of the antrum. A mature follicle has thick thecal layers and normally develops from a primordial follicle over a period of about 90 days.
Follicular Atresia
Most ovarian follicles undergo the degenerative process called atresia, in which follicular cells and oocytes die and are disposed of by phagocytic cells. Follicles at any stage of development, including nearly mature follicles, may become atretic (Figure 22–9). Atresia involves apoptosis and detachment of the granulosa cells, autolysis of the oocyte, and collapse of the zona pellucida. Early in this process, macrophages invade the degenerating follicle and phagocytose the debris, followed later by fibroblasts. Although follicular atresia takes place from before birth until a few years after menopause, it is most prominent just after birth, when levels of maternal hormones decline rapidly, and during both puberty and pregnancy, when qualitative and quantitative hormonal changes occur again.
FIGURE 22–9 Atresia.
During a typical menstrual cycle, one follicle becomes dominant and develops farther than the others. The dominant follicle usually reaches the most developed stage of follicular growth and undergoes ovulation, while the other primary and antral follicles undergo atresia. Although their oocytes are never directly used, the large growing follicles produce much estrogen before becoming atretic follicles each month. As described later, this estrogen stimulates preparation of the reproductive tract to transport and sustain the embryo if the oocyte from the dominant follicle is fertilized.
Ovulation & Its Hormonal Regulation
Ovulation is the hormone-stimulated process by which the oocyte is released from the ovary. Ovulation normally occurs midway through the menstrual cycle, that is, around the 14th day of a typical 28-day cycle. In the hours before ovulation, the mature dominant follicle bulging against the tunica albuginea develops a whitish or translucent ischemic area, the stigma, in which tissue compaction has blocked blood flow. In humans usually only one oocyte is liberated during each cycle, but sometimes either no oocyte or two or more simultaneous oocytes may be expelled.
Just before ovulation the oocyte completes the first meiotic division, which it began and arrested in prophase during fetal life (Figure 22–10). The chromosomes are equally divided between the two daughter cells, but one of these retains almost all of the cytoplasm. That cell is now the secondary oocyte and the other becomes the first polar body, a very small nonviable cell containing a nucleus and a minimal amount of cytoplasm. Immediately after expulsion of the first polar body, the nucleus of the oocyte begins the second meiotic division but arrests at metaphase and never completes meiosis unless fertilization occurs (Figure 22–10).
FIGURE 22–10 Oogenesis.
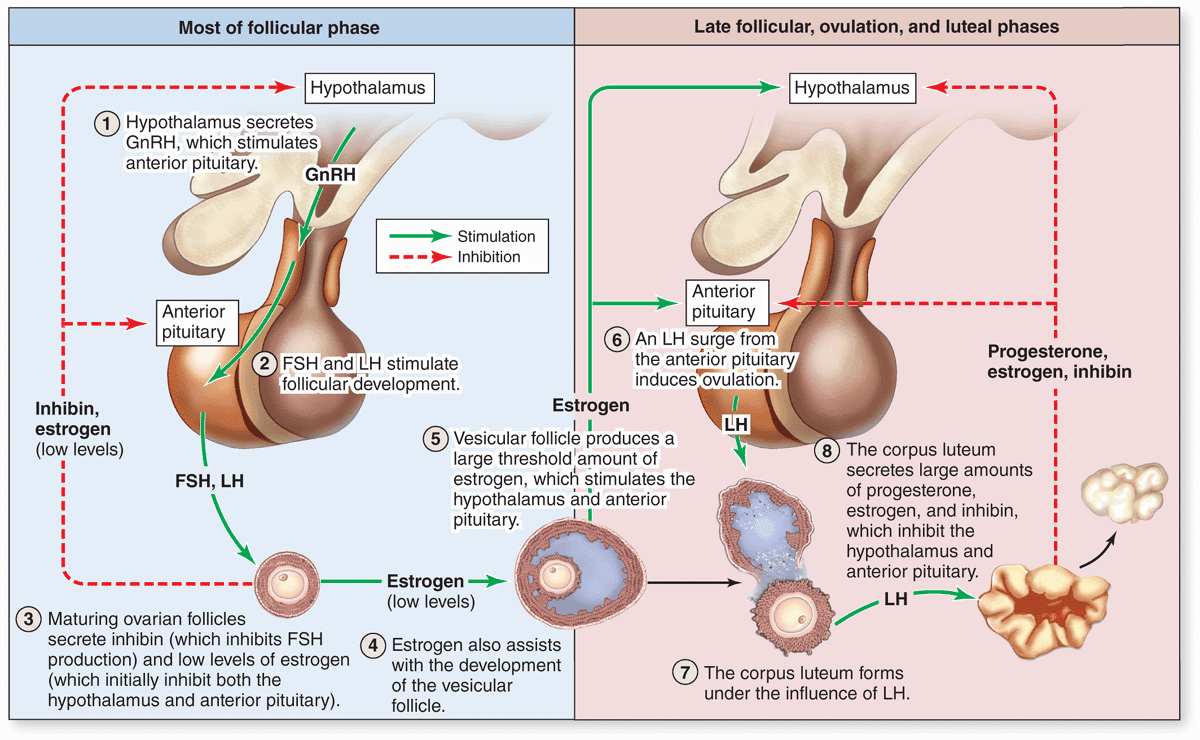
As mentioned before, follicular development depends on FSH from pituitary gonadotrophs, whose secretion is stimulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. Figure 22–11 summarizes the main hormonal interactions that regulate follicular growth as well as the ovulation and formation of the corpus luteum. Note that negative feedback of estrogen and progesterone on the hypothalamus and anterior pituitary is reinforced by a polypeptide hormone, inhibin, also produced by granulosa and luteal cells. In the days preceding ovulation, the dominant vesicular follicle secretes higher levels of estrogen which stimulate more rapid pulsatile release of GnRH from the hypothalamus.
FIGURE 22–11 Hormonal regulation of ovarian function.
The increased level of GnRH causes a surge of LH release from the pituitary gland that rapidly triggers a sequence of major events in and around the dominant follicle:
 Meiosis I is completed by the primary oocyte, yielding a secondary oocyte and the first polar body which degenerates (Figure 22–10).
Meiosis I is completed by the primary oocyte, yielding a secondary oocyte and the first polar body which degenerates (Figure 22–10).
 Granulosa cells are stimulated to produce much greater amounts of both prostaglandin and extracellular hyaluronan. This hydrophilic GAG loosens these cells and rapidly increases the volume, pressure, and viscosity of the follicular fluid.
Granulosa cells are stimulated to produce much greater amounts of both prostaglandin and extracellular hyaluronan. This hydrophilic GAG loosens these cells and rapidly increases the volume, pressure, and viscosity of the follicular fluid.
 Ballooning at the stigma, the ovarian wall weakens as activated plasminogen (plasmin) from broken capillaries degrades collagen in the tunica albuginea and surface epithelium.
Ballooning at the stigma, the ovarian wall weakens as activated plasminogen (plasmin) from broken capillaries degrades collagen in the tunica albuginea and surface epithelium.
 Smooth muscle contractions begin in the theca externa, triggered by prostaglandins diffusing from follicular fluid.
Smooth muscle contractions begin in the theca externa, triggered by prostaglandins diffusing from follicular fluid.
The increasing pressure with the follicle and weakening of the wall lead to rupture of the ovarian surface at the stigma. The oocyte and corona radiata, along with follicular fluid, are expelled by the local smooth muscle contractions. The ovulated secondary oocyte adheres loosely to the ovary surface in the viscous follicular fluid and, as described later, is drawn into the opening of the uterine tube where fertilization may occur. If not fertilized within about 24 hours, the secondary oocyte begins to degenerate. Cells of the ovulated follicle that remain in the ovary redifferentiate under the influence of LH and give rise to the corpus luteum (Figure 22–11).
Corpus Luteum
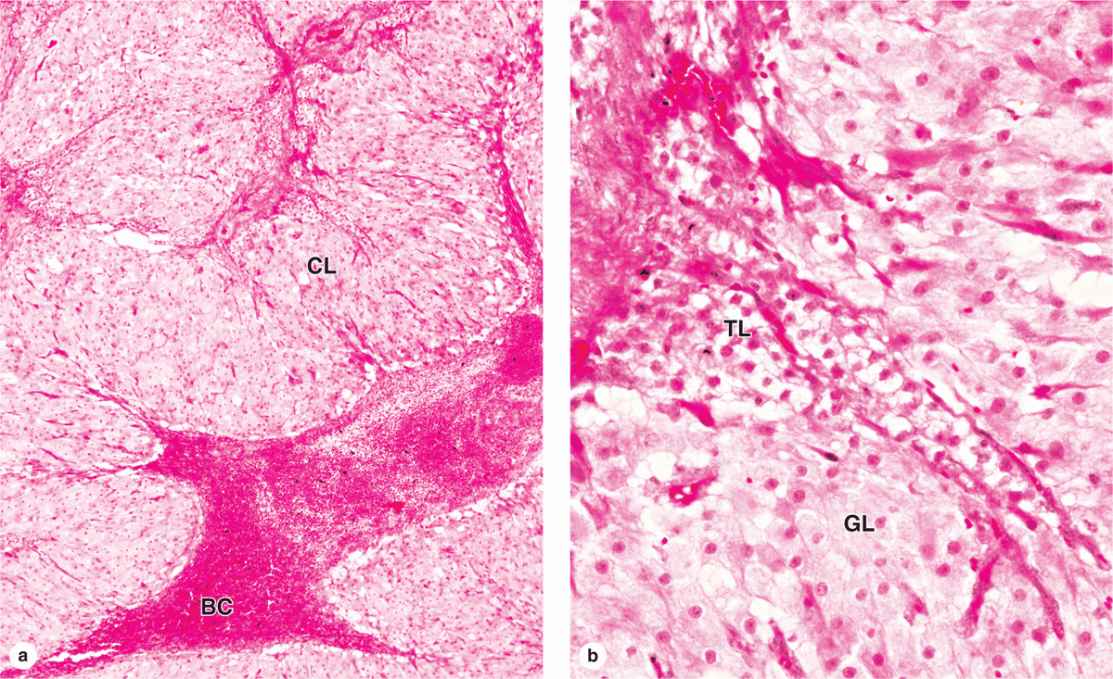
After ovulation, the granulosa cells and theca interna of the ovulated follicle reorganize to form a larger temporary endocrine gland, the corpus luteum (L., yellowish body), in the ovarian cortex. Ovulation is followed immediately by the collapse and folding of the granulosa and thecal layers of the follicle’s wall, and blood from disrupted capillaries typically accumulates as a clot in the former antrum (Figure 22–12). The granulosa is now invaded by capillaries. Cells of both the granulosa and theca interna change histologically and functionally under the influence of LH, becoming specialized for more extensive production of progesterone in addition to estrogens.
FIGURE 22–12 Corpus luteum.
Granulosa cells increase greatly in size (20-35 μm in diameter), without dividing, and eventually comprise about 80% of the corpus luteum. They are now called granulosa lutein cells (Figure 22–12) and have lost many features of protein-secreting cells to expand their role in aromatase conversion of androstenedione into estradiol. The former theca interna forms the rest of the corpus luteum, as theca lutein cells (Figure 22–12). These cells are half the size of the granulosa lutein cells and are typically aggregated in the folds of the wall of the corpus luteum, which, like all endocrine glands, becomes well vascularized. LH causes these cells to produce large amounts of progesterone as well as androstenedione.
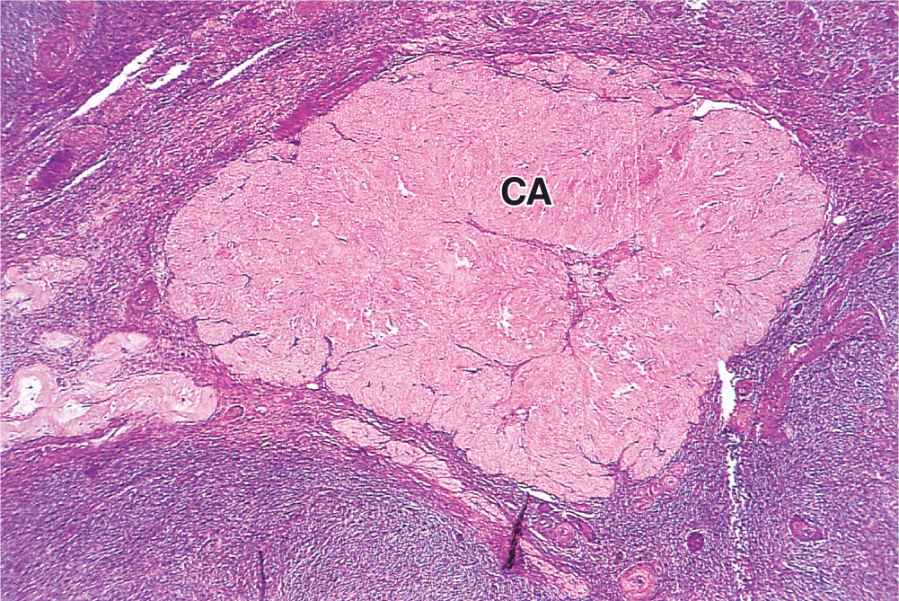
The short-term fate of the corpus luteum depends on whether a pregnancy occurs. The ovulatory LH surge causes the corpus luteum to secrete progesterone for 10 to 12 days. Without further LH stimulation and in the absence of pregnancy, both major cell types of the corpus luteum cease steroid production and undergo apoptosis, with regression of the tissue. A consequence of the decreased secretion of progesterone is menstruation, the shedding of part of the uterine mucosa. Estrogen produced by the active corpus luteum inhibits FSH release from the pituitary. However, after the corpus luteum degenerates, the blood steroid concentration decreases and FSH secretion increases again, stimulating the growth of another group of follicles and beginning the next menstrual cycle. The corpus luteum that persists for part of only one menstrual cycle is called a corpus luteum of menstruation. Remnants from its regression are phagocytosed by macrophages, after which fibroblasts invade the area and produce a scar of dense connective tissue called a corpus albicans (L., white body) (Figure 22–13).
FIGURE 22–13 Corpus albicans.
If pregnancy occurs, the uterine mucosa must not be allowed to undergo menstruation because the embryo would be lost. To prevent the drop in circulating progesterone, trophoblast cells of the implanted embryo produce a glycoprotein hormone called human chorionic gonadotropin (HCG) with targets and activity similar to that of LH. HCG maintains and promotes further growth of the corpus luteum, stimulating secretion of progesterone to maintain the uterine mucosa. This corpus luteum of pregnancy becomes very large and is maintained by HCG for 4 to 5 months, by which time the placenta itself produces progesterone (and estrogens) at levels adequate to maintain the uterine mucosa. It then degenerates and is replaced by a large corpus albicans.
UTERINE TUBES
The paired uterine tubes, or oviducts, supported by ligaments and mesenteries that allow considerable mobility, each measure about 10 to 12 cm in length (Figure 22–14). Each opens into the peritoneal cavity near the ovary, with regions in the following sequence:
FIGURE 22–14 Uterine tubes and uterus.
 MEDICAL APPLICATION
MEDICAL APPLICATION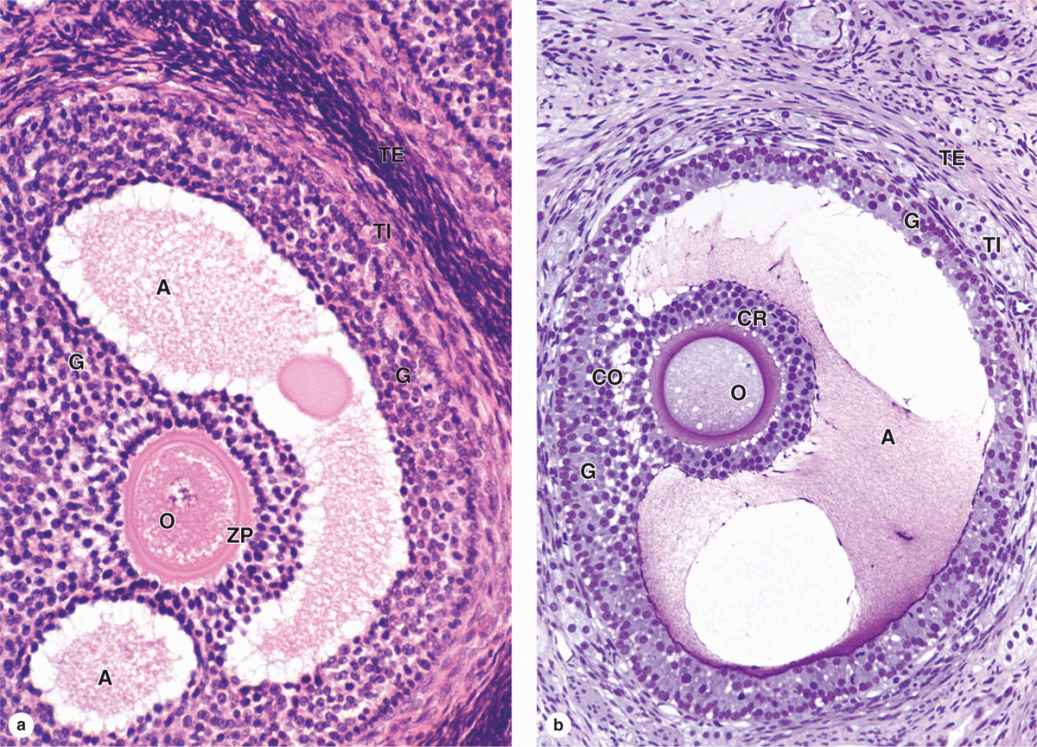
 MEDICAL APPLICATION
MEDICAL APPLICATION