Microfilaments (Actin Filaments)
Cells and extracellular material together comprise all the tissues that make up the organs of multicellular animals. In all tissues, cells themselves are the basic structural and functional units, the smallest living parts of the body. Animal cells are eukaryotic (Gr. eu, good, + karyon, nucleus), with distinct membrane-limited nuclei surrounded by cytoplasm containing various membrane-limited organelles. In contrast, the smaller prokaryotic cells of bacteria typically have a cell wall around the plasmalemma and lack other membranous structures, including an envelope around their DNA. In multicellular organisms, different cells become specialized by concentrating specific organelles and specializing in various basic cellular activities generally found to more limited extents in all eukaryotic cells.
CELL DIFFERENTIATION
The human organism consists of hundreds of different cell types, all derived from the zygote, the single cell formed by the merger of a spermatozoon with an oocyte at fertilization. The first zygotic cellular divisions produce cells called blastomeres, and as part of the early embryo’s inner cell mass blastomeres give rise to all tissue types of the fetus. Explanted to tissue culture cells of the inner call mass are called embryonic stem cells. During their specialization process, called cell differentiation, cells synthesize increased quantities of specific proteins and become very efficient in specialized functions, often changing their shape accordingly. For example, muscle cell precursors elongate into fiber-like cells containing large arrays of actin and myosin. All animal cells contain and use actin filaments and myosins, but muscle cells are specialized for using these proteins to convert chemical energy into forceful contractions.
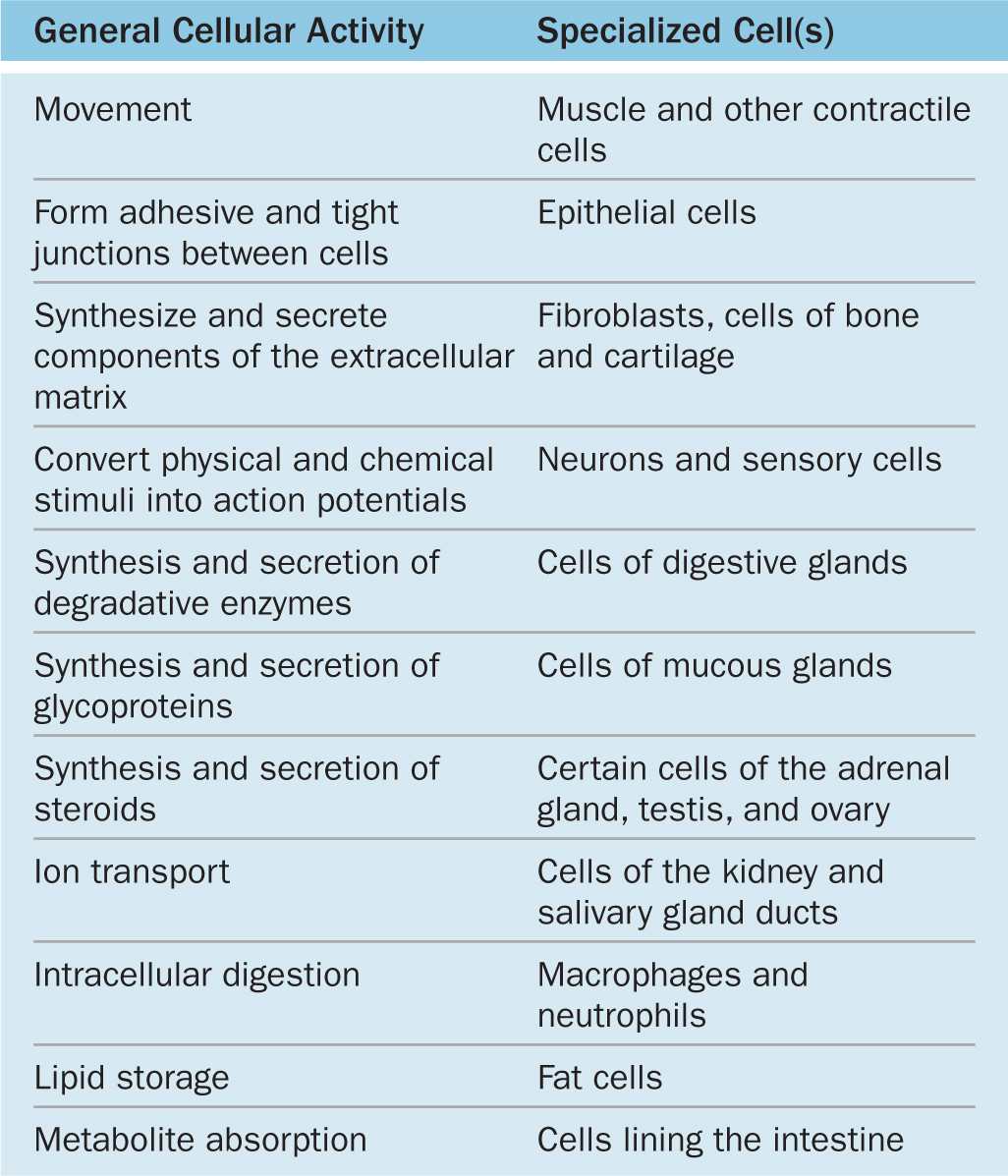
Major cellular functions performed by specialized cells in the body are listed in Table 2–1. It is important to understand that the functions listed there can be performed by most cells of the body; specialized cells have greatly expanded their capacity for one or more of these functions during differentiation. Changes in cells’ microenvironments under normal and pathologic conditions can cause the same cell type to have variable features and activities. Cells that appear similar structurally often have different families of receptors for signaling molecules such as hormones and extracellular matrix (ECM) components, causing them to behave differently. For example, because of their diverse arrays of receptors, breast fibroblasts and uterine smooth muscle cells are exceptionally sensitive to female sex hormones while most other fibroblasts and smooth muscle cells are insensitive.
TABLE 2–1 Differentiated cells typically specialize in one activity.
CYTOPLASMIC ORGANELLES
The cell is composed of two basic parts: cytoplasm (Gr. kytos, cell, + plasma, thing formed) that surrounds the nucleus (L. nux, nut). The outermost component of the cell, separating the cytoplasm from its extracellular environment, is the plasma membrane (plasmalemma). Although the plasma membrane defines the outer limit of the cell, a continuum exists between the interior of the cell and extracellular macromolecules. The plasma membrane contains proteins called integrins linked to both cytoplasmic protein filaments and ECM components. These linkages produce a continuous exchange of influences, in both directions, between the ECM and the cytoplasm.
The cytoplasm consists largely of a fluid component, cytosol, bathing metabolically active structures, the organelles, which may be membranous (such as mitochondria) or nonmembranous protein complexes (such as ribosomes and proteasomes). In addition to the organelles, there are protein components of the cytoplasmic cytoskeleton, which determines the shape and motility of eukaryotic cells. Among the minor cytoplasmic structures are inclusions that are generally deposits of carbohydrates, lipids, or pigments.
Cytosol also contains hundreds of enzymes, such as those of the glycolytic pathway, that produce building blocks for larger molecules and break down small molecules to liberate energy. All the machinery converging on the ribosomes for protein synthesis (mRNA, transfer RNA, enzymes, and other factors) is also contained within the cytosol. Oxygen, CO2, electrolytic ions, low-molecular-weight substrates, metabolites, and waste products all diffuse through cytosol, either freely or bound to proteins, entering or leaving organelles where they are used or produced.
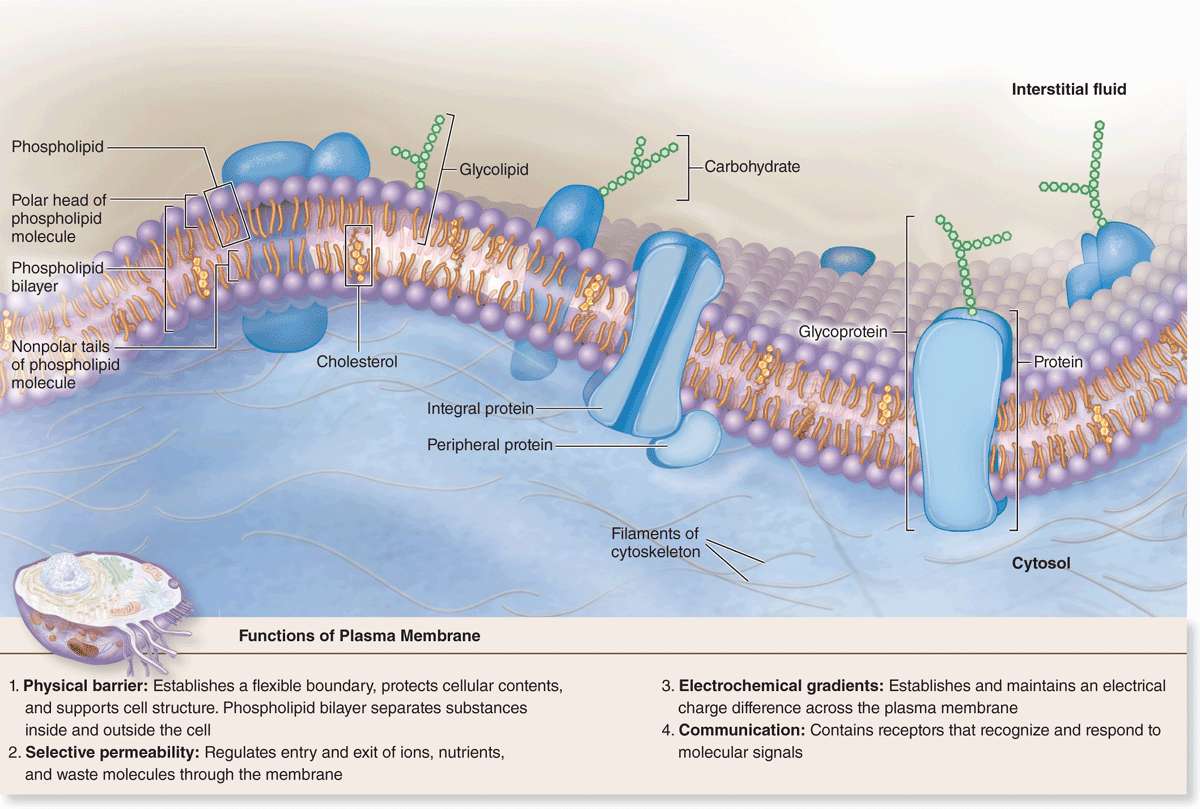
Plasma Membrane
The limiting membranes that envelop all eukaryotic cells are made of phospholipids, cholesterol, proteins, and oligosaccharide chains covalently linked to phospholipid and protein molecules. The plasma membrane (cell membrane) functions as a selective barrier regulating the passage of materials into and out of the cell and facilitating the transport of specific molecules. One important role of the cell membrane is to keep constant the ion content of cytoplasm, which differs from that of the extracellular fluid. Membranes also carry out a number of specific recognition and signaling functions, playing a key role in the interactions of the cell with its environment.
Membranes range from 7.5 to 10 nm in thickness and consequently are visible only in the electron microscope. The line between adjacent cells sometimes seen faintly with the light microscope is formed by plasma membrane proteins plus extracellular material, which together can reach a dimension visible by light microscopy.
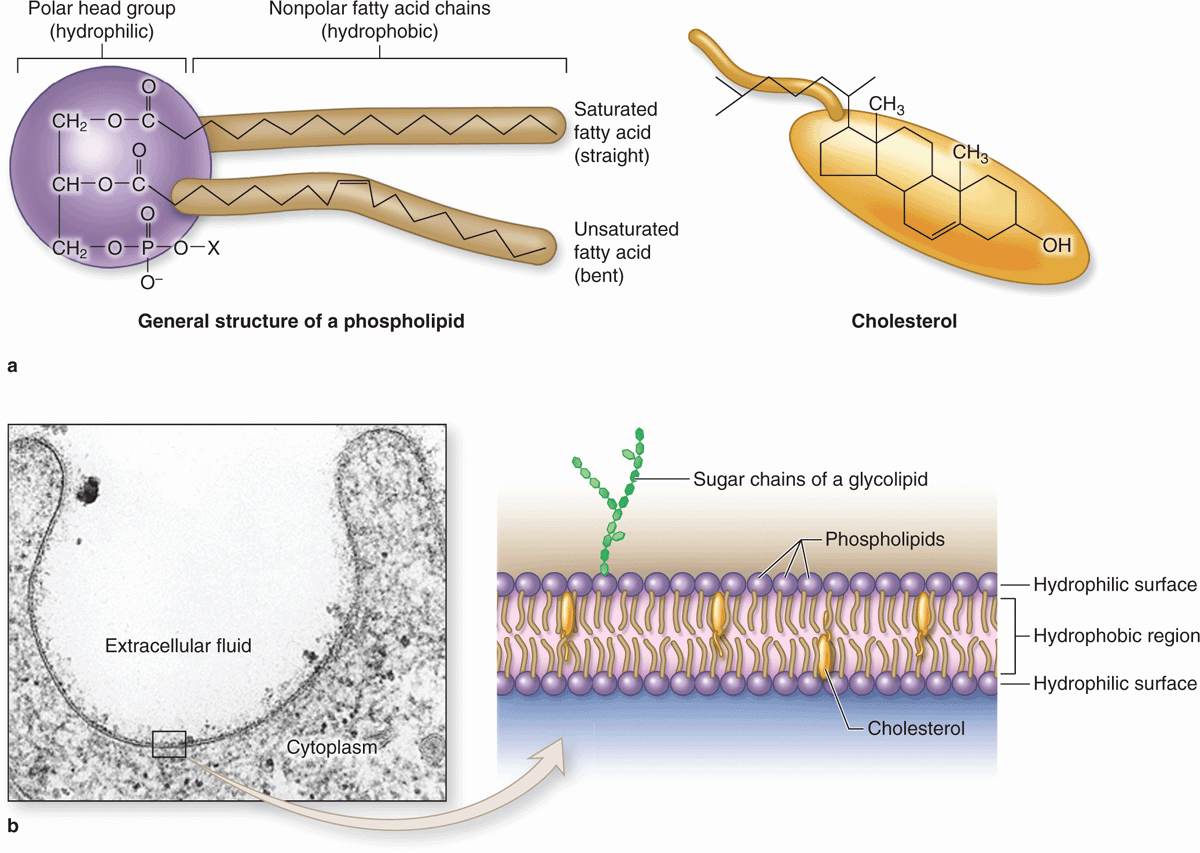
Membrane phospholipids are amphipathic, consisting of two nonpolar (hydrophobic or water-repelling) long-chain fatty acids linked to a charged polar (hydrophilic or water-attracting) head that bears a phosphate group (Figure 2–1a). Phospholipids are most stable when organized into a double layer (bilayer) with the hydrophobic fatty acid chains directed toward the middle away from water and the hydrophilic polar head groups facing the water (Figure 2–1b). Molecules of cholesterol, a sterol lipid, insert at varying densities among the closely-packed phospholipid fatty acids, restricting their movement, and modulating the fluidity and movement of all membrane components. The phospholipids in each half of the bilayer are different. For example, in the well-studied membranes of red blood cells phosphatidylcholine and sphingomyelin are more abundant in the outer half, while phosphatidylserine and phosphatidylethanolamine are more concentrated in the inner layer. Some of the outer lipids, known as glycolipids, include oligosaccharide chains that extend outward from the cell surface and contribute to a delicate cell surface coating called the glycocalyx (Figures 2–1b and 2–2). With the transmission electron microscope (TEM) the cell membrane—and all other organellar membranes—may exhibit a trilaminar appearance after fixation in osmium tetroxide; osmium binding the polar heads of the phospholipids, the outer sugar chains, and associated membrane proteins produces the two dark outer lines enclosing the light band of osmium-free fatty acids (Figure 2–1b).
FIGURE 2–1 Lipids in membrane structure.
FIGURE 2–2 Proteins associated with the membrane lipid bilayer.
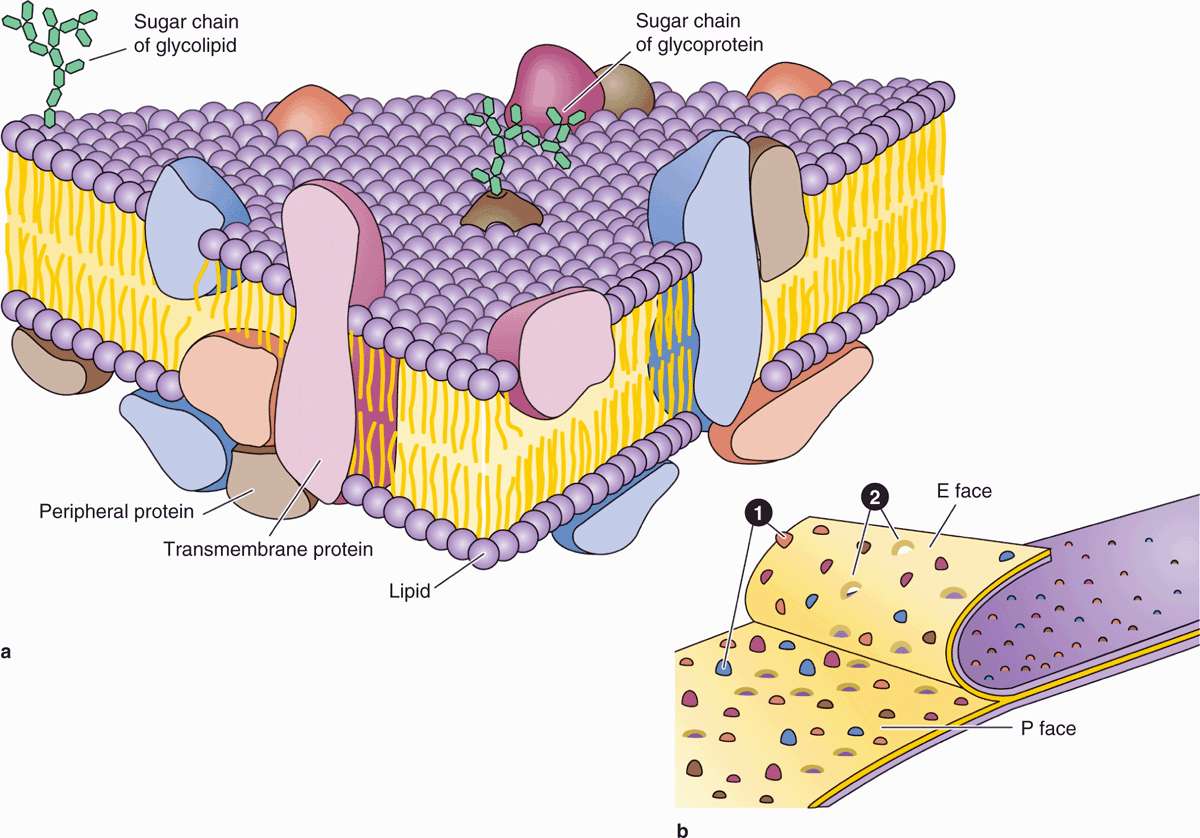
Proteins are major constituents of membranes (~50% by weight in the plasma membrane). Integral proteins are directly incorporated within the lipid bilayer itself, whereas peripheral proteins exhibit a looser association with one of the two membrane surfaces, particularly the inner (Figure 2–2). The loosely bound peripheral proteins can be easily extracted from cell membranes with salt solutions, whereas integral proteins can be extracted only by using detergents to disrupt lipids. The poly-peptide chains of many integral proteins span the membrane several times, from one side to the other, and are accordingly called multipass transmembrane proteins. Integration of the proteins within the lipid bilayer is mainly the result of hydrophobic interactions between the lipids and nonpolar amino acids present on the outer region of the proteins.
Freeze-fracture electron microscope studies of membranes show that parts of many integral proteins protrude from both the outer or inner membrane surface (Figure 2–2b). Like those of glycolipids, the carbohydrate moieties of glycoproteins project from the external surface of the plasma membrane and contribute to the glycocalyx (Figure 2–3). They are important components of proteins acting as receptors, which participate in important interactions such as cell adhesion, cell recognition, and the response to protein hormones. As with lipids, the distribution of membrane polypeptides is different in the two surfaces of the cell membranes. Therefore, all membranes in the cell are asymmetric.
FIGURE 2–3 Membrane proteins.
Studies with labeled membrane proteins of cultured cells reveal that many such proteins are not bound rigidly in place and are able to move laterally (Figure 2–4). Such observations as well as data from biochemical, electron microscopic, and other studies showed that membrane proteins comprise a moveable mosaic within the fluid lipid bilayer, the well-established fluid mosaic model for membrane structure (see Figure 2–2a). However, unlike the lipids, many membrane proteins are restricted in their lateral diffusion by attachment to cyto-skeletal components. In most epithelial cells, tight junctions between the cells (see Chapter 4) also restrict lateral diffusion of unattached transmembrane proteins and outer layer lipids, producing specific membrane domains.
FIGURE 2–4 Experiment demonstrating the fluidity of membrane proteins.
Membrane proteins functioning as components of large enzyme complexes are also less mobile, especially those involved in the transduction of signals from outside the cell. Such protein complexes are located in specialized membrane patches termed lipid rafts having higher concentrations of cholesterol and saturated fatty acids which reduce lipid fluidity. This together with the presence of scaffold proteins that maintain spatial relationships between enzymes and signaling proteins allows the proteins assembled within lipid rafts to remain in close proximity and interact more efficiently.
Transmembrane Proteins & Membrane Transport
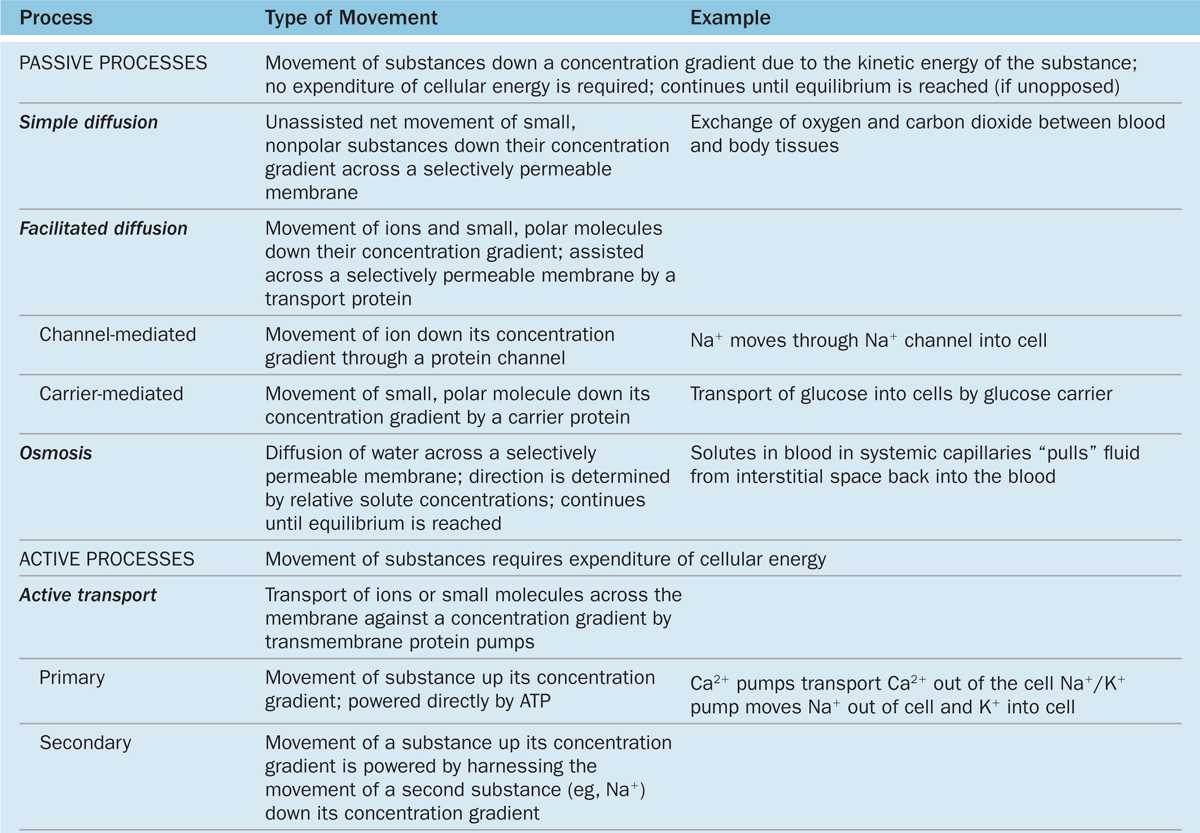
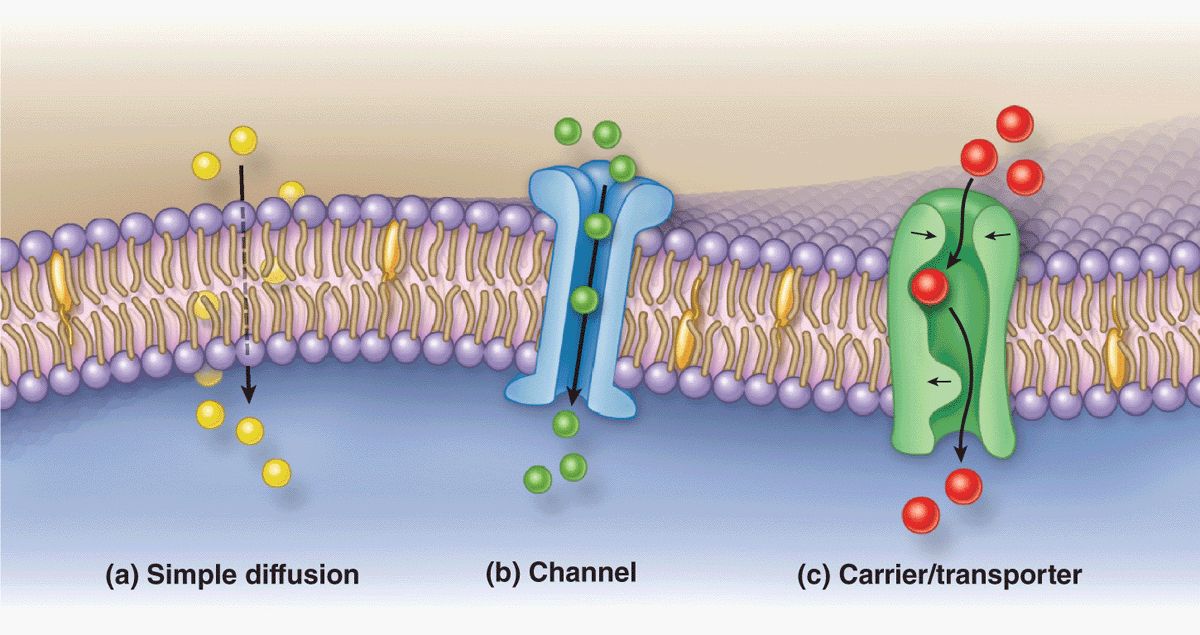
The plasma membrane is the site where materials are exchanged between the cell and its environment, with many molecules moving through the membrane by the general mechanisms shown in Figure 2–5. Small, lipophilic (fat-soluble) molecules can pass through lipid bilayers by simple diffusion. Ions such as Na+, K+, and Ca2+ cross membranes by passing through integral membrane proteins that act as ion channels or ion pumps. Transmembrane diffusion of water molecules (by osmosis) involves their passive movement through multipass transmembrane proteins called aquaporins. Other ions and many molecules only cross membranes after binding to carrier or transporter proteins, which are integral membrane proteins in which conformational changes deliver the bound molecule to the other side (Figure 2–5). While simple diffusion is passive (requiring no energy), ion pumps and carrier proteins involve active transport, using energy from the breakdown of adenosine triphosphate (ATP). These transport processes are summarized with further details and examples in Table 2–2.
FIGURE 2–5 Major mechanisms by which molecules cross membranes.
TABLE 2–2 Mechanisms of transport across the plasma membrane.
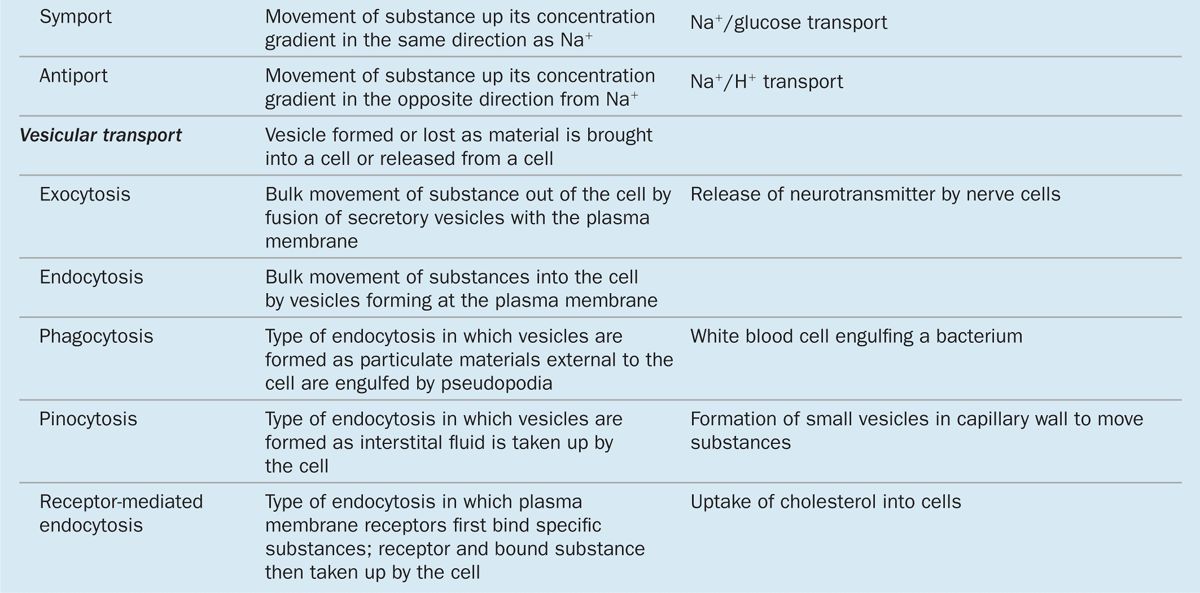
Vesicular Transport: Endocytosis & Exocytosis
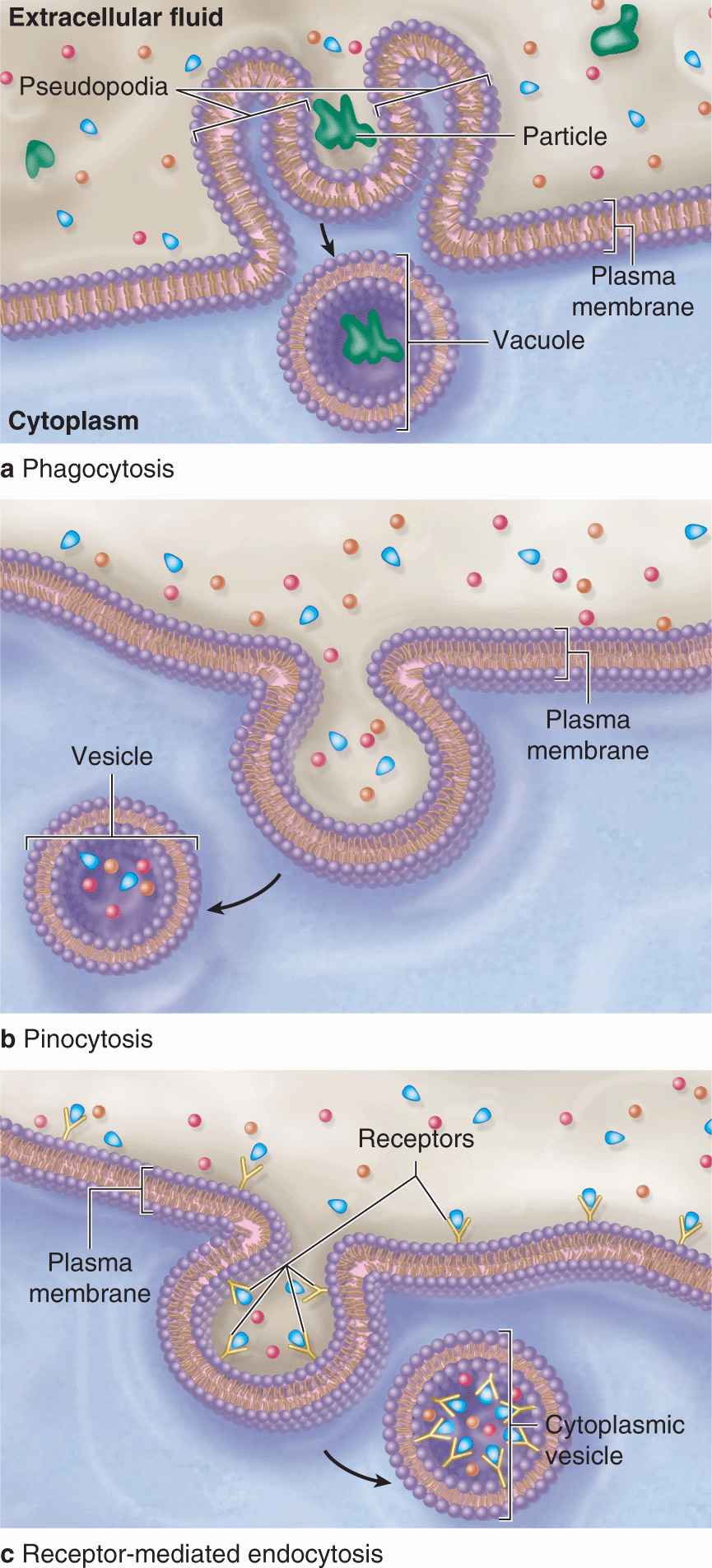
Bulk uptake of material also occurs across the plasma membrane in a general process called endocytosis, an active process involving folding and fusion of the membrane to form vesicles that enclose the material transported. Cells show three general types of endocytosis, summarized in Table 2–2 and Figure 2–6.
FIGURE 2–6 Three major forms of endocytosis.
1. Phagocytosis: Phagocytosis literally means “cell eating.” Certain white blood cells, such as macrophages and neutrophils, are specialized for engulfing and removing particulate matter such as bacteria, protozoa, dead cells, and unneeded extracellular constituents. When a bacterium becomes bound to the surface of a neutrophil, cytoplasmic extensions are extended from the cell (in an actindependent process) and to surround the bacterium. The membranes of these extensions meet and fuse, enclosing the bacterium in an intracellular vacuole called a phagosome, which then fuses with a lysosome for degradation of the contents as discussed later in this chapter.
2. Fluid-phase endocytosis: In fluid-phase pinocytosis (“cell drinking”), smaller invaginations of the cell membrane form and entrap extracellular fluid and its dissolved contents. Pinocytotic vesicles (~80 nm in diameter) then pinch off inwardly from the cell surface. In most cells such vesicles usually fuse with lysosomes. In many very thin cells, however, pinocytotic vesicles may move to the opposite cell surface where they fuse with the membrane and release their contents outside the cell. This accomplishes bulk transfer of material across the cell in a process termed transcytosis.
3. Receptor-mediated endocytosis: Receptors for many substances, such as low-density lipoproteins and protein hormones, are integral membrane proteins at the cell surface. High-affinity binding of such ligands to their receptors causes these proteins to aggregate in special membrane regions that then invaginate and pinch off internally as vesicles.
As shown in Figure 2–7, the formation and fate of vesicles emerging from receptor-mediated endocytosis is regulated by specific peripheral membrane coat proteins. The occupied receptors associate with other proteins on the cytoplasmic membrane surface and begin invagination as coated pits. The electrondense coating on the cytoplasmic surface of such pits contains several polypeptides, the major one being clathrin. In a coated pit clathrin molecules interact like the struts in a geodesic dome, forming that region of cell membrane into a cage-like invagination that is pinched off into the cytoplasm as a coated vesicle (Figure 2–7) containing the ligands and their receptors internally. Another type of receptor-mediated endocytosis very prominent in endothelial cells uses invaginations called caveolae (L. caveolae, little caves) that involve the membrane protein caveolin.
FIGURE 2–7 Receptor-mediated endocytosis involves regulated membrane trafficking.
In all these endocytotic processes, the vesicles or vacuoles produced quickly enter and fuse with the endosomal compartment, a dynamic system of membranous vesicles (Figure 2–7) of various sizes and shapes located in the cytoplasm near the cell surface (early endosomes) or deeper in the cytoplasm (late endosomes). The clathrin molecules separated from the coated vesicles recycle back to the cell membrane to participate in the formation of new coated pits. The membrane of endosomes contains ATP-driven H+ pumps that acidify their interior.
While phagosomes and pinocytotic vesicles soon fuse with lysosomes, molecules penetrating the endosomal compartment after receptor-mediated endocytosis may also be directed down other pathways (Figure 2–7a). The acidic pH of early endosomes causes many ligands to uncouple from their receptors, after which the two molecules are sorted into separate vesicles. The receptors may be returned to the cell membrane to be reused. Low-density lipoprotein receptors, for example, are recycled several times. The ligands typically are transferred to late endosomes. However, some ligands are returned to the extracellular milieu with their receptors and both are used again. An example of this activity is the iron-transport protein transferrin: ferric ions dissociate from transferrin at low endosomal pH and the free protein and the receptor both return to the cell surface. Other endosomes may release their entire contents at a separate domain of the cell membrane (transcytosis), which is especially important in epithelial cells.
Bulk movement of large molecules from inside to outside the cell can involve the form of vesicular transport called exocytosis. In this process a membrane-limited cytoplasmic vesicle fuses with the plasma membrane, resulting in the release of its contents into the extracellular space without compromising the integrity of the plasma membrane (Figure 2–7a). Exocytosis is triggered in many cells by transient increase in cytosolic Ca2+. The pathways and process of membrane fusion during exocytosis are highly regulated and involve interactions between several specific membrane proteins. Exocytosis of stored products from epithelial cells usually occurs specifically at the apical domains of cells, constituting a major mechanism of glandular secretion (see Chapter 4).
Protein secretion involving exocytosis may follow two pathways:
 Constitutive secretion is used for products that are released from cells continuously, as soon as synthesis is complete, such as procollagen for the ECM.
Constitutive secretion is used for products that are released from cells continuously, as soon as synthesis is complete, such as procollagen for the ECM.
 Regulated secretion occurs in response to signals coming to the cells, such as the release of digestive enzymes from pancreatic cells in response to specific stimuli.
Regulated secretion occurs in response to signals coming to the cells, such as the release of digestive enzymes from pancreatic cells in response to specific stimuli.
Portions of the cell membrane become part of the endocytotic vesicles or vacuoles during endocytosis; during exocytosis, membrane is returned to the cell surface. This process of membrane movement and recycling is called membrane trafficking (Figure 2–7a). Trafficking and sorting of membrane components occur continuously in most cells and are not only crucial for maintaining the cell but also for physiologically important processes such as reducing blood lipid levels.
Subpopulations of vacuoles among the early and late endosomes in many cells accumulate small vesicles and tubules within their lumens by further invaginations of their limiting membranes, becoming multivesicular bodies. While multi-vesicular bodies may merge with lysosomes for selective degradation of their contents, this organelle may also fuse with the plasma membrane and release the intralumenal vesicles outside the cell. The small (<120 nm diameter) vesicles released (called exosomes) allow transfer of membrane proteins and other materials to nearby cells.
Signal Reception & Transduction
Cells in a multicellular organism communicate with one another to regulate tissue and organ development, to control their growth and division, and to coordinate their functions. Many cells form communicating junctions that couple adjacent cells and allow the exchange of ions and small molecules (see Chapter 4). Through these channels, called gap junctions, signals may pass directly from cell to cell without reaching the extracellular fluid.
Cells also use nearly two dozen families of receptor proteins to detect and respond to extracellular molecules and physical stimuli of all types. Only cells with receptors for a specific ligand are target cells for that molecule. Each cell type in the body contains a distinctive set of receptor proteins that enable it to respond to a complementary set of signaling molecules in a specific, programmed way. Signal molecules can take different routes:
 In endocrine signaling, the signal molecules (called hormones) are carried in the blood to target cells throughout the body.
In endocrine signaling, the signal molecules (called hormones) are carried in the blood to target cells throughout the body.
 In paracrine signaling, the chemical mediators are rapidly metabolized after release so that they act only on local cells very close to the source.
In paracrine signaling, the chemical mediators are rapidly metabolized after release so that they act only on local cells very close to the source.
 In synaptic signaling, a special kind of paracrine interaction, neurotransmitters act only on adjacent cells through special contact areas called synapses (see Chapter 9).
In synaptic signaling, a special kind of paracrine interaction, neurotransmitters act only on adjacent cells through special contact areas called synapses (see Chapter 9).
 In autocrine signaling, signals bind receptors on the same cell type that produced the messenger molecule.
In autocrine signaling, signals bind receptors on the same cell type that produced the messenger molecule.
 In juxtacrine signaling, important in early embryonic tissue interactions, signaling molecules such as proteins remain part of a cell membrane and bind surface receptors of the target cell when the two cells make direct physical contact.
In juxtacrine signaling, important in early embryonic tissue interactions, signaling molecules such as proteins remain part of a cell membrane and bind surface receptors of the target cell when the two cells make direct physical contact.
Receptors for hydrophilic signaling molecules, including most hormones and neurotransmitters, are usually transmembrane proteins in the plasmalemma of target cells, frequently as part of lipid rafts. Three important functional classes of such receptors are shown in Figure 2–8:
FIGURE 2–8 Major types of membrane receptors.
 Channel-linked receptors open upon ligand binding to allow ion transfer across the membrane.
Channel-linked receptors open upon ligand binding to allow ion transfer across the membrane.
 Enzymatic receptors, in which ligand binding induces catalytic activity in associated peripheral proteins.
Enzymatic receptors, in which ligand binding induces catalytic activity in associated peripheral proteins.
 G-protein–coupled receptors upon ligand binding change an associated “G protein” that then binds the guanine nucleotide GTP and is released to activate other cytoplasmic proteins.
G-protein–coupled receptors upon ligand binding change an associated “G protein” that then binds the guanine nucleotide GTP and is released to activate other cytoplasmic proteins.
The hydrophilic ligands (or first messengers) binding such receptor proteins often begin a process of signal transduction, activating a series of intracellular intermediaries producing changes in either the cytoplasm, the nucleus, or both. Channel-mediated ion influx or activation of kinases can activate downstream proteins, amplifying the signal. Activated G proteins target ion channels or other membrane-bound effectors that also propagate the signal further into the cell (Figure 2–8). One such effector protein is the enzyme adenyl cyclase that generates large quantities of second messenger molecules, such as cyclic adenosine monophosphate (cAMP). Other second messengers include 1, 2-diacyglycerol (DAG), and inositol 1, 4, 5-triphosphate (IP3). The ionic changes or second messengers amplify the first signal and trigger a cascade of enzymatic activity, usually including kinases, leading to changes in gene expression or cell behavior. Second messengers may diffuse through the cytoplasm or be retained locally by scaffold proteins for more focused amplification of activity.
Hydrophobic signaling molecules, such as steroids and thyroid hormones, bind reversibly to carrier proteins in the plasma for transport through the body. Such hormones are lipophilic and, once released from their carrier proteins, pass by diffusion through the plasma membrane of the target cell and bind to specific intracellular receptor proteins. With many steroid hormones, receptor binding activates that protein, enabling the complex to move into the nucleus and bind with high affinity to specific DNA sequences. This generally increases the level of transcription from specific genes. Each steroid hormone is recognized by a different member of a family of homologous receptor proteins.
Ribosomes
Ribosomes are small electron-dense particles, about 20 × 30 nm in size. Ribosomes found in the cytosol are composed of four segments of rRNA and approximately 80 different proteins. Bound to mRNA, all ribosomes have two subunits of different sizes and act to catalyze the process of protein translation.
In eukaryotic cells, the rRNA molecules of both subunits are synthesized within the nucleus. Their numerous proteins are synthesized in the cytoplasm but then enter the nucleus and associate with rRNAs. The assembled large and small subunits then leave the nucleus and enter the cytoplasm to participate in protein synthesis.
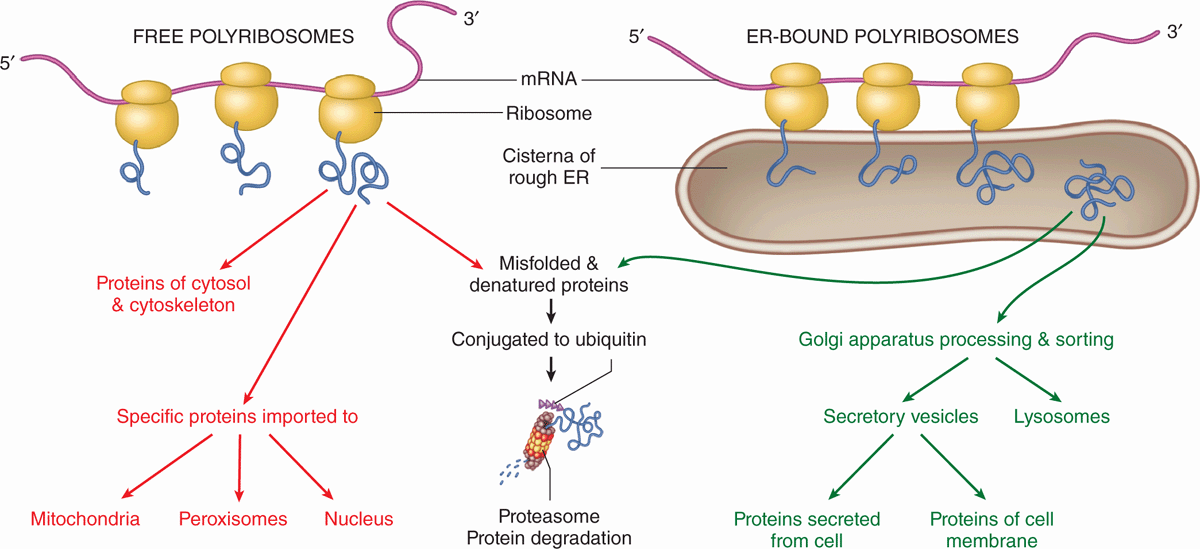
The large and small ribosomal subunits come together by binding an mRNA strand (Figure 2–9), and typically many ribosomes occupy a single mRNA to form polyribosomes (or polysomes). With the nucleotide sequence of the mRNA specifying the polypeptide sequence being synthesized, ribosomes assemble the protein chain from amino acids ferried in by tRNA. The compact core of each ribosome contains the rRNA molecules that not only provide structural support but also position tRNAs in the correct “reading frame” and catalyze the formation of the peptide bonds. The more peripheral proteins of the ribosome seem to function primarily to stabilize the catalytic RNA core.
FIGURE 2–9 Polyribosomes: free or bound to the endoplasmic reticulum.
Polyribosomes are intensely basophilic because of the numerous phosphate groups of the constituent RNAs that act as polyanions. Thus, cytoplasmic regions that stain intensely with hematoxylin and basic dyes, such as methylene and toluidine blue, indicate sites of active protein synthesis.
Proper folding of new proteins is guided by protein chaperones (often present in chaperonin complexes); denatured proteins or those that cannot be refolded properly are conjugated to the protein ubiquitin that targets them for breakdown by proteasomes (discussed later). As indicated in Figure 2–9, proteins synthesized for use within the cytosol (eg, glycolytic enzymes) or for import into the nucleus and certain other organelles are synthesized on polyribosomes existing as isolated cytoplasmic clusters. Polyribosomes attached via the large subunits to the membranes of the endoplasmic reticulum (ER) translate mRNA coding for membrane proteins of the ER, the Golgi apparatus, or the cell membrane; enzymes to be stored in lysosomes; and proteins to undergo exocytosis from secretory vesicles.
Endoplasmic Reticulum
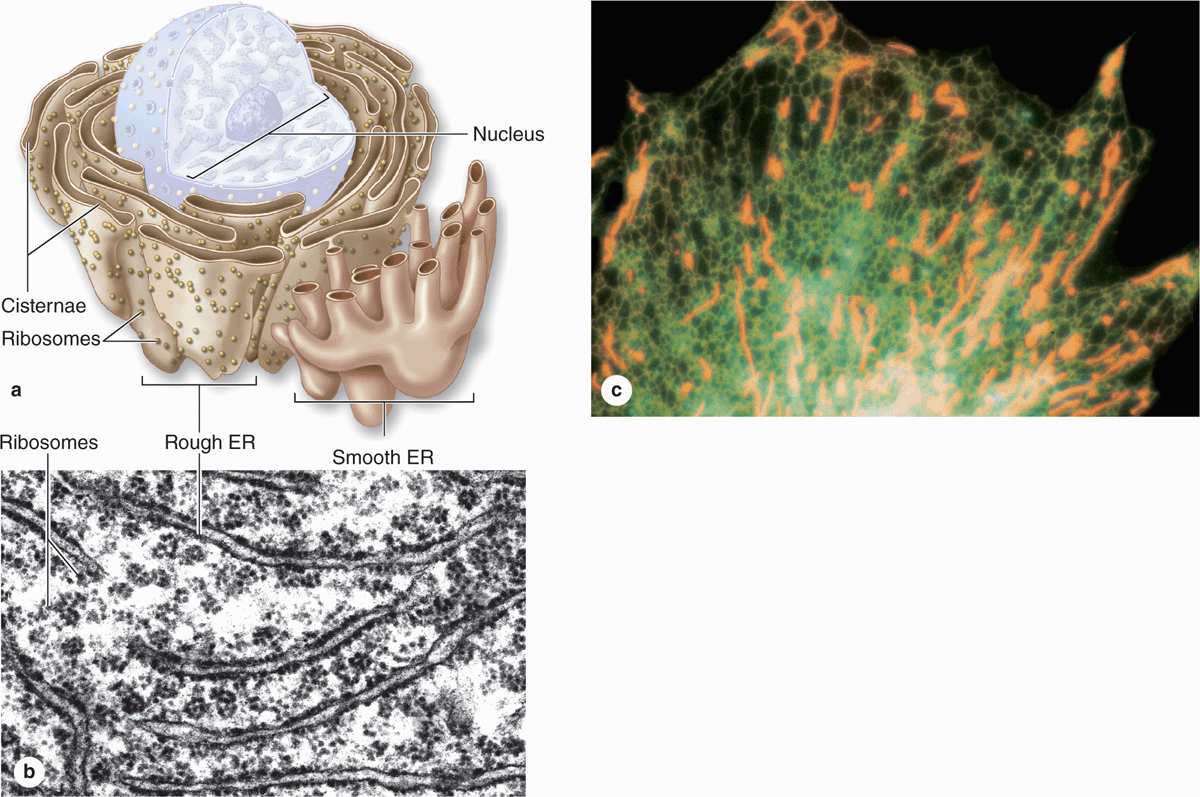
The cytoplasm of most cells contains a convoluted membranous network called the endoplasmic reticulum (ER). This network (reticulum) extends from the surface of the nucleus to the cell membrane and encloses a series of intercommunicating channels and sacs called cisternae (Figure 2–10). With a membrane surface up to 30 times that of the cell membrane, the ER provides a major site for vital cellular activities, including biosynthesis of proteins and lipids. In addition, the ER cisternae (L. cisternae, reservoirs), which appear as separated spaces in electron microscopic sections, comprise a continuous internal cell compartment that collects newly synthesized proteins for modification and delivery into pathways leading to other organelles and for secretion. In most cells over a quarter of all proteins are imported into the lumen of the ER or integrated into ER membranes.
FIGURE 2–10 Rough and smooth endoplasmic reticulum.
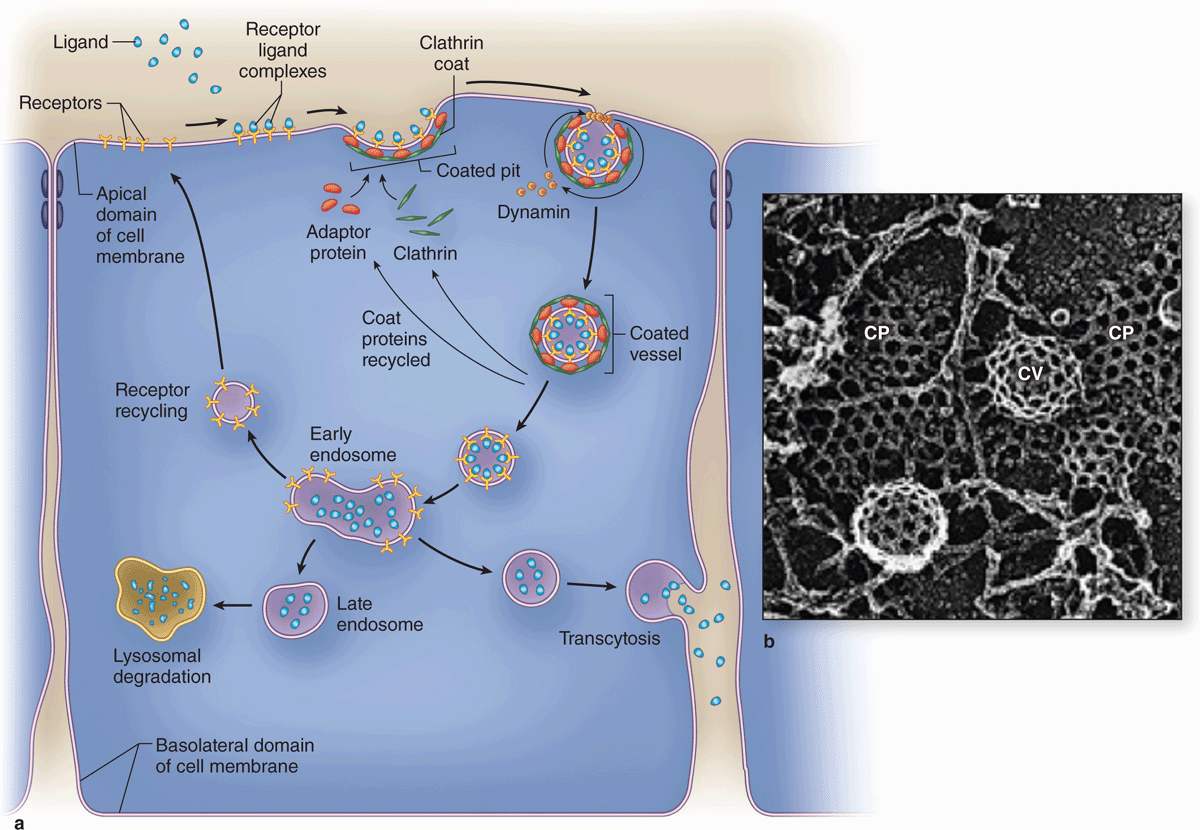
 Receptors and ligands may be carried to late endosomes and then to lysosomes for degradation.
Receptors and ligands may be carried to late endosomes and then to lysosomes for degradation. Ligands may be released internally and the receptors recycled to the cell surface.
Ligands may be released internally and the receptors recycled to the cell surface. Vesicles may move to and fuse with another cell surface, where the ligands are released again outside the cell (transcytosis).
Vesicles may move to and fuse with another cell surface, where the ligands are released again outside the cell (transcytosis).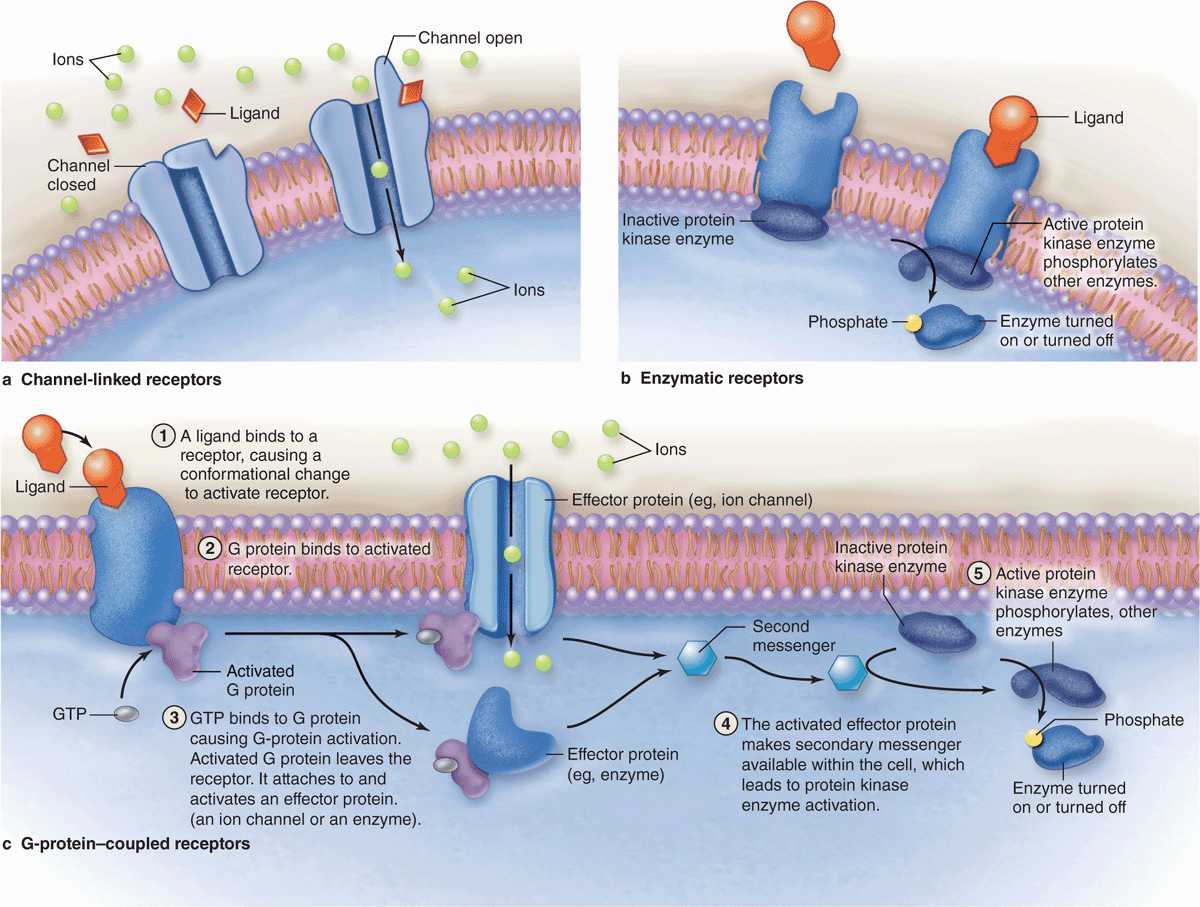
 MEDICAL APPLICATION
MEDICAL APPLICATION