Chapter 22 Volatile oils and resins
VOLATILE OILS
Volatile or essential oils, as their name implies, are volatile in steam. They differ entirely in both chemical and physical properties from fixed oils. With the exception of oils such as oil of bitter almonds, which are produced by the hydrolysis of glycosides, these oils are contained largely as such in the plant. They are secreted in oil cells, in secretion ducts or cavities or in glandular hairs (see Chapter 42). They are frequently associated with other substances such as gums and resins and themselves tend to resinify on exposure to air.
Composition of volatile oils
Evaluation
The volatile oil content of crude drugs is commonly determined by distillation (Chapter 16).
Biogenesis
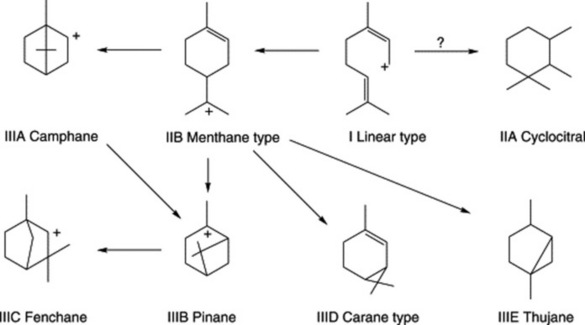
The origin of metabolites with phenylpropane and terpenoid structures has been discussed in Chapter 18. In medicinal essential oils the number of the former is limited but for the monoterpenes which arise at the geranyl pyrophosphate (GPP) level of terpenoid synthesis there are numerous examples. Analyses show that these oils commonly contain 40–80 monoterpenoids, many in relatively small proportion. A major constituent of one oil may be a minor one in another.
Three groups of monoterpenoid structures are involved: (1) acyclic or linear; (2) monocyclic; and (3) bicyclic. In the plant they are sequentially derived from limonene in this order as illustrated in Fig. 22.1. Relatively few enzymes, termed cyclases, appear to determine the skeletal class (e.g. menthanes, pinanes, thujanes etc.) and it is possible that these serve as rate-limiting enzymes. However, regulatory factors for the control of synthesis operate not only at the biosynthetic enzyme level per se but in a hierarchical manner up to the whole-organism level.
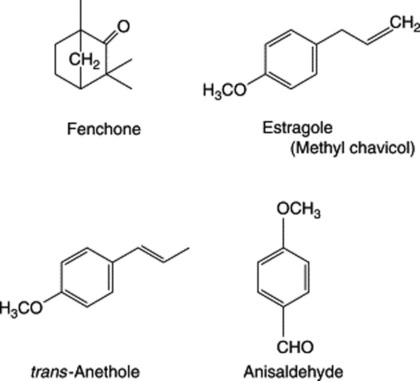
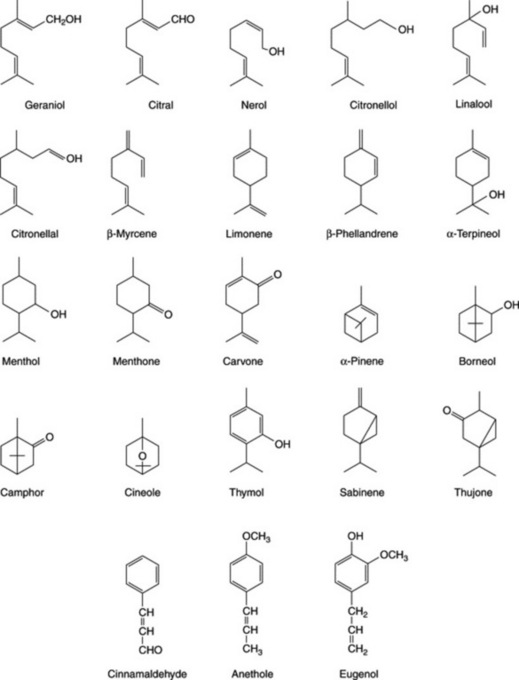
Because of the trans geometry of the double bond at the C-2 of GPP, direct cyclization to limonene is not possible and for Mentha spp. it has been shown that (−)-limonene synthase located within the oil glands catalyses the isomerization of GPP to enzyme-bound (+)-3S-linalyl pyrophosphate with subsequent cyclization to (−)-limonene. However, many enzymatic steps are involved in the subsequent modifications and interconversions of these monoterpenes. Some components of volatile oils are sesquiterpenes (C15H24) (q.v.) and they include cadinene, zingiberene (structure, Fig. 18.17) and caryophyllene. The formulae of some of the more common constituents of pharmaceutical volatile oils are given in Fig. 22.2.
Table 22.1 may be used to compare the compositions of important volatile oils. The classification is arbitrary, since an oil may contain a number of compounds all about equally important but belonging to different chemical classes. The substance used for classification is not necessarily the one present in greatest amount. Thus, nutmeg is classified on its myristicin and lemon on citral, although these constituents form only a small percentage of these oils.
Table 22.1 Composition of volatile oils.
| Name | Botanical name | Important constituents |
|---|---|---|
| Terpenes or sesquiterpenes | ||
| Tea-tree | Melaleuca alternifolia | Cyclic monoterpenes |
| Turpentine | Pinus spp. | Terpenes (pinenes, camphene) |
| Juniper | Juniperus communis | Terpenes (pinene, camphene); sesquiterpene (cadinene); alcohols |
| Cade (Juniper Tar Oil) | Juniperus oxycedrus | Sesquiterpenes (cadinene); phenols (guaiacol, cresol) |
| Alcohols | ||
| Coriander | Coriandrum sativum | Linalol (65–80% alcohols); terpenes |
| Otto of rose | Rosa spp. | Geraniol, citronellol (70–75% alcohols); esters |
| Geranium | Pelargonium spp. | Geraniol; citronellol; esters |
| Indian or Turkish geranium (Palmarosa) | Cymbopogon spp. | Geraniol (85–90%) |
| Sandalwood | Santalum album | Santalols (sesquiterpene alcohols), esters, aldehydes |
| Esters and alcohols | ||
| Bergamot | Citrus bergamia | Linalyl acetate, linalol |
| Lavender | Lavandula officinalis | Linalol; linalyl acetate (much); ethyl-pentyl ketone |
| Rosemary | Rosmarinus officinalis | Borneol and linalol (10–18%); bornyl acetate, etc. (2–5%); terpenes; cineole |
| Pumilio pine | Pinus mugo var. pumilio | Bornyl acetate (about 10%); terpenes; sesquiterpenes |
| Peppermint | Mentha piperita | Menthol (about 45%); menthyl acetate (4–9%) |
| Aldehydes | ||
| Cinnamon bark | Cinnamomum verum Presl. | Cinnamaic aldehyde (60–75%); eugenol; terpenes |
| Cassia | Cinnamomum cassia | Cinnamic aldehyde (80%) |
| Lemon | Citrus limon | Citral (over 3.5%); limonene (about 90%) |
| Lemon grass | Cymbopogon spp. | Citral and citronellal (75–85%); terpenes |
| ‘Citron-scented’ eucalyptus | Eucalyptus citriodora | Citronellal (about 70%) |
| Ketones | ||
| Spearmint | Mentha spicata and M. cardiaca | Carvone (55–70%); limonene, esters |
| Caraway | Carum carvi | Carvone (60%); limonene, etc. |
| Dill | Anethum graveolens | Carvone (50%); limonene, etc. |
| Sage | Salvia officinalis | Thujone (about 50%); camphor; cineole etc. |
| Wormwood | Artemisia absinthium | Thujone (up to 35%); thujyl alcohol; azulenes |
| Phenols | ||
| Cinnamon leaf | Cinnamomum verum Presl. | Eugenol (up to 80%) |
| Clove | Syzygium aromaticum (L.) Merr & L. M. Perry | Eugenol (85–90%); acetyl eugenol, methylpentyl ketone, vanillin |
| Thyme | Thymus vulgaris | Thymol (20–30%) |
| Horsemint | Monarda punctata | Thymol (about 60%) |
| Ajowan | Trachyspermum ammi | Thymol (4–55%) |
| Ethers | ||
| Anise and Star-anise | Pimpinella anisum and Illicium verum | Anethole (80–90%); chavicol methyl ether, etc. |
| Fennel | Foeniculum vulgare | Anethole (60%); fenchone, a ketone (20%) |
| Eucalyptus | Eucalyptus globulus | Cineole (over 70%); terpenes, etc. |
| Cajuput | Melaleuca spp. | Cineole (50–60%); terpenes, alcohols and esters |
| Camphor | Cinnamomum camphora | After removal of the ketone camphor contains safrole; terpenes, etc. |
| Parsley | Petroselinum sativum | Apiole (dimethoxysafrole) |
| Indian dill | Peucedanum soja | Dill-apiole (dimethoxysafrole) |
| Nutmeg | Myristica fragrans | Myristicin (methoxysafrole) up to 4%; terpenes (60–85%); alcohols, phenols |
| Peroxides | ||
| Chenopodium | Chenopodium ambrosioides var. anthelmintica | Ascaridole (60–77%), an unsaturated terpene peroxide |
| Non-terpenoid and derived from glycosides | ||
| Mustard | Brassica spp. | Glucosinolates |
| Wintergreen | Gaultheria procumbens | Methyl salicylate |
| Bitter almond | Prunus communis var. amara | Benzaldehyde and HCN (from amygdalin) |
Preparation of volatile oils
All the official volatile oils are extracted by distillation with the exception of oil of lemon and oil of cade. The distillation of volatile oils by means of water or steam has long been practised, but modern plants possess many advantages over the older stills, in which charring and undesirable decomposition of the oil often took place. Modern volatile oil stills contain the raw material on perforated trays or in perforated baskets. The still contains water at the base which is heated by steam coils, and free steam under pressure may also be passed in. Tough material such as barks, seeds and roots may be comminuted tofacilitate extraction but flowers are usually placed in the still without further treatment as soon as possible after collection. Distillation is frequently performed in the field.
Extraction of oils used in perfumery
Certain oils used in perfumery, such as oil of rose, are prepared by steam distillation as described above but many of the flower perfumes require other treatment. An important centre for the extraction of flower oils is Grasse, in the south of France. Here the oils are extracted by enfleurage, by digestion in melted fats, by pneumatic methods or by means of solvents. In the enfleurage process glass plates are covered with a thin layer of fixed oil or fat upon which the fresh flowers are spread. The volatile oil gradually passes into the fat and the exhausted flowers are removed and replaced by a fresh supply. Formerly the flowers had to be picked off by hand but this is now done mechanically. Only a small percentage of the flowers, which resist the action of the machine, require removal by the fingers or by means of a vacuum cleaner. The pneumatic method, which is similar in principles to the enfleurage process, involves the passage of a current of warm air through the flowers. The air, laden with suspended volatile oil, is then passed through a spray of melted fat in which the volatile oil is absorbed. In the digestion process the flowers are gently heated in melted fat until exhausted, when they are strained out and the perfume-containing fat is allowed to cool. It will be seen that in each of the above processes the volatile oil has now been obtained in a fatty base. The volatile oil is obtained from this by three successive extractions with alcohol. The alcoholic solutions may be put on the market as flower perfumes or the oil may be obtained in a pure form by recovery of the alcohol. Solvent extraction is based on the Soxhlet principle (see Chapter 16).
Oil of rose
PEPPERMINT LEAF AND PEPPERMINT OIL
Microscopical characters
The microscopy of peppermint leaves is typical of the family, showing numerous diacytic stomata on the lower surface (Fig. 42.2G), three- to eight-celled clothing trichomes with a striated cuticle (Fig. 42.4C), and two types of glandular trichome, one with a unicellular base and small single-celled head and the other with a multicellular head characteristic of the family (Fig. 42.5E). Calcium oxalate is absent.
There is a 5% limit of stems over 1 mm in diameter for the official leaves, and as mints are very susceptible to most diseases, there is a 10% limit of leaves infected by Puccinia menthae.
OIL OF PEPPERMINT
Biogenesis of peppermint monoterpenoids
A proposed pathway for the formation of monoterpenes in peppermint is given in Fig. 22.3. A number of enzymes involved in the reactions have been characterized. The hydrolase system involving (−)-limonene-3-hydroxylase in the formation of the alcohol (−)-trans-isopiperitenol is cytochrome-P450-dependent and is associated with the oil gland microsomal fraction. The remaining steps are catalysed by operationally soluble enzymes of the oil cells. It will be noted that (+)-pulegone is a branching point for the formation of menthol stereoisomers.
DEMENTHOLIZED MINT OIL BP
This is cited as the volatile oil from Mentha arvensis var. piperascens from which the menthol has been partially removed. The two commercial oils, Brazilian and Chinese, differ somewhat in their ranges of ester and alcohol contents; standards are given for each. For both, the cineole:limonene ratio, as determined by GC, is less than unity.
SPEARMINT OIL
Constituents
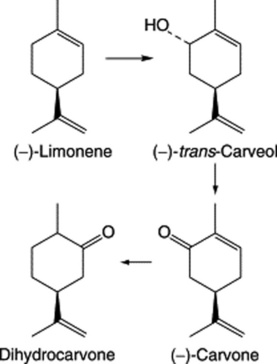
Oil of spearmint contains (−)-carvone, (−)-limonene, phellandrene and esters. As with M. × piperita limonene is the precursor of the monoterpenoids and in this case the action of a (−)-limonene-6-hydroxylase predominates to give the alcohol (−)-trans-carveol which is oxidized to carvone (Fig. 22.4). Dihydrocarvone is formed later in the season and is absent from plantlets produced by shoot-tip culture. Again like peppermint, oil production is influenced by the age of plant, time of collection, chemical varieties and hybridization.
SAGE LEAF
Constituents
The volatile oil of sage contains about 50% of α- and β-thujone together with cineole, borneol and other constituents (Fig. 22.2). Varieties and other species of sage contain differing amounts of thujone.
SAGE OIL
Sage oils are produced commercially by steam distillation from a number of Salvia species but the oil composition is not uniform, as illustrated by the three species considered here. For this reason, the BP/EP specifies one species, S. sclarea L., the clary sage, as the source of the official oil. The plant is a native of Mediterranean regions and had been introduced into England by the the 14th century.
The oil is widely used for flavouring and perfumery purposes.
ROSEMARY OIL
LEMON BALM
Lemon balm yields only a small quantity of volatile oil (0.06–0.4%), which none the less gives the plant, when crushed, its strong lemon-like odour. Principal components of the oil are the aldehydes citral (composed of the isomers geranial and neral) and citronellal. Other components in smaller proportions are citronellol, nerol and the sesquiterpene β-caryophyllene; in all, over 70 constituents have been reported. Due to the low yield of oil from the plant, lemon balm oil is subject to adulteration with lemon-grass oil (Cymbopogon citratus), lemon-scented verbena oil (Aloysia triphylla) or various citrus products.
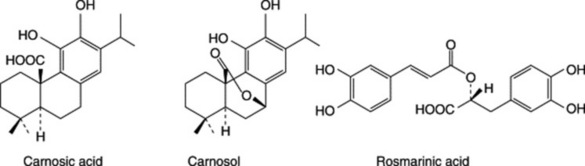
The BP/EP drug is assayed on its total content ( 4.0%) of hydroxycinnamic acid derivatives expressed as rosmarinic acid (p. 270); these are mainly structurally related to caffeic acid. Other constituents are flavonoids, principally luteolin glycosides (Table 21.5).
4.0%) of hydroxycinnamic acid derivatives expressed as rosmarinic acid (p. 270); these are mainly structurally related to caffeic acid. Other constituents are flavonoids, principally luteolin glycosides (Table 21.5).
THYME
Both species have similar morphological and microscopical characteristics and can be difficult to distinguish in the dried state. Stems above 15 mm in length and over 1 mm in diameter are limited to 10% by the pharmacopoeia. The grey–green leaves are slightly hairy on the upper surface and densely so on the lower surface, up to 12 mm long, and 3 mm wide, opposite, sessile and ovate to lanceolate in shape with slightly rolled edges. Under the microscope, both species show on the lower surface volatile oil-containing glandular trichomes typical of the Labiatae and numerous warty-walled clothing trichomes. The characteristic elbow-shaped trichomes of T. vulgaris are illustrated in Fig. 42.4. Numerous thick bundles of fibres are apparent in the powder of T. zygis.
OREGANO
There is a large number of marjorams, and various varieties are cultivated extensively for ornamental and culinary purposes. Two medicinally used species described in the BP/EP are Origanum onites L. (syn. Majoram onites) the pot marjoram or Greek oregano, and O. vulgare L. subsp. hirtum (Link) Ietsw., a subspecies of the wild marjoram, or oregano, family Labiatae. The dried leaves and flowers are separated from the stems; a mixture of both species may be used. Both have a strong, thyme-like odour. Both appear similar in the dried state but the leaves of O. onites are yellowish–green whereas those of O. vulgare are more distinctly green. In the powdered form, both show typical laminaceous characteristics.
LAVENDER FLOWER
LAVENDER OIL
Lavender oil types
Species of Lavandula other than the above are also cultivated. L. stoechas has a markedly different odour and of its 51 volatile components, fenchone, pinocaryl acetate, camphor, eucalyptol and myrthenol predominate. Large producers are Spain and France. Oil from wild plants growing in the Algiers region of Algeria contained as significant constituents fenchone (31.6%), camphor (22.4%), p-cymene (6.5%) lavandulyl acetate (3.0%) and α-pinene (1.0%). Fifty-four components amounting to ca 73% of the oil were identified (T. Dob et al., Pharm Biol., 2006; 44, 60).
CARAWAY FRUIT
Macroscopical characters
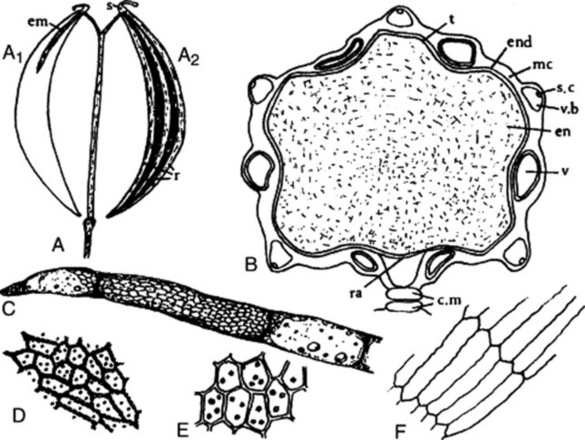
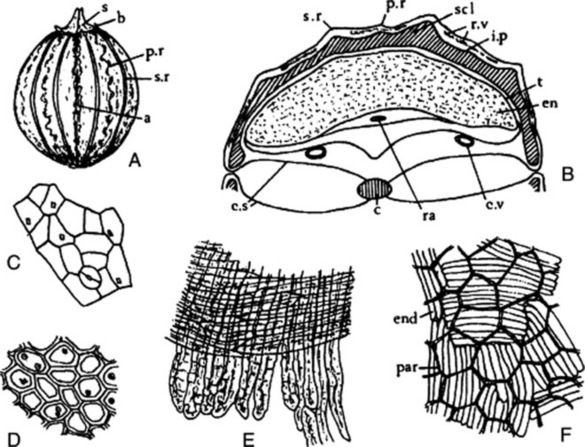
The commercial drug (Fig. 22.5) usually consists of mericarps separated from the pedicels. The fruits are slightly curved, brown and glabrous, about 4–7 mm long, 1–2.3 mm wide and tapered at both ends; they are crowned with a stylopod often with style and stigma attached. Each mericarp shows five almost equal sides, five narrow primary ridges, and, when cut transversely, four dorsal and two commissural vittae. They have a characteristic aromatic odour and taste.
Microscopical characters
A transverse section of a caraway mericarp (Fig. 22.5) shows five primary ridges, in each of which is a vascular strand with associated pitted sclerenchyma and having a single secretory canal at the outer margin of each. The six vittae which appear somewhat flattened and elliptical in transverse section may attain a width of 350 μm; they extend from the base of the fruit to the base of the stylopod. They are lined with small, dark reddish-brown cells and contain a pale yellow or colourless oleoresin (Fig. 22.5B, C). The raphe lies on the inner side of the endosperm, which is non-grooved. Occupying the majority of the transverse section is the endosperm, with thickened cellulose walls (having also deposits of a β-(1,4)-mannan as a reserve polysaccharide) and containing fixed oil and aleurone grains having one or two microrosettes of calcium oxalate. The embryo, which is situated near the apex of the mericarp, will only be seen in sections passing through that region.
More detailed examination shows that the outer epidermis of the pericarp is glabrous (cf. aniseed) and has a striated cuticle (cf. fennel). The mesocarp consists of more or less collapsed parenchyma and lacks the reticulated cells of fennel. The endodermis (or inner epidermis of the pericarp) (Fig. 22.5F) consists of a single layer of elongated cells, arranged more or less parallel to one another and not showing the ‘parquetry’ arrangement of coriander.
CARAWAY OIL
The volatile oil (Caraway Oil BP/EP) consists largely of the ketone carvone and the terpene limonene (formulae, Fig. 22.4) with small quantities of dihydrocarvone, carveol and dihydrocarveol. As there is a demand for pure carvone, there is a considerable amount of decarvonized oil available for adulteration.
DILL AND DILL OIL
Constituents
Monoterpene glycosides have been isolated from the water-soluble fraction of the fruits.
Uses
Like caraway, dill is used as a carminative and flavour; it is much used in infant’s gripe water.
CORIANDER AND CORIANDER OIL
Macroscopical characters
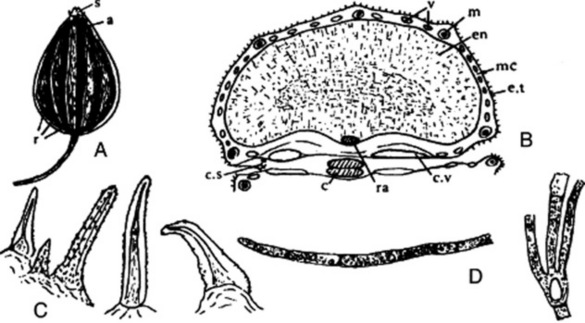
The drug (Fig. 22.6A) usually consists of the whole cremocarps, which, when ripe, are about 2.3–4.3 mm diameter and straw-yellow. Each consists of two hemispherical mericarps united by their margins. Considerable variation exists in coriander. The Indian variety is oval, but the more widely distributed spherical varieties vary in size from the Ukrainian 2.3–3.7 mm to the Moroccan 4.0–4.3 mm. The apex bears two divergent styles. The 10 primary ridges are wavy and inconspicuous; alternating with these are eight more prominent, straight, secondary ridges. The fruits have an aromatic odour and a spicy taste. They are somewhat liable to insect attacks.
Microscopical characters
A transverse section of a fully ripe fruit shows only two mature vittae in each mericarp, both on the commissural surface (Fig. 22.6B). The numerous vittae present in the immature fruit on the dorsal surface of each mericarp gradually join and are eventually compressed into slits. The outer part of the pericarp, which possesses stomata and prisms of calcium oxalate, is more or less completely thrown off. Within the vittae-bearing region of the mesocarp a thick layer of sclerenchyma is formed, which consists of pitted, fusiform cells. These sclerenchymatous fibres tend in the outer layers to be longitudinally directed and in the inner layers to be tangentially directed. In the region of the primary ridges more of the fibres are longitudinally directed; in the secondary ridges nearly all are tangentially directed. Traversing the band of sclerenchyma and corresponding in position to the primary ridges are small vascular strands composed of a small group of spiral vessels. The mesocarp within the sclerenchymatous band is composed of irregular polygonal cells with lignified walls. The inner epidermis of the pericarp is composed of ‘parquetry’ cells, which in the powder are often seen united to the cells of the inner mesocarp. The testa is composed of brown flattened cells. The endosperm is curved and consists of parenchymatous cells containing fixed oil and aleurone grains. The latter contain rosettes of calcium oxalate 3–10 mm diameter (see Fig. 22.6 C–F).
Constituents
The unripe plant has an unpleasant, mousy odour, which is also present in oil distilled from unripe fruits (mainly aldehydes such as n-decanal contained in peripheral vittae). Marked changes occur in volatile oil composition during ontogenesis; the peripheral vittae flatten and lose their oil, all of which is then produced by the commissural vittae. During ordinary storage of the fruits, the oil composition undergoes considerable alteration.
ANISEED AND ANISEED OIL
Macroscopical characters
The drug (Fig. 22.7A) consists of greyish-brown, pear-shaped, somewhat compressed cremocarps, which are usually attached to pedicels 2–12 mm in length. The cremocarps are 3–6 mm long and 2–3 mm broad. The Spanish (Alicante) and Italian are distinguished by their large size and light colour, while the German and ‘Russian’ are smaller, more ovoid and darker. Each mericarp has five somewhat wavy ridges and is slightly pubescent on the dorsal surface. They have an aromatic odour and a sweet, aromatic taste.
Microscopical characters
Microscopical examination shows that the epidermis bears numerous papillae and unicellular hairs. On the dorsal surface of each mericarp are from 15 to 45 branched vittae. A small amount of vascular tissue and reticulated parenchyma is present. The endosperm is slightly concave on the commissural surface and contains protein and fixed oil (see Fig. 22.7B–D).
STAR ANISE FRUIT AND OIL
The genuine fruits of I. verum should yield a minimum of 7.0% volatile oil containing not less than 86.0% of trans-anethole. More recently, they have been employed for the extraction of shikimic acid (see Fig. 19.5), which is the starting material for the synthesis of the antiviral drug Tamiflu (Roche). As a consequence, the plant is in some danger of overexploitation and other sources of the acid are being investigated (q.v.).
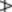 4.0%, carvone
4.0%, carvone 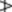 1.0%. The ratio of the cineole to limonene contents exceeds 2.
1.0%. The ratio of the cineole to limonene contents exceeds 2.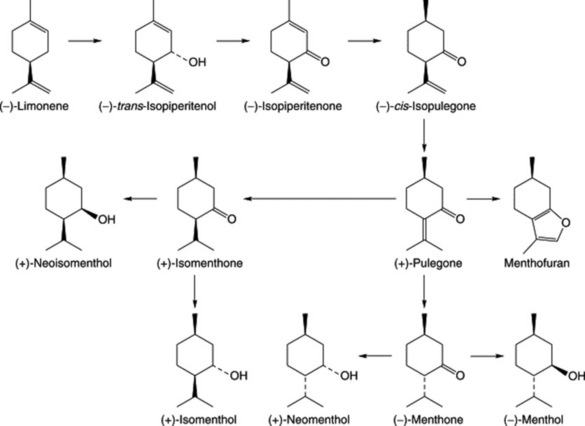
 2.0%), which is almost identical with that obtained from star-anise fruits. A number of water-soluble constituents have been isolated from the fruits including various glucosides, see E. Fujimatu et al., Phytochemistry, 2003, 63 (5).
2.0%), which is almost identical with that obtained from star-anise fruits. A number of water-soluble constituents have been isolated from the fruits including various glucosides, see E. Fujimatu et al., Phytochemistry, 2003, 63 (5).