Nonneoplastic or inflammatory skin diseases encompass a wide array of pathologic processes ranging from autoimmune to infectious to diseases of unknown etiology. In contrast to neoplastic surgical pathology, the histopathology of inflammatory skin diseases frequently does not exhibit a one-to-one correlation with a single diagnosis and requires correlation with the clinical presentation for a definitive diagnosis. In many instances, the dermatologist is neither looking for nor needs a specific histologic diagnosis. For instance, if the clinical differential diagnosis is between atopic dermatitis and psoriasis, the diagnosis of spongiotic dermatitis conveys the essential information to the clinician and guides appropriate therapy. Although the diagnosis of many inflammatory skin diseases requires correlation with the clinical features, there are critical diagnoses, such as toxic epidermal necrolysis and staphylococcal scalded skin syndrome, which the surgical pathologist may be asked to differentiate.
The most accurate interpretation of the histopathology of inflammatory skin disease is accomplished if the pathologist is cognizant of the clinical differential diagnosis as well as the histopathologic differential diagnosis. The pathologist must insist that an accurate clinical differential diagnosis or impression be submitted in addition to other data such as the age and sex of the patient and the anatomic site of the biopsy. Although dermatopathology specimens should be interpreted objectively, the final interpretation should always be correlated with the clinical findings.
In this chapter, we have divided nonneoplastic skin diseases into various groups based on histopathologic patterns of inflammation (
Tables 1.1 and
1.2). This approach is popular because it furnishes a basis for structured learning of these diseases without a prior knowledge of clinical dermatology. Like all classifications, this approach is not perfect, and it falls short at times because of the incredible complexity of the pathologic processes. Few diseases fit exclusively into only one category. Perhaps the best way to use this morphologic approach is to use the metaphor of a framework and superimposed templates. Think of each pattern as the framework and the specific histologic features of each disease as a template. Mentally superimposing the template then results in a modification of the original pattern. For example, in the diagnosis of lichenoid drug reaction, the pattern of lichenoid interface dermatitis is the framework. Superimposing a template of parakeratosis, eosinophils, and plasma cells over the framework leads to the diagnosis of lichenoid drug eruption. Of course, one must learn to recognize the basic patterns for this system to work effectively.
One final important point concerning the histopathologic interpretation of inflammatory skin diseases is that the lesions are dynamic and change is an intrinsic quality. It must be remembered that a biopsy “captures” the histopathology of the lesion at one point in its evolution. Many inflammatory skin diseases may only be readily diagnosed microscopically at certain points within the spectrum of changes. If a lesion is biopsied early or late in its evolution, the microscopic findings may be nondiagnostic.
SPECIMEN PREPARATION
Careful gross processing of skin biopsies is critical for accurate microscopic interpretation. The shave, punch, and elliptical biopsy techniques are most frequently used to obtain skin specimens for microscopic examination.
The elliptical excision is preferred when the disease process involves the deep dermis or subcutis. Superficial fascia can only be obtained reliably with this technique. In our experience, the “bread-loaf” method (sequential serial sectioning) of cutting skin ellipses is best because it is simple, can be performed rapidly, and ensures adequate sampling of the tissue. The skin ellipse is cut perpendicular to the long axis of the specimen at approximately 3-mm intervals. If the cut surface is marked with ink and each tissue slice is embedded in a separate cassette, it is easy to decide which block to recut if additional sections are needed. Some dermatopathologists prefer to section skin ellipses longitudinally in nonneoplastic lesions. For the most part, the choice is a personal one.
The punch biopsy tool is best used to obtain a cylinder of skin that includes the epidermis, dermis, and a small amount of subcutis. Punch biopsies 4 mm in diameter or greater should be bisected before embedding. Smaller punch biopsy specimens are difficult to bisect and should be embedded intact.
The shave or tangential technique (blade parallel to the skin surface) is of limited value for the study of inflammatory skin diseases because only epidermis and superficial dermis are consistently sampled by this method. Shave biopsy specimens should be bisected or trisected if large enough so that a straight edge is available for microtome sectioning. Applying ink to the cut edge with an applicator stick enables the histotechnologist to identify the cut edge.
Ten percent buffered formalin is an excellent generalpurpose fixative for skin specimens. Fixation in B5 solution results in the greater preservation of nuclear detail and is especially useful for evaluating lymphocytic infiltrates clinically suspicious for cutaneous lymphoma.
Hematoxylin and eosin (H&E) is the most commonly used routine stain in dermatopathology, but most special stains used
in general surgical pathology are also employed. Specific uses of histochemical stains and immunohistochemistry will be discussed along with the diseases in which their use is of value (
1,
2,
3 and
4).
In dermatopathology, it is important to recreate a threedimensional mental picture of the two-dimensional microscopic sections. In addition, many inflammatory skin diseases are particularly zonal in their microscopic architecture. Therefore, it is frequently helpful to make either step or serial sections from the paraffin block to maximize the yield of information obtainable from the microscopic sections.
Transverse sectioning of punch biopsies from the scalp is frequently used in the diagnosis of alopecia. A detailed discussion of this technique is beyond the scope of this chapter (
5,
6 and
7).
NORMAL HISTOLOGY
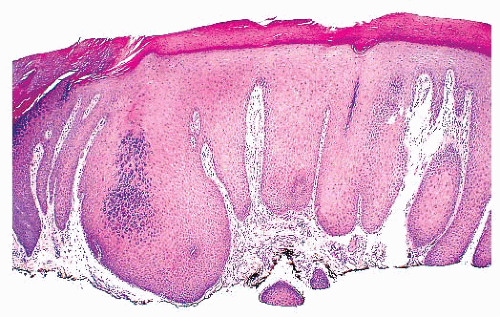
The skin comprises three structures: the epidermis, dermis, and subcutis. The superficial fascia marks the deep boundary between the skin and the underlying soft tissues. Regional anatomic variation of the skin is readily apparent if one compares a specimen from the scalp with one from the palm.
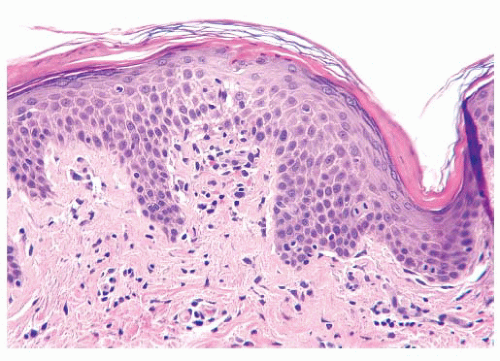
The epidermis is derived from ectoderm and composed of four layers or strata. The stratum corneum is the outermost layer of the epidermis. The fully keratinized cells of this layer are flattened and devoid of nuclei. On acral surfaces (palms and soles), a thin stratum lucidum (clear zone) is present between the stratum corneum and the stratum granulosum. The stratum granulosum is named for the prominent deeply basophilic keratohyalin granules found in flattened keratinocytes. Deep to the stratum granulosum is the stratum spinosum, which is characterized by abundant eosinophilic cytoplasm, ovoid nuclei, and intercellular bridges. The stratum basale (basal cell layer) is the undulating row of cuboidal to columnar cells with minimal cytoplasm and contains proliferating cells for epidermal renewal (
8). The basal cells attach to the basement membrane of the epidermis. The stratum spinosum and the basal cell layer are collectively referred to as the
stratum malpighii.
Although the majority of cells in the epidermis are keratinocytes, other cell types are present. Melanocytes are neural crest-derived dendritic clear cells situated in the basal zone. The primary function of melanocytes is the production of melanin. Langerhans cells are antigen-processing dendritic cells that are usually interspersed among the keratinocytes of the stratum spinosum but are virtually impossible to see in routine sections. Immunohistochemistry for S-100 protein is positive in both melanocytes and Langerhans cells, but Langerhans cells also express CD1a (
9). Merkel cells are sparsely present neuroendocrine cells in the epidermis and function in mechanoreception. Merkel cells are not readily apparent in routine sections (
9).
The basement membrane zone that separates the epidermis from the dermis appears to be a homogeneous eosinophilic band with light microscopy but displays a multilayered, complex arrangement at the ultrastructural level. The basement membrane zone is composed of four main layers: the basal cell hemidesmosome, the lamina lucida, the lamina densa, and the sublamina densa (
8).
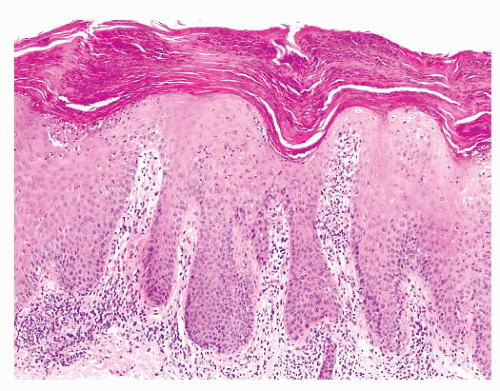
The most superficial level of the dermis is called the papillary dermis because it is located between the downward projections of the epidermis (rete ridges). The dermal papillae have a complex “hand-in-glove” relationship with the epidermal rete ridges. The deep border of the papillary dermis extends to the superficial vascular plexus and reticular dermis. Although it is a small part of the dermis quantitatively, the papillary dermis is important in many inflammatory skin diseases, and it functions as an anatomic buffer zone between the epidermis and reticular dermis.
In addition to its superficial location, the papillary dermis is characterized by a collagen pattern that is distinctively delicate and pale in H&E-stained sections. The adventitial dermis, the fine collagen fibers that invest adnexal structures, blood vessels, and nerves, is continuous with the papillary dermis.
The reticular dermis makes up the bulk of the dermis. Here, the collagen fibers are large, coarse, and brightly eosinophilic. Most of the adnexal structures are found within the reticular dermis. Progressively narrowing projections of the reticular dermis extend in a netlike manner into the subcutis to form the retinacular dermis.
The subcutis is the deepest layer of the skin. It is composed of collagenous septa and lobules of adipocytes. The fibrous septa connect the retinacular dermis with the superficial fascia to which the skin is anchored (
10).
The cutaneous adnexa include hair follicles, sebaceous glands, eccrine glands, and apocrine glands (
10). The hair follicle is a complex structure (
5). The anagen, or growing, hair follicle can be divided into several parts. The deepest part, the hair bulb, is formed from both ectoderm (hair matrix) and mesoderm (dermal papilla). The isthmus extends from the superficial part of the hair bulb to the sebaceous duct. The infundibulum connects the isthmus and sebaceous duct to the epidermis. The intraepidermal portion of the hair follicle is called the
acrotrichium. The sebaceous lobules empty into the follicle via the short sebaceous duct. An arrector pili muscle attaches to the isthmus below the entrance of the sebaceous duct at an area of the follicle termed the
bulge.
The telogen, or resting, hair follicle lacks the well-defined components of the anagen, or growing, follicle. Instead, only a small ball of basaloid keratinocytes is located below the level of the sebaceous duct (
5).
Sebaceous glands have a widespread distribution and are typically associated with hair follicles (
10). In the eyelids, they are not associated with hair follicles and are known as the
meibomian glands and
glands of Zeis. Sebaceous glands secrete a lipid material known as
sebum.
The eccrine glands are present at essentially all sites and are composed of a deep dermal or subcutaneous coil, mid dermal coiled and straight ducts, and an intraepidermal acrosyringium (
10). Cuboidal epithelial cells surrounded by myoepithelial cells line eccrine ducts. The eccrine glands’ primary function is thermal regulation.
Apocrine glands are much more limited in distribution than eccrine glands and are primarily present in the axillary and anogenital regions (
10). Modified apocrine glands are present in the external ear canal as ceruminous glands and in the eyelid as a gland of Moll. The apocrine epithelium consists of eosinophilic, cuboidal to columnar cells with decapitation secretion. Myoepithelial cells surround the outer portion of the apocrine glands and ducts. Their dermal duct usually ends in the follicular infundibulum, only rarely opening into the surface epidermis. The function of apocrine glands in humans is unknown.
The dermis is rich in blood vessels and lymphatics. The skin vasculature is supplied by perforating arteries of subcutaneous adipose tissue and skeletal muscle. The capillaries, arterioles, and venules of the superficial vascular plexus are located at the junction of the papillary and reticular dermis. Vessels extend from this plexus into the adventitial dermis of the adnexa and also penetrate through the reticular dermis to connect with the deep vascular plexus composed of larger vessels at the level of the deep reticular dermis. From the deep vascular plexus, vessels extend into the fibrous septa of the subcutis (
10).
Aside from specialized end organs such as the Meissner and pacinian corpuscles, the nerves of the dermis are inconspicuous (
10). They progressively decrease in caliber as they become more superficial. In the deep dermis, they usually course adjacent to blood vessels.