6 • Myoepithelial cells are an important component of certain secretory glands (see Ch. 5), where they function to expel secretions from glandular acini. • Pericytes are smooth muscle-like cells that surround blood vessels (see Ch. 8). • Myofibroblasts are cells that have a contractile role in addition to being able to secrete collagen. This type of cell is generally inconspicuous in normal tissues but becomes important following tissue damage during the process of healing and repair, leading to formation of a scar. • Skeletal muscle is responsible for the movement of the skeleton as well as organs such as the globe of the eye and the tongue. Skeletal muscle is often referred to as voluntary muscle since it is capable of voluntary (conscious) control. The arrangement of the contractile proteins gives rise to the appearance of prominent cross-striations in some histological preparations and so the name striated muscle is often applied to skeletal muscle. The highly developed functions of the cytoplasmic organelles of muscle cells has led to the use of a special terminology for some muscle cell components: plasma membrane or plasmalemma = sarcolemma; cytoplasm = sarcoplasm; endoplasmic reticulum = sarcoplasmic reticulum. • Smooth muscle is so named because, unlike other forms of muscle, the arrangement of contractile proteins does not give the histological appearance of cross-striations. This type of muscle forms the muscular component of visceral structures such as blood vessels, the gastrointestinal tract, the uterus and the urinary bladder, giving rise to the alternative name of visceral muscle. Since smooth muscle is under inherent autonomic and hormonal control, it is also described as involuntary muscle. • Cardiac muscle has many structural and functional characteristics intermediate between those of skeletal and smooth muscle and provides for the continuous rhythmic contractility of the heart. Although striated in appearance, cardiac muscle is readily distinguishable from skeletal muscle and should not be referred to by the term ‘striated muscle’. Muscle cells of all three types are surrounded by an external lamina (see Ch. 4). In all muscle cell types, contractile forces developed from the internal contractile proteins are transmitted to the external lamina via link proteins which span the muscle cell membrane. The external lamina binds individual muscle cells into a single functional mass. Skeletal muscle contraction is controlled by large motor nerves, individual nerve fibres branching within the muscle to supply a group of muscle fibres, collectively described as a motor unit. Excitation of any one motor nerve results in simultaneous contraction of all the muscle fibres of the corresponding motor unit. The structure of neuromuscular junctions is described in Fig. 7.12. The vitality of skeletal muscle fibres is dependent on maintenance of their nerve supply which, if damaged, results in atrophy of the fibres (see below). Skeletal muscle contains highly specialised stretch receptors known as neuromuscular spindles which are shown in Fig. 7.23. FIG. 6.1 Skeletal muscle FIG. 6.2 Skeletal muscle FIG. 6.3 Skeletal muscle FIG. 6.4 Skeletal muscle embryogenesis FIG. 6.5 Skeletal muscle FIG. 6.6 Skeletal muscle FIG. 6.7 The arrangement of myofilaments in the sarcomere FIG. 6.8 The conducting system for contractile stimuli in skeletal muscle
Muscle
Introduction
Skeletal Muscle
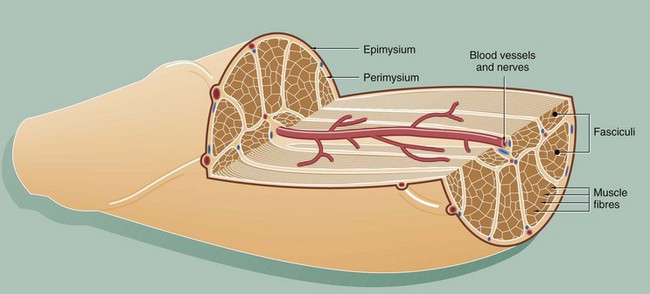
This diagram illustrates the arrangement of the basic components which make up a typical skeletal muscle. The individual muscle cells (muscle fibres) are grouped together into elongated bundles called fasciculi or fascicles with delicate supporting tissue called endomysium occupying the spaces between individual muscle fibres.
Each fascicle is surrounded by loose collagenous tissue called perimysium. Most muscles are made up of many fasciculi, and the whole muscle mass is invested in a dense collagenous sheath called the epimysium. Large blood vessels and nerves enter the epimysium and divide to ramify throughout the muscle in the perimysium and endomysium.
The size of the fasciculi reflects the function of the muscle concerned. Muscles responsible for fine, highly controlled movements (e.g. external muscles of the eye) have small fasciculi and a relatively greater proportion of perimysial supporting tissue. In contrast, muscles responsible for gross movements only (e.g. muscles of the buttocks) have large fasciculi and relatively little perimysial tissue. Muscle fibres are anchored to the supporting tissue so that contractile force can be transmitted. The connective tissue framework contains both collagen and elastic fibres. This connective tissue becomes continuous with that of the tendons and muscle attachments (see Ch. 10), which distribute and direct the motive forces of the muscle to bone, skin, etc., as appropriate.
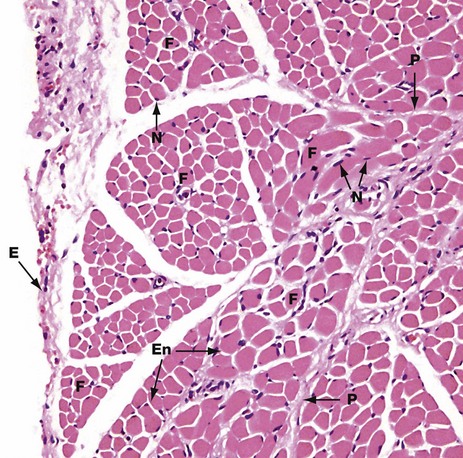
H&E (MP)
This micrograph shows the general arrangement of muscle fibres in skeletal muscle. Here, there are several distinct fasciculi.
The individual pink-stained muscle cells (fibres) are cut in transverse section and appear polygonal in shape, with nuclei N lying at the peripheries of the cells. The spaces between the cells are occupied by small amounts of barely visible endomysial supporting tissue. The endomysium, which consists mainly of reticulin fibres and a small amount of collagen, conveys numerous small blood vessels, lymphatics and nerves throughout the muscle.
Surrounding the individual fasciculi F is the perimysium P, composed of collagen and through which larger vessels and nerves run. The epimysium E is a collagenous sheath that binds the fascicles into a single muscle. The endomysium En is barely visible as the delicate support tissue surrounding each muscle fibre.
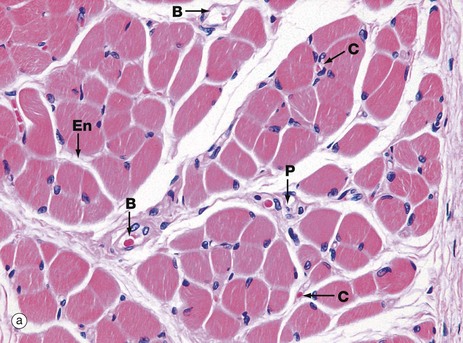
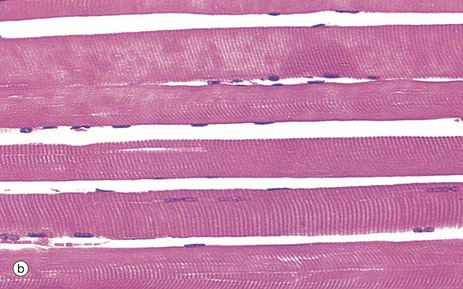
(a) H&E, TS (HP) (b) H&E, LS (HP)
These micrographs show skeletal muscle from human limb muscles. Micrograph (a) in transverse section shows the muscle to be made up of numerous small fasciculi. The spaces between the fasciculi are filled with loose collagenous tissue, the perimysium P, which is continuous with the delicate endomysium En, separating individual muscle fibres in each fasciculus. The supporting tissue of skeletal muscle also contains elastin fibres (not distinguishable in this preparation) which are most numerous in muscles attached to soft tissues as in the tongue and face. Note the rich network of capillaries C in the endomysium. Small blood vessels B and nerves run in the perimysium.
Micrograph (b) demonstrates the characteristic histological features of skeletal muscle fibres in longitudinal section. Skeletal muscle fibres are extremely elongated, unbranched cylindrical cells with numerous flattened nuclei located at fairly regular intervals just beneath the sarcolemma (plasma membrane).
Each muscle fibre has multiple nuclei arranged at the cell periphery. In transverse section, as in micrograph (a), most muscle fibre profiles appear to contain only a single nucleus, while some do not include any because the plane of section has cut between the zones containing a nucleus.
In routine histological preparations stained with H&E, it is often possible to see the striations in skeletal muscle when cut in longitudinal section. Special stains are required for better resolution of these structures (see Fig. 6.6a).
(a) H&E (MP) (b) H&E (HP)
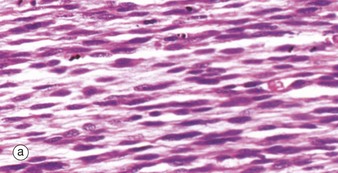
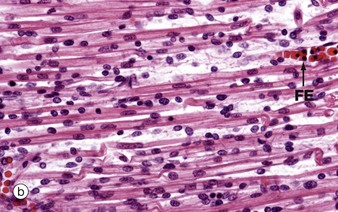
During embryological development, mesenchymal cells in each myotome differentiate into long, mononuclear skeletal muscle precursors called myoblasts, which then proliferate by mitosis. Subsequently, the myoblasts fuse end to end forming elongated multinucleate cells called myotubes which may eventually contain up to 100 nuclei. These myotubes then synthesise contractile proteins to form myofilaments and so cross-striations gradually become visible. Proliferating myoblasts and early developing myotubes are illustrated in micrograph (a). Micrograph (b) illustrates a slightly later stage in development, and here there is a suggestion of very early cross-striation in some of the myotubes. Note the nucleated fetal erythrocytes FE within a small vessel on one edge of the image.
Mature muscle cells can regenerate if damaged, by proliferation of stem cells which remain in adult muscles. These muscle stem cells resemble myoblasts and are called satellite cells. They enter mitosis after muscle damage, and several fuse to form differentiated muscle fibres. Muscle fibres which have formed as a result of regeneration after damage have nuclei in the centre of the fibre rather than at the periphery.
(a) Iron haematoxylin, TS (HP) (b) Schematic diagram
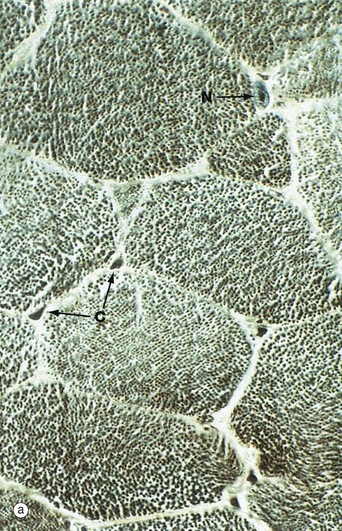
Micrograph (a) shows a transverse section through several skeletal muscle fibres at very high magnification. The plane of section includes only one skeletal muscle cell nucleus N. Note the presence of erythrocytes in endomysial capillaries C.
In preparations such as this, the transversely sectioned muscle fibres appear packed with numerous dark dots. These represent the cut ends of myofibrils, elongated cylindrical structures which lie parallel to one another in the sarcoplasm.
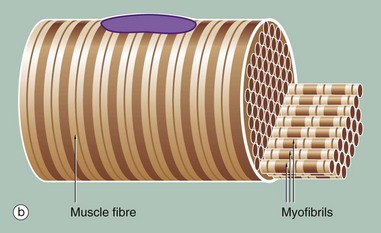
Figure (b) shows part of a single muscle fibre. The diagram illustrates that each myofibril exhibits a repeating pattern of cross-striations which is a product of the highly ordered arrangement of the contractile proteins within it; detail of this arrangement can only be seen using electron microscopy (see Fig. 6.6). Furthermore, the parallel myofibrils are each arranged with their cross-striations in register, giving rise to the regular striations which may be seen with light microscopy in longitudinal sections of skeletal muscle as in Fig. 6.3.
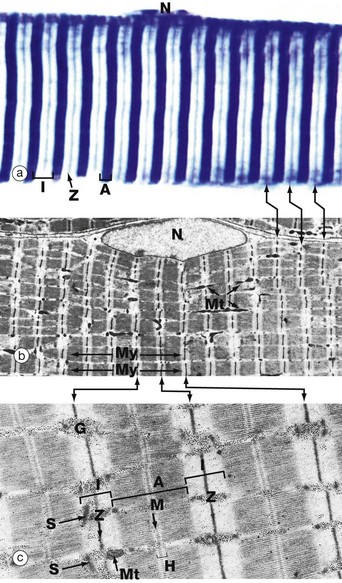
(a) Heidenhain’s haematoxylin (HP) (b) EM ×2860 (c) EM ×18 700
This series of micrographs shows the arrangement of the contractile proteins within skeletal muscle and explains the striations seen with light microscopy.
Micrograph (a) shows the striations of a skeletal muscle fibre at a magnification close to the limit of resolution. They are composed of alternating broad light I bands (isotropic in polarised light) and dark (anisotropic) A bands. Fine dark lines called Z lines (Zwischenscheiben) Z can be seen bisecting the I bands. Note the nucleus N at the periphery of the cell.
Micrograph (b) shows the electron microscopic appearance of muscle with a nucleus N situated in a similar position. The sarcoplasm is filled with myofibrils My oriented parallel to the long axis of the cell. These are separated by a small amount of sarcoplasm containing rows of mitochondria Mt in a similar orientation. Each myofibril has prominent regular cross-striations arranged in register with those of the other myofibrils and corresponding to the I, A and Z bands seen in light microscopy. The Z bands are the most electron-dense and divide each myofibril into numerous contractile units called sarcomeres, arranged end to end.
With further magnification in micrograph (c), the arrangement of the contractile proteins (myofilaments) may be seen in each sarcomere. The dark A band is bisected by the lighter H (Heller) band, which is further bisected by a more dense M (Mittelscheibe) line. Irrespective of the degree of contraction of the muscle fibre, the A band remains constant in width. In contrast, the I and H bands narrow during contraction, and the Z lines are drawn closer together. These findings are explained by the sliding filament theory (Fig. 6.7). Mitochondria Mt and numerous glycogen granules G provide a rich energy source in the scanty cytoplasm between the myofibrils. The mature muscle cell contains little rough endoplasmic reticulum; it contains, however, a smooth membranous system S which is involved in activation of the contractile mechanism (see Figs 6.8 to 6.10).
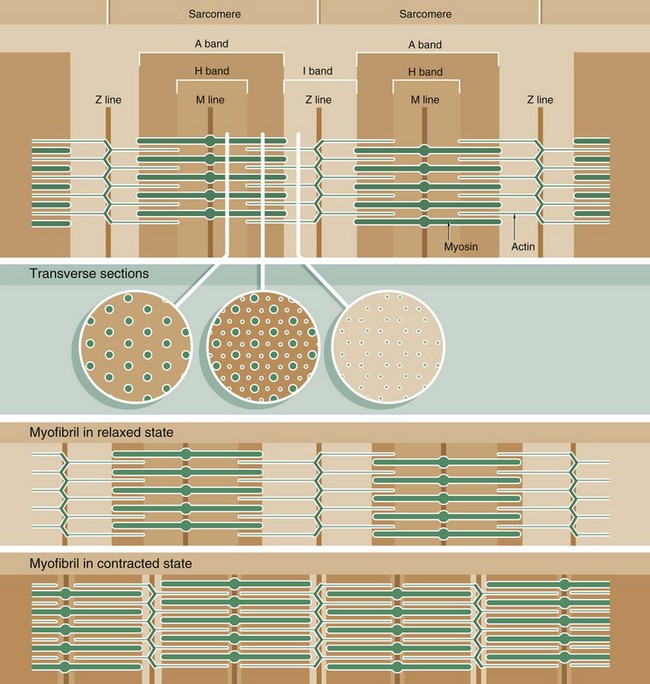
Muscle function is intimately linked to its microscopic structure. The sarcomere consists of two types of myofilaments, thick filaments and thin filaments. Each type remains constant in length irrespective of the state of contraction of the muscle. The thick filaments, which are composed mainly of the protein myosin, are maintained in register by their attachment to a disc-like zone represented by the M line. Similarly, the thin filaments, which are composed mainly of the protein actin, are attached to a disc-like zone represented by the Z line. The I and H bands, both areas of low electron density, represent areas where the thick and thin filaments do not overlap one another.
The sliding filament mechanism of muscle contraction proposes that contraction occurs through a sliding movement of the thick and thin filaments over one another as illustrated above. Myosin molecules possess ATP-activated side projections or head groups which can bind to actin to form cross-bridges, temporary physical linkages between the thick and thin filaments. Once bound to the adjacent actin molecules, these myosin head groups generate movement by a change in protein configuration, triggered by energy from the hydrolysis of ATP, pulling the myosin thick filaments over the thin actin filaments and so shortening the length of the sarcomere. When another ATP molecule binds to the myosin head group, it detaches from actin, breaking the cross-bridge, and the head group then moves back to its original configuration, ready for the next cross-bridge cycle. This process can be likened to multiple small strokes from the oars of a boat steadily producing movement of the craft.
A large number of accessory proteins are also present in the sarcomere where they play roles in filament alignment and in regulation of contraction.
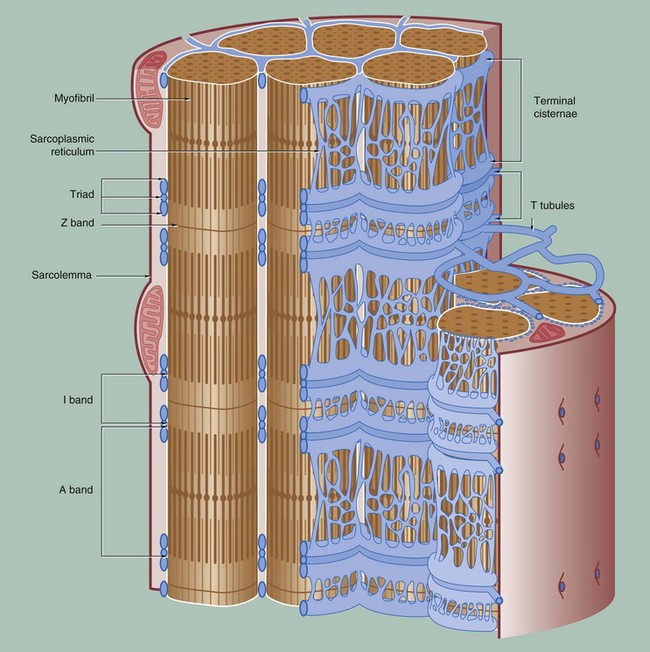
To permit the synchronous contraction of all sarcomeres in the muscle fibre, a system of tubular extensions of the muscle cell plasma membrane (sarcolemma) extends transversely into the muscle cell to surround each myofibril at the region of the junction of the A and I bands. Known as the T tubule system, its lumen is continuous with the extracellular space. (In amphibian skeletal muscle, which was the first to be studied, the T tubules are disposed at the Z bands and the same applies in cardiac muscle.)
Between the T tubules, a second membrane system derived from smooth endoplasmic reticulum, the sarcoplasmic reticulum, forms a membranous network which embraces each myofibril. On either side of each T tubule, the sarcoplasmic reticulum exhibits a flattened cisternal arrangement, each pair of terminal cisternae and a T tubule forming a triad near the junction of the I and A bands of each sarcomere.
Calcium ions are concentrated within the lumen of the sarcoplasmic reticulum. Depolarisation of the sarcolemma of the muscle fibre is rapidly disseminated throughout the sarcoplasm by the T tubule system. This promotes the release of Ca2+ ions from the sarcoplasmic reticulum into the sarcoplasm surrounding the myofilaments; Ca2+ ions then activate the sliding filament mechanism as described above, resulting in muscle contraction.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine
