Treating a patient in an intensive care unit (ICU) setting presents a series of unique and complicated issues. The feeling of loss of control that may occur when one is admitted to a hospital may be amplified for the ICU patient. The introduction of multiple tubes, the fear and anxiety associated with a serious medical illness, and the sudden loss of the ability to talk if a tracheostomy tube or mechanical ventilation is required may all contribute to a feeling of helplessness. The continued advances of medical technology that have led to lifesaving techniques for individuals with stroke, traumatic brain injury (TBI), spinal cord injury (SCI), pulmonary disease, neurodegenerative disease, trauma, and cardiopulmonary conditions mean that the ICU patient may be of any age and have any one of a myriad of disorders that affect communication or swallowing.
The involvement of speech-language pathologists (SLPs) in the care of ICU patients has increased over the past several years. For this reason it is imperative that clinicians have a working knowledge of the breathing and speaking changes that occur in individuals who require endotracheal tubes, tracheostomy tubes, or noninvasive ventilatory assistance. In the earliest stages of admission to an ICU, medical stability is the primary concern; SLPs may be asked to assist at this early stage by identifying a mode of communication or establishing a safe method of nutritional intake for a patient. The current trend of limiting hospital stays to decrease medical costs may be one of the reasons for early consultation among a variety of health care specialists who would previously have delayed consultation until a patient was transferred to a regular medical unit.
This chapter familiarizes the practicing SLP with some of the more common terminology and issues specific to the ICU environment. Our discussion includes an explanation of the basic processes of normal respiration and mechanical ventilation as well as common illnesses seen in an ICU setting. Because the role of SLPs working with tracheostomized patients has become more commonplace, we present a review of various methods of voicing with tracheostomy tubes and speaking valves. In addition, we discuss decision-making issues regarding a patient’s candidacy for oral feeding and various methods of testing for and treating dysphagia, including some ethical considerations related to feeding severely ill patients. The importance of a team approach to diagnosing and treating ICU patients is emphasized throughout. This information will help the practicing SLP to understand the complexities of working with an ICU patient and thus facilitate effective communication and swallowing care for individuals in an ICU setting.
16.1 Normal Respiration
In most cases, admission to a medical ICU indicates a problem in respiration. To fully understand such disorders, it is essential for us to have sound knowledge of the normal respiratory process.
The respiratory system is relatively easy to comprehend when one understands the function of its two major components: the lungs and the respiratory, or ventilatory, pump. The lungs are primarily involved in gas exchange; they are responsible for moving oxygen from the atmosphere into the alveoli and then into the pulmonary capillary blood and for moving carbon dioxide from the pulmonary capillary blood to the alveoli, where it can be exhaled into the atmosphere. Hence, the lungs ensure a constant supply of oxygen to the blood, which is then distributed to the cells, tissues, and organs of the body, providing the necessary maintenance of normal biochemical and metabolic function. 1 Likewise, the ability of the lungs to excrete carbon dioxide, the major waste product of metabolism, prevents accumulation of this potentially deadly gas in the body. Diseases that primarily affect the working tissue of the lung (alveoli), such as pneumonia, congestive heart failure, pulmonary hemorrhage, etc., interfere with pulmonary gas exchange and lead to low blood oxygen, called hypoxemia, or occasionally to elevated blood carbon dioxide levels, or hypercapnia. 2
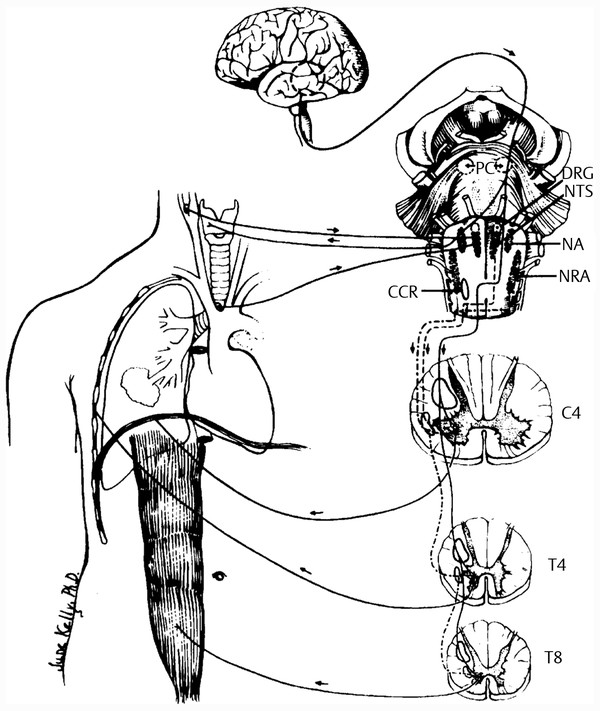
Ventilation is the process of eliminating carbon dioxide from the body through the lungs. The function of the respiratory, or ventilatory, pump of the respiratory system is more complex than that of the lungs and involves the elements that are included in the bellows function of the system. The respiratory cycle begins in the brainstem in the respiratory centers of the pons and medulla, with the generation of a nerve impulse and the transmission of that stimulus to the respiratory muscles, primarily the diaphragm ( ▶ Fig. 16.1). The contraction and flattening of the diaphragm result in the shortening of its muscle fibers as it descends in the thorax. When this occurs, intrapleural pressure becomes more negative as intrathoracic volume increases. This increase in negative intrapleural pressure is transmitted to the alveoli and allows intra-alveolar pressure to be lower than atmospheric pressure. The gradient between the atmosphere and alveoli causes air to move into the lungs until alveolar and atmospheric pressures are equal. 3 At end-inspiration, when atmospheric pressure equals intra-alveolar pressure, airflow into the lungs ceases and expiration begins.
Fig. 16.1 Summary of the route of respiration. (From American College of Chest Physicians. The diagnosis and management of neuromuscular diseases causing respiratory failure. Chest 1991;99:1485–1494, with permission.)
Usually, expiration is a passive process dependent on the elastic recoil of the lungs, the presence or absence of airway resistance, and the use of the abdominal and intercostal expiratory muscles. At end-expiration, intrapleural pressure has increased back to –2.0 cm H2O and the whole process is repeated. 4 The negative intrapleural pressure at end-expiration is created by the opposing forces of the lungs, which want to collapse, and the chest wall, which wants to spring outward. These opposing forces keep the lungs expanded and prevent them from collapsing, create negative intrapleural pressure at end-expiration, and allow a volume of air in the lungs prior to the next inspiration, termed functional residual capacity (FRC).
Functional residual capacity is the beginning point for a new respiratory cycle. Therefore, the normal respiratory cycle in a spontaneously breathing person depends on opposite pressure and volume changes that allow air to move in and out of the lungs. Tidal volume is the volume of air moving into the lungs depending on their compliance. If the lung is stiff, as in idiopathic pulmonary fibrosis, less volume moves into the lung. Conversely, if pulmonary compliance is great, such as in emphysema or asthma, a greater amount of air constitutes the patient’s tidal volume.
16.2 Mechanical Ventilation
Patients in an ICU setting are frequently assisted in their respiration by mechanical ventilation. Respiratory failure, whether secondary to hypoxemia, hypercapnia, or both, is the primary reason that critically ill patients eventually require ICU care and mechanical ventilation. 5 The majority of patients seen in an ICU have a fulminant course with a dramatic presentation, and the institution of mechanical ventilation can be life-saving once the primary insult is treated or reversed. However, in some cases patients with neuromuscular disease or chest wall abnormalities experience slowly progressive, chronic respiratory failure and are unable to be weaned from the ventilator.
Most adult patients requiring mechanical ventilation in an ICU will be ventilated via positive-pressure, volume-cycled ventilators. This means that gas that is above atmospheric pressure is delivered into the lungs, raising intra-alveolar pressure during inspiration. During inspiration from a ventilator, a preset tidal volume is delivered to the patient, and both intrathoracic volume and pressure increase. Therefore, intrathoracic pressure and volume move in the same direction, unlike that of a spontaneously breathing individual. The amount of delivered tidal volume is constant and the pressure is variable, depending on lung compliance and airway resistance. 6 Airway pressure will be high if lung compliance is low (e.g., idiopathic pulmonary fibrosis) or airway resistance is high (e.g., tumor, foreign-body aspiration, increased pulmonary secretions), or both. At end-inspiration, an exhalation port valve is opened and exhalation is essentially passive, dependent on lung elastic recoil, airway resistance, and the use of expiratory muscles as in a spontaneously breathing patient.
The next breath is either triggered by the patient or automatically given to the patient by the ventilator. If the patient is alert or able to initiate a breath, the patient sets the rate of breathing. This mode of mechanical ventilation is referred to as the assist-control (AC) mode and is the preferred mode of early ventilation in patients who develop respiratory failure from a variety of causes. If the patient is unable to set the respiratory rate, the physician can determine the number of minimum breaths per minute. The product of delivered tidal volume and respiratory rate is termed minute volume or minute ventilation; this value varies from patient to patient depending on the underlying disease process. All ventilator settings are adjusted by a physician, usually a pulmonologist/intensive care physician or respiratory therapist or nurse who follows the orders of a physician. Some patients are uncomfortable with artificial ventilation, which causes them to be anxious and agitated. In those instances, sedation, and in extreme cases, drug-induced paralysis, may be necessary to ensure good patient-ventilator synchrony.
There are other types of noninvasive modes of respiration that may be implemented in less ill patients who may not require conventional mechanical ventilation or to forestall more conventional ventilation in patients whose primary disease process can be reversed or controlled relatively quickly. These alternative modes of ventilation deliver positive pressure to the airway via a tight-fitting nasal mask, full facial mask or nasal pillows, which are meant to augment the oxygenation and ventilatory processes and reduce the patient’s work of breathing. 7 Noninvasive ventilation may also serve as a bridge to more normal spontaneous ventilation as a patient’s medical status improves and he or she is ready to be weaned from full mechanical ventilation. Noninvasive airway ventilatory management that is typically seen in an ICU setting may include continuous positive airway pressure, pressure-support ventilation, and inspiratory/expiratory positive airway pressure (BiPAP, bilevel positive airway pressure).
16.2.1 Continuous Positive Airway Pressure
Continuous positive airway pressure (CPAP) is the application of positive pressure to the airways throughout the respiratory cycle (i.e., during both inspiration and expiration). This mode of ventilation can be used only in a spontaneously breathing person. The amount of airway pressure applied is determined by a physician and is usually between 5 and 10 cm H2O. CPAP may be applied through the ventilator in an intubated patient or via a tight-fitting nasal or full face mask in a nonintubated patient. It can be used to assist in weaning a patient from mechanical ventilation or for breath support in patients with frank or impending respiratory failure. CPAP works by splinting open atelectatic alveoli, increasing end-expiratory lung volume, and assisting the inspiratory muscles in reducing the work of breathing. 8
16.2.2 Pressure-Support Ventilation
Pressure-support ventilation (PSV), which is patient initiated, has been relatively recently described and used. It is a physician-determined mode that is pressure-cycled and flow-limited in a spontaneously breathing patient with an intact respiratory center. That is, pressure is applied to the airway only during the inspiratory phase of the respiratory cycle (pressure-cycled), and the pressure applied (determined by the physician) ceases once the inspiratory flow drops below a certain level (e.g., 5 L/min [flow-limited]). Exhalation is then passive and no pressure is applied during this part of the respiratory cycle. PSV can be used to entirely support the patient or to assist in the weaning of the patient from mechanical ventilation. The more pressure used, the more the patient is assisted and the less the work of breathing for the patient.
Like CPAP, PSV can be used via a mask or through an endotracheal tube in an intubated patient. PSV reduces the work of breathing by unloading the inspiratory muscles. It may be of special value in patients who have increased inspiratory resistance (e.g., central airway mass/tumor, etc.). 9
16.2.3 Inspiratory/Expiratory Positive Airway Pressure
BiPAP, or bilevel positive airway pressure, is a technique of noninvasive ventilation in which pressure applied to the airway can be adjusted for both the inspiratory and expiratory phases of the respiratory cycle. Hence, the differential pressures may be used to assist in the ventilation of patients with different disease processes. This modality is used in a spontaneously breathing patient by a tight-fitting nasal or full face mask or nasal pillows. The pressure applied during inspiration is inspiratory positive airway pressure (IPAP) and during expiration is expiratory positive airway pressure (EPAP). IPAP is very similar to PSV and EPAP is similar to positive end-expiratory pressure (PEEP). BiPAP helps to unload the respiratory muscles, reduce the work of breathing, splint open collapsed alveoli, and raise end-expiratory volume. BiPAP is often used in patients who require partial ventilatory support or in those who have impending respiratory failure who might be spared endotracheal intubation if the primary disorder can be quickly reversed. 10
16.2.4 Mechanical Ventilation Weaning
Although the literature is replete with articles suggesting the best approaches or proper timing for weaning a patient from mechanical ventilation, this process is as much an art as a science. 11 Numerous weaning parameters have been established to aid physicians in the decision-making process, but these parameters do not always predict a successful outcome. 12 As a general rule, the less time a patient is mechanically ventilated and the more quickly reversible the primary insult is, the more accurate the weaning guidelines are for predicting successful weaning. In addition, younger patients and surgical patients tend to wean more quickly from mechanical ventilation than older patients, patients with multiple or complex medical problems, or those with multiple organ dysfunction.
A rapid shallow breathing index, which provides a ratio of respiratory frequency divided by tidal volume in liters, seems to predict successful weaning in most patients if this ratio is less than 105. 13 Ultimately, many experienced critical care physicians base the decision to wean on general impressions of patient status. However, as a general principle, weaning usually occurs when patients assume a greater part of the work of breathing as their clinical condition improves and when gas exchange, as assessed by arterial blood gases, shows stable oxygenation and ventilation.
16.3 Medical Conditions that Require Treatment in an Intensive Care Unit Setting
This section reviews the most common pulmonary and neurologic illnesses that are typically seen in an ICU setting.
16.3.1 Pulmonary Disease
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) encompasses a group of lung diseases characterized by slow progression and long continuance as well as the presence of expiratory airflow limitation or obstruction. Emphysema and chronic bronchitis are two of the more important diseases that fall under this large category, but chronic bronchial asthma, cystic fibrosis, bronchiectasis, small airway disease, and bronchiolitis all fulfill the broad definition of COPD. About 16 million people in the United States have emphysema and chronic bronchitis, and another 16 million suffer from asthma (total 32 million, or 11 to 12.5% of the U.S. population). 14 The differences in these diseases are based on histologic, physiologic, radiologic, clinical, and other criteria.
Emphysema
Emphysema is characterized by the permanent destruction and enlargement of the distal air spaces, most commonly caused by cigarette smoking (active or passive). 15 Patients tend to be limited mostly by dyspnea and have significant air trapping and hyperinflation. Because they work so hard to maintain normal or near-normal blood gases, they are often referred to as “pink puffers.” They are usually thin and cachectic and rely on pursed-lip breathing.
Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS, formerly adult respiratory distress syndrome) is a catastrophic disease characterized by significant and acute lung injury leading to respiratory failure, usually within 48 hours of the insult. The majority of ARDS patients have profound impairment in gas exchange, are severely hypoxemic, and spend variable amounts of time on a mechanical ventilator. ARDS is most commonly caused by pneumonia, aspiration, polytrauma, multiple transfusions, gram-negative sepsis, and pancreatitis. Other organ system involvement and failure occur, and the mortality is high (40%). 16
Pulmonary Infections
A myriad of bacterial, viral, fungal, and parasitic pathogens can cause severe lung injury and respiratory failure, requiring mechanical ventilation. Two recently recognized infections, both caused by respiratory viruses, have received considerable attention. Severe acute respiratory syndrome (SARS) and swine influenza have received significant and justified attention just in the last 10 years. SARS is caused by a coronavirus and in 2002–2003 an outbreak that began in Hong Kong affected 8,422 individuals and caused 916 deaths worldwide. 17 Most cases develop into ARDS or atypical pneumonia. Swine influenza (pig flu, swine flu, H1N1, etc.), recognized in 2009, is caused by swine influenza virus (SIV). Swine flu can cause a variety of flulike symptoms but the most common cause of death is respiratory failure. Pneumonia, sepsis, and multiple organ system failure are common, with deaths occurring in the very young and the elderly.
16.3.2 Cardiopulmonary Illness
Congestive Heart Failure
Congestive heart failure (CHF) is caused by several diseases in which impairments in cardiac output allow blood to pool in the pulmonary veins, capillaries, and pulmonary arteries. This pooling of blood leads to lung congestion and impairments of gas exchange. Etiologies that can cause CHF include myocardial infarction, valvular heart disease, hypertensive heart disease, cardiomyopathy, and pericardial diseases. The condition can be acute or chronic and almost all patients suffer dyspnea, fatigue, exercise intolerance, and lower extremity edema.
Cardiac Failure
Cardiac failure is a nonspecific condition of cardiac or heart dysfunction caused by a variety of diseases, including hypertensive and ischemic heart disease, pericardial disease, and valvular disease. During cardiac failure, cardiac output is impaired and end-organ perfusion suffers. Patients with cardiac failure may develop stroke, kidney disease, liver failure, and other organ dysfunction. A majority of these patients also have CHF.
Pulmonary Edema
Pulmonary edema is a condition of excess lung fluid that often leads to respiratory failure because the fluid-filled alveoli are unable to participate in normal gas exchange. CHF and ARDS are causes of pulmonary edema. Important in the evolution of this disease is the type of accumulated lung fluid. Protein-poor edema fluid (as in CHF) is more responsive to diuretic therapy than is protein-rich edema fluid (as in ARDS). Pulmonary edema usually causes dyspnea, fatigue, weakness, exercise intolerance, and peripheral edema.
16.3.3 Neurologic Disease
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a degenerative neuromuscular disease that involves the upper motor neurons in the cortex and lower motor neurons in the brainstem and spinal cord. 18 To date, the etiology of ALS is unknown. Viral, toxic, and genetic causes have been proposed. 18 In the United States, the ratio of men to women who acquire the nonfamilial form of the illness is 2:1, and the cause of death is usually respiratory failure or pneumonia. 18 Although muscle cramping is commonly the initial symptom of the disease, nearly one-quarter of patients present with dysarthria as their initial symptom. 19, 20 The illness eventually involves the musculature of speech, swallowing, and respiration. Patients who have made the decision to undergo a tracheotomy and/or mechanical ventilation to assist or prolong their respiratory status may be seen in the ICU. If a patient is anarthric, speech via a tracheostomy tube will not be functional and alternative methods of communication are necessary.
Guillain-Barré Syndrome
Adams and Victor 21 describe this illness as a rapidly progressing ascending inflammatory disease that affects people of all ages and both sexes. The usual clinical presentation is muscle weakness, which can result in total muscle paralysis and death due to respiratory failure within a few days of onset. Facial diplegia occurs in approximately half of the cases and arises along with other cranial nerve signs after the arms are affected. The use of plasmapheresis within 2 weeks of onset has been known to expedite recovery, including the length of time patients require mechanical ventilation. The focus of medical treatment is respiratory assistance and careful nursing care as the disease resolves naturally. Recovery is complete in the majority of cases. A flaccid form of dysarthria is seen with this disorder, and careful speech assessment and short-term speech and swallowing treatment may be necessary as the patient recovers.
Stroke
Many forms of stroke require ICU placement for a patient. Stroke may result from an intracranial hemorrhage or a thrombotic or embolic event. Patients who develop brainstem strokes are likely to spend their acute phase of hospitalization in an ICU because respiration may be compromised. As the course of the illness progresses, the patient should be followed closely from a communication and swallowing standpoint.
Traumatic Brain Injury
A patient with an acute TBI sometimes has an evaluation for basic cognitive stimulation in the ICU by an SLP. Because intubation and eventual tracheostomy are common occurrences in such situations, thorough diagnostic therapy from a communication and swallowing standpoint is imperative. TBI patients may exhibit rapid changes in neurologic status in a short period of time, and thus constant contact with nursing and medical staff are necessary for anticipating the patient’s ability to try new methods of communication and feeding, particularly as this relates to voicing and swallowing with a tracheostomy tube.
Spinal Cord Injury
Patients with acute SCI above the level of C3 likely have breathing problems and require mechanical ventilation. 22 As the patient stabilizes, communication and swallowing issues arise. Communication may progress from simple eye blinks for yes/no responses to voicing with a tracheostomy tube as the patient’s condition improves. Ongoing monitoring and diagnostic therapy are necessary to provide the most effective and efficient methods of communication for the patient.
Laryngeal Injury
According to Tucker, 23 the larynx is relatively protected within the neck by the sternocleidomastoid muscles laterally, the cervical vertebrae and other muscles of the neck posteriorly, and the mandible from above. Laryngeal injuries have several causes, ranging from blunt trauma to the larynx, as seen in seat belt or steering wheel injuries in car accidents, to penetrating trauma from bullet or knife wounds. Blunt injury to an older, more calcified larynx may result in arytenoid dislocation or fracture of the cartilaginous structures of the larynx, whereas the same blow may produce little damage to a young, flexible larynx.
The ICU patient with a laryngeal injury commonly has a tracheostomy tube. It is important to discuss the full extent of the patient’s laryngeal injuries and the surgical repair procedures with the physician to anticipate short- and long-term voicing conditions. Counseling regarding voice use in the early stages of hospitalization is recommended, with close monitoring, and voice therapy, with or without the presence of a tracheostomy tube, may be necessary.
16.4 Tracheotomy
During their initial days in the ICU patients may be ventilated via nasal or oral intubation with an endotracheal tube. However, many of these patients require a tracheotomy to facilitate respiration. Decisions about the proper timing and indications for changing the airway access to a tracheotomy and insertion of a tracheostomy tube seem to be less controversial than in the past, but a variety of opinions still exist. The trend in recent years has been toward earlier tracheotomy after initial nasal or oral intubation in critically ill patients. For spontaneously breathing patients, a tracheotomy is performed to improve ventilation. The tracheotomy is usually performed at or below the second or third tracheal ring. A tracheotomy is usually performed in cases when upper respiratory obstruction occurs owing to illness, injury, or surgery. 24 Tracheostomy tubes provide direct access to the lower respiratory tract. The patient inhales and exhales directly via the tracheostomy tube, thus bypassing the nose and mouth.
16.4.1 Components of a Tracheostomy Tube
There are three basic parts to a tracheostomy tube: the obturator, the outer cannula, and the inner cannula ( ▶ Fig. 16.2). 24 The obturator is used for insertion of the outer cannula of the tracheostomy tube and is replaced by the inner cannula once the outer cannula is securely in place. The inner cannula remains in place to collect secretions that could potentially obstruct the airway. At times these secretions may be viscous or may collect and dry within the inner cannula. If the inner cannula is not in place and secretions become encrusted within the outer cannula, the potential for airway obstruction exists, which, in extreme cases, may require a total tracheostomy tube change. For this reason it is highly recommended that the inner cannula remain in place and undergo cleaning on a regular basis. Disposable inner cannulas are available that may be replaced without cleaning, and single cannula tracheostomy tubes are also available that are made of a silicone and do not require the use of an inner cannula. The silicone material is intended to reduce the risk of encrustation of secretions. 25
Fig. 16.2 Shiley outer cannula, inner cannula, and obturator (left to right). (Courtesy of Mallinckrodt Medical, Inc.)

16.4.2 Types of Tracheostomy Tubes
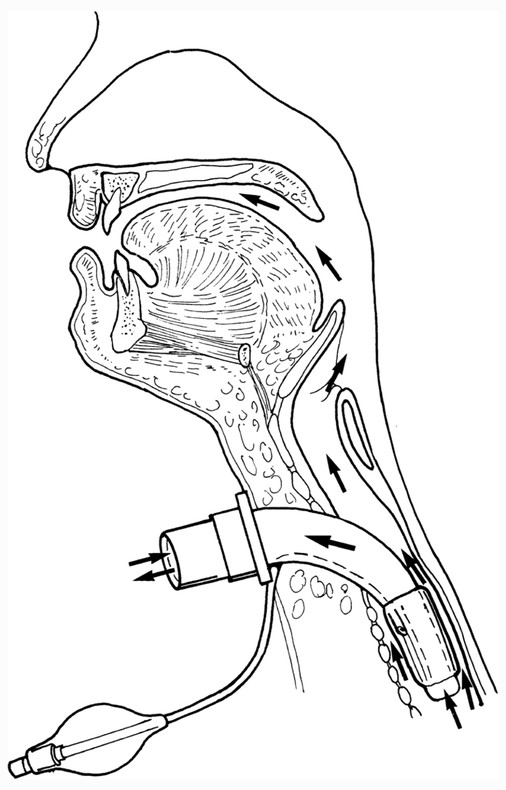
Tracheostomy tubes may be cuffless ( ▶ Fig. 16.2) or cuffed ( ▶ Fig. 16.3). The cuff is inflated and deflated via a syringe that inserts into the pilot balloon. When the cuff is inflated, the route of air travel is directly via the trachea to the lower airway, thus bypassing the nose and mouth, as shown in ▶ Fig. 16.4. In most cases, as a patient’s medical status improves, the cuff may be deflated or a cuffless tracheostomy tube may be inserted. In either of these scenarios, air is then able to pass through the upper airway and vocal folds, enabling the patient to voice with a tracheostomy tube, as shown in ▶ Fig. 16.5.
Fig. 16.3 Shiley cuffed tracheostomy tube. From left to right: outer cannula, inner cannula, obturator. (Courtesy of Mallinckrodt Medical, Inc.)
Fig. 16.4 Route of airflow to and from the lower airway (arrows) with an inflated tracheostomy tube cuff.
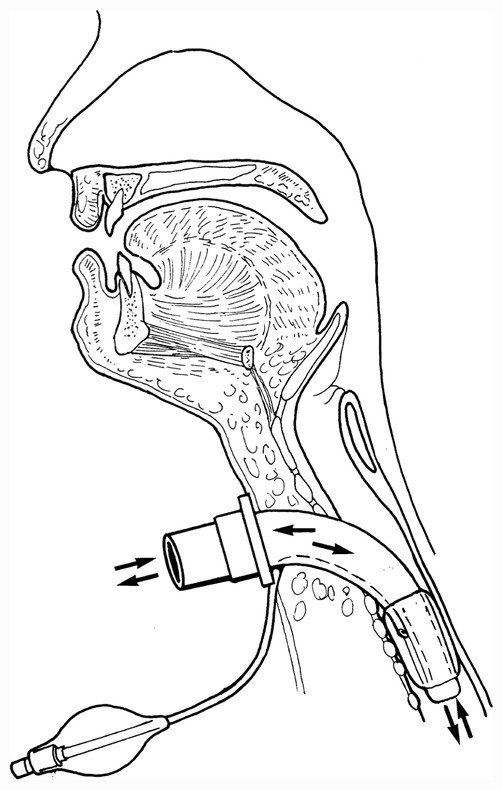
Fig. 16.5 Route of airflow to the upper airway as well as the lower airway (arrows) when a tracheostomy tube cuff is deflated.
Many manufacturers offer tracheostomy tubes of varying sizes, yet, as noted in ▶ Table 16.1, tube sizes are usually not comparable among the different manufacturers. For example, a standard No. 6 Shiley tracheostomy tube (Mallinckrodt Medical, Inc., Irvine, CA), does not have the same dimensions as a No. 6 tracheostomy tube made by Portex (Smiths Medical, Hythe, Kent, UK). For this reason, it is important to know the inner and outer cannula dimensions, and not just the given size of the tube, when substituting tubes from one manufacturer to another.
16.4.3 Advantages to Tracheostomy Tube Placement
As with any procedure, there are advantages and potential complications to the placement of a tracheostomy tube. In addition to the primary advantage of providing airway access, advantages to the placement of a tracheostomy tube include 24, 25
Ease of access to the lower respiratory tract for suctioning
A decrease in the risk of laryngeal granuloma and subglottic stenosis owing to the elimination of constant pressure of an orotracheal tube at the level of the vocal folds and subglottis
Improved weaning from mechanical ventilation because (a) the design and placement of a tracheostomy tube lessens the resistance to airflow that is seen with endotracheal tubes; and (b) air volume is delivered directly to the lungs, thereby reducing the amount of dead space in the airway (areas within the airway that do not participate in gas exchange) and allowing for greater volumes of inspired air available for oxygen exchange
An increase in patient comfort compared with nasal or orotracheal intubation
Improved options for oral communication and swallowing compared with nasal or orotracheal intubation
Improved oral hygiene owing to improved access to the oral cavity without the presence of an endotracheal tube
16.4.4 Complications of Tracheostomy Tube Placement
The following are some complications of tracheostomy tube placement 24, 25, 26, 27, 28:
Loss of taste and smell owing to an absence of airflow through the nose and mouth
An increase in secretions secondary to the presence of the tracheostomy tube as the body reacts to the presence of a foreign object
Tracheal granuloma secondary to abrasion at the stoma site
Tracheomalacia, a softening or degeneration of the elastic and connective tissues of the trachea, which may occur when there is any trauma to the tracheal walls that exposes the cartilage and leads to tissue breakdown
Tracheal stenosis occurs when the trachea narrows as part of the healing process after trauma. Stenosis may occur at the stoma site or near the cuff of the tracheostomy tube. Tracheal stenosis at the stoma site may result from frequent infections, tube changes, a stoma that is too large and begins to close as part of the healing process, or continuous tugging on the tracheostomy tube from ventilator tubing. Constant pressure on the tracheal mucosa or abrasive movements of the cuff as the tube moves up and down during breathing, swallowing, or changes in body position may contribute to tracheal stenosis at the cuff site.
Limitation of laryngeal elevation with a cuffed tracheostomy tube during swallowing, which may lead to aspiration
Tracheoesophageal fistula, a connection between the trachea and the esophagus, may occur from pressure on the anterior esophageal wall from an overinflated tracheostomy tube cuff in conjunction with the presence of a large nasogastric feeding tube. Necrosis of the tracheal and esophageal walls then takes place. Infection and poor nutrition may contribute to this problem.
Tracheoinnominate fistula occurs when a tracheostomy tube is inserted too low within the trachea, usually below the third cartilaginous ring. The tube may impinge on the innominate artery, causing eventual erosion, the creation of a fistula, and potentially life-threatening hemorrhage. This is a dangerous but rare complication of a tracheostomy tube.
Respiratory infection may occur owing to changes in bacterial colonization within the airway following tracheotomy. Tracheal wound infections, a severely ill patient, and a reduction in tracheal mucociliary clearance after tracheotomy all contribute to the risk of pneumonia in tracheostomized patients.
Adult Tracheostomy Tube Cross Reference | |||||||||||||||||
BIVONA | ID | OD | APPX | APPX | Length | SHILEY | ID | OD | APPX | APPX | Length | PORTEX | ID | OD | APPX | APPX | Length |
CODE NO. | mm | mm | French | Jackson | mm | CODE NO. | mm | mm | French | Jackson | mm | CODE NO. | mm | mm | French | Jackson | mm |
850150 | 5.0 | 7.3 | 22 | 3 | 60 | ||||||||||||
850160 | 6.0 | 8.7 | 26 | 4 | 70 | 4DCT | 5.0 | 8.5 | 26 | 4 | 67 | 503060 | 5.0 | 8.5 | 26 | 4 | 67 |
850170 | 7.0 | 10.0 | 30 | 6 | 80 | 6DCT | 7.0 | 10.0 | 30 | 6 | 78 | 503070 | 6.0 | 9.9 | 30 | 6 | 73 |
850180 | 8.0 | 11.0 | 33 | 7 | 88 | 503080 | 7.0 | 11.3 | 34 | 7 | 78 | ||||||
850190 | 9.0 | 12.3 | 37 | 8 | 98 | 8DCT | 8.5 | 12.0 | 36 | 8 | 84 | 503090 | 8.0 | 12.6 | 38 | 8 | 84 |
850195 | 9.5 | 13.3 | 40 | 10 | 98 | 10DCT | 9.0 | 13.0 | 39 | 10 | 84 | 503100 | 9.0 | 14.0 | 42 | 10 | 84 |
850150 | 5.0 | 7.3 | 22 | 3 | 60 | 5SCT | 5.0 | 7.0 | 21 | 3 | 58 | ||||||
850160 | 6.0 | 8.7 | 26 | 4 | 70 | 6SCT | 6.0 | 8.3 | 24.9 | 4 | 67 | 530060 | 6.0 | 8.3 | 24 | 4 | 55 |
850170 | 7.0 | 10.0 | 30 | 6 | 80 | 7SCT | 7.0 | 9.6 | 28.8 | 6 | 80 | 530070 | 7.0 | 9.7 | 30 | 6 | 75 |
850180 | 8.0 | 11.0 | 33 | 7 | 88 | 8SCT | 8.0 | 10.9 | 32.7 | 7 | 89 | 530080 | 8.0 | 11.0 | 33 | 7 | 82 |
850190 | 9.0 | 12.3 | 37 | 8 | 98 | 9SCT | 9.0 | 12.1 | 36.3 | 8 | 99 | 530090 | 9.0 | 12.4 | 36 | 8 | 87 |
850195 | 9.5 | 13.3 | 40 | 10 | 98 | 10SCT | 10.0 | 13.3 | 39.9 | 10 | 105 | 530100 | 10.0 | 13.8 | 40 | 10 | 98 |
670150 | 5.0 | 7.3 | 22 | 3 | 60 | ||||||||||||
670160 | 6.0 | 8.7 | 26 | 4 | 70 | 4FEN | 5.0 | 8.5 | 26 | 4 | 67 | 513060 | 5.0 | 8.5 | 26 | 4 | 67 |
670170 | 7.0 | 10.0 | 30 | 6 | 80 | 6FEN | 7.0 | 10.0 | 30 | 6 | 78 | 513070 | 6.0 | 9.9 | 30 | 6 | 73 |
670180 | 8.0 | 11.0 | 33 | 7 | 88 | 513080 | 7.0 | 11.3 | 34 | 7 | 78 | ||||||
670190 | 9.0 | 12.3 | 37 | 8 | 98 | 8FEN | 8.5 | 12.0 | 36 | 8 | 84 | 513090 | 8.0 | 12.6 | 38 | 8 | 84 |
670195 | 9.5 | 13.3 | 40 | 10 | 98 | 10FEN | 9.0 | 13.0 | 39 | 10 | 84 | 513100 | 9.0 | 14.0 | 42 | 10 | 84 |
Uncuffed Tracheostomy Tube Cross Reference | |||||||||||||||||
BIVONA | ID | OD | APPX | APPX | Length | SHILEY | ID | OD | APPX | APPX | Length | PORTEX | ID | OD | APPX | APPX | Length |
CODE NO. | mm | mm | French | Jackson | mm | CODE NO. | mm | mm | French | Jackson | mm | CODE NO. | mm | mm | French | Jackson | mm |
60N025 | 2.5 | 4.0 | 12 | 000 | 30 | ||||||||||||
60N030 | 3.0 | 4.7 | 14 | 00 | 32 | 00NT | 3.1 | 4.5 | 14 | 00 | 30 | 553025 | 2.5 | 4.5 | 13 | 00 | 30 |
0NT | 3.4 | 5.0 | 15 | 0 | 32 | 553030 | 3.0 | 5.2 | 15 | 0 | 32 | ||||||
60N035 | 3.5 | 5.3 | 16 | 0/1 | 34 | ||||||||||||
INT | 3.7 | 5.5 | 17 | 1 | 34 | 553035 | 3.5 | 5.8 | 16 | 1 | 34 | ||||||
60N040 | 4.0 | 6.0 | 18 | 2 | 36 | ||||||||||||
60P025 | 2.5 | 4.0 | 12 | 000 | 38 | ||||||||||||
60P030 | 3.0 | 4.7 | 14 | 00 | 39 | 00PT | 3.1 | 4.5 | 14 | 00 | 39 | 555025 | 2.5 | 4.5 | 13 | 00 | 30 |
0PT | 3.4 | 5.0 | 15 | 0 | 40 | 555030 | 3.0 | 5.2 | 15 | 00 | 36 | ||||||
60P035 | 3.5 | 5.3 | 16 | 0/1 | 40 | ||||||||||||
1PT | 3.7 | 5.5 | 17 | 1 | 41 | 555035 | 3.5 | 5.8 | 16 | 01 | 40 | ||||||
60P040 | 4.0 | 6.0 | 18 | 2 | 41 | 2PT | 4.1 | 6.0 | 18 | 2 | 42 | 555040 | 4.0 | 6.5 | 18 | 02 | 44 |
60P045 | 4.5 | 6.7 | 20 | 3_ | 42 | ||||||||||||
3PT | 4.8 | 7.0 | 21 | 3 | 44 | 555045 | 4.5 | 7.1 | 19 | 03 | 48 | ||||||
60P050 | 5.0 | 7.3 | 22 | 3_ | 44 | 555050 | 5.0 | 7.7 | 21 | 4_ | 50 | ||||||
60P055 | 5.5 | 8.0 | 24 | 4 | 46 | 4PT | 5.5 | 8.0 | 24 | 4 | 46 | 555055 | 5.5 | 8.3 | 23 | 4_ | 52 |
60A150 | 5.0 | 7.3 | 22 | 3 | 60 | ||||||||||||
60A160 | 6.0 | 8.7 | 26 | 4 | 70 | 4CFS | 5.0 | 8.5 | 26 | 4 | 67 | 550060 | 6.0 | 8.3 | 24 | 4 | 55 |
60A170 | 7.0 | 10.0 | 30 | 6 | 80 | 6CFS | 7.0 | 10.0 | 30 | 6 | 78 | 550070 | 7.0 | 9.7 | 30 | 6 | 75 |
60A180 | 8.0 | 11.0 | 33 | 7 | 88 | 550080 | 8.0 | 11.0 | 33 | 7 | 82 | ||||||
60A190 | 9.0 | 12.3 | 37 | 8 | 98 | 8CFS | 8.5 | 12.0 | 36 | 8 | 84 | 550090 | 9.0 | 12.4 | 36 | 8 | 87 |
60A195 | 9.5 | 13.3 | 40 | 10 | 98 | 10CFS | 9.0 | 13.0 | 39 | 10 | 84 | 550100 | 10.0 | 13.8 | 40 | 10 | 98 |
Source: Courtesy of Bivona Medical Technologies. | |||||||||||||||||
16.4.5 Communication with a Tracheostomy Tube
Patient Assessment
Before recommending voicing with a tracheostomy tube, patients should be assessed to determine whether they are viable candidates for use of this mode of communication. Evaluation includes a review of the patient’s medical condition, vocal fold status, oral-motor status, and general communicative/cognitive condition.
Medical History
A thorough review of the patient’s medical history facilitates anticipating any problems with verbal communication from a linguistic/cognitive, speech, and voice standpoint. In particular, the patient’s respiratory and neurologic status should be discussed thoroughly with the patient’s physician.
Otolaryngologic Examination
A laryngeal evaluation is important to determine the presence of vocal fold pathology. Vocal fold paralysis or granulation tissue in the posterior commissure of the glottis are common sequelae of endotracheal intubation. These findings may not preclude the use of a tracheostomy tube for voicing but will prepare the patient for changes in expected vocal quality.
Oral-Motor Status
This evaluation is necessary for determining if a patient is a candidate for verbal communication. Severely restricted lingual, labial, and buccal movements secondary to neurologic impairment or head and neck surgery may limit the effectiveness of voicing with a tracheostomy tube, making alternative modes of communication necessary. In many cases, patients with mild to moderate dysarthria are able to effectively communicate verbally via their tracheostomy tubes.
Voicing Options for Patients with Deflated or Cuffless Tracheostomy Tubes
Several different options are possible for voicing with a tracheostomy tube. In all cases, the cuff must be deflated or a cuffless tube must be in place. Medical clearance is always necessary when manipulating a tracheostomy tube. When a tracheostomy tube is in place and the cuff is inflated, air is being inhaled and exhaled directly to and from the trachea. Therefore, to redirect the air through the vocal folds, the cuff must be deflated so that air can then travel around the distal end of the tracheostomy tube and up through the vocal folds. The proximal opening of the tube should be occluded during voicing so airflow will not escape through the tracheostomy tube to the atmosphere outside, but will be completely redirected through the vocal folds and out the patient’s mouth and nose. There are several ways of doing this:
Keep the inner cannula in place while using digital occlusion over the proximal opening of the tracheostomy tube. The patient inhales, the inner cannula is digitally occluded, and the patient voices on exhalation.
If the tube is fenestrated, use a fenestrated inner cannula or remove a nonfenestrated inner cannula in order for air to flow both around the distal end of the outer cannula and up through the fenestration, thus allowing for greater airflow and a stronger voice while the proximal end of the outer cannula is digitally occluded or capped. Because air will flow through the path of least resistance, it is important to occlude the outer cannula for more efficient use of breath support and a louder voice, otherwise air will flow both out of the tracheostomy tube and the patient’s mouth and nose, and a weak, breathy voice will occur.
Remove the inner cannula in a cuffless or cuff-deflated nonfenestrated tube and use digital occlusion or capping of the outer cannula for voicing.
Use a one-way speaking valve with the inner cannula in place.
In the rare case when a fenestrated tube is in place and cuff deflation is not an option from a medical standpoint, the inner cannula may be removed for short periods and voicing may occur through the fenestration alone when the outer cannula is digitally occluded during exhalation. However, if the cuff cannot be deflated, this is probably the result of an excess of oropharyngeal and tracheal secretions. Therefore, thorough suctioning prior to removal of the inner cannula should be done to limit the risk of the patient’s aspirating secretions that may fall through the fenestration. This method usually produces a weaker or strained voice because airflow is limited to travel through the small fenestration, and this method is recommended for vocalization of only a few phrases, for a short time, with close monitoring of the patient’s respiratory status.
One-Way Speaking Valves
Although digital occlusion of a tracheostomy tube is usually effective for voicing under the proper conditions, the patient is at risk for infection if he or she or the person occluding the tracheostomy tube is not wearing gloves. Also, digital occlusion may not be an option if the patient or caregiver is unable to occlude the tube because of extremity weakness. A speaking valve eliminates the need for digital occlusion of a tracheostomy tube for voicing or swallowing. Speaking valves continue to allow inhalation via the tracheostomy tube, but exhalation occurs via the nose and mouth as the valve closes and air is then redirected to the upper airway. Many long-term tracheostomized patients become accustomed to airflow through the tracheostomy tube, which bypasses the nose and mouth. When the patient is ready to begin weaning from a tracheostomy tube, a speaking valve may be less anxiety provoking than sudden capping of the tracheostomy tube (and redirection of inhalation and exhalation through the upper airway). The valve thus may act as an intermediate step between full breathing from a tracheostomy tube and capping of the tube.
All speaking valves must be used with a completely deflated cuff or cuffless tracheostomy tube. In addition, the use of a speaking valve is not recommended with foam-cuffed tracheostomy tubes because a foam cuff usually does not completely deflate and spontaneously reinflates. If a cuff is inflated while a speaking valve is in place, the patient is not able to exhale. Air becomes trapped beneath the level of the cuff, unable to travel to the upper airway owing to the inflated cuff and unable to travel through the outer opening of the tracheostomy tube because of the seal of the valve, which occurs during exhalation. ▶ Fig. 16.6 and ▶ Fig. 16.7 demonstrate the route of airflow when a speaking valve is attached to a tracheostomy tube when the cuff is deflated and when it is inflated. The amount of pressure required to open a speaking valve varies between valves. 29 The following are common valves currently on the market.
Fig. 16.6 Route of inhalation through a speaking valve and exhalation via the upper airway (arrows) with a speaking valve in place and the tracheostomy tube cuff fully deflated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


