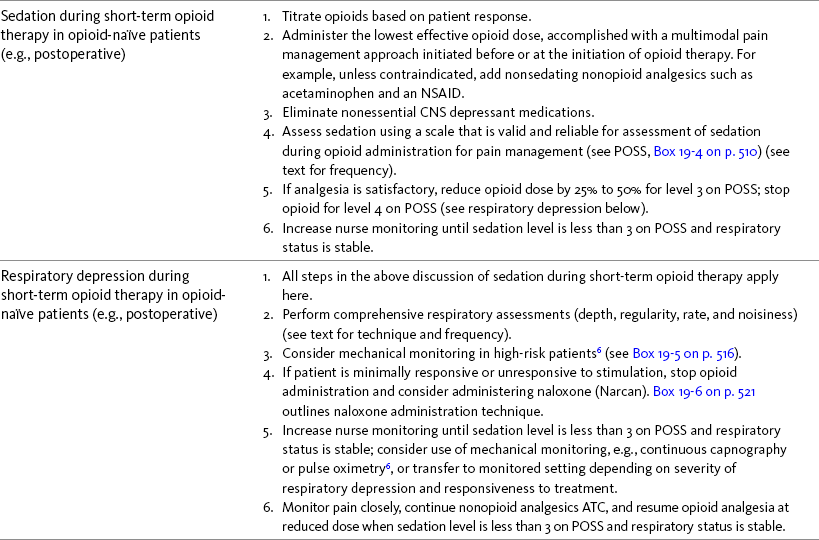
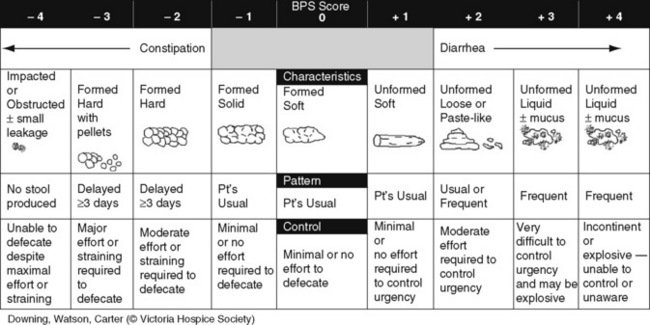
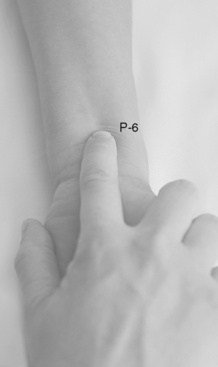
Chapter 19 Nausea and Vomiting in Patients Receiving Long-Term Opioid Therapy Postoperative Nausea and Vomiting (PONV) Sedation or Cognitive Impairment During Long-Term Opioid Therapy Sedation During Short-Term Opioid Therapy in Opioid-Naïve Patients Respiratory Depression During Long-Term Opioid Therapy Respiratory Depression in Opioid-Naïve Patients The following is a discussion of many of the opioid adverse effects. Table 19-1 is a guide to preventing and managing the common ones. See Table 11-1 on p. 285 for information on the specific opioid receptor binding sites of each adverse effect. The simple activity of chewing gum also seems to stimulate bowel motility (Schuster, Grewal, Greaney, et al., 2006). In a small prospective study, 34 patients undergoing elective open sigmoid resections were randomized into two groups: gum chewing or a control group. The patients chewing sugarless gum three times a day for one hour passed flatus, had their first bowel movement, and were discharged significantly sooner than the control group. More studies are needed, but gum chewing appears harmless and may help with constipation. Other tips on managing constipation are listed in Table 19-1. Tables containing the various classifications and properties of laxatives and the amount of fiber in common foods can be found in Curry, C. E., & Butler, D. M. (2006). Constipation. In R. R. Berardi, A. L. Kroon, J. H. McDermott, et al. (Eds.), Handbook of nonprescription drugs. An interactive approach to self care, ed 15, pp. 299-326, Washington DC, American Pharmacists Association. Tools with established reliability and validity for assessment of bowel function and constipation (Downing, Kuziemsky, Lesperance, et al., 2007; Goodman, Low, Wilkinson, 2005; Hinrichs, Huseboe, 2001; McMillan, Williams, 1989) are available. Figure 19-1 provides the Victoria Bowel Performance Scale (BPS), a simple-to-use 9-point tool. See also Form IV-1 on p. 546 at the end of Section IV. It contains valuable information that should be given to all patients receiving opioid therapy. A systematic review of 20 studies that were conducted on the use of methylnaltrexone and alvimopan for treatment of constipation concluded that further research with larger numbers of patients and varying types of pain are required (Becker, Galandi, Blum, 2007). Another analysis of 22 studies concluded that there is insufficient evidence for the safety and efficacy of naloxone or nalbuphine for the treatment of opioid-induced bowel dysfunction and that further research is needed to fully assess the role of alvimopan and methylnaltrexone in therapy (McNicol, Boyce, Schumann, et al., 2008). Following is a discussion of some of the research on methylnaltrexone. See pp. 491-493, Postoperative Ileus, for more on alvimopan. Methylnaltrexone was studied in a randomized, parallel-group, repeated dose, dose-ranging trial that included a one-week double-blind phase followed by an open-label phase for up to 3 weeks (Portenoy, Thomas, Moehl Boatwright, et al., 2008). The patients (N = 33) in this study had terminal or end-stage diseases and were receiving palliative care and long-term opioid therapy with stable doses for at least 2 weeks (mean and median opioid morphine equivalent dose = 289.9 mg and 180 mg, respectively) and reported ongoing constipation. They were randomized to receive 1, 5, 12.5, or 20 mg of SC methylnaltrexone. Doses between 5 mg and 20 mg (0.05 to 0.5 ) induced a bowel movement within 4 hours of drug administration significantly more often than a dose of 1 mg (less than 0.05 ), and there was no dose-response relationship above 5 mg/day. Approximately 50% of the patients responded with doses 5 mg or more within 4 hours and maintained favorable effects with repeated doses. These doses produced effective and rapid relief of constipation without producing pain flare or opioid withdrawal symptoms. All of the patients in this study experienced at least one adverse effect related to treatment; however, most were mild and not related to the dose of methylnaltrexone; abdominal pain was the most common. More recent research (N = 52) showed methylnaltrexone (0.15 ) given SC every other day for 2 weeks to patients with advanced illness resulted in a higher percentage of patient-rated improvements in bowel status, prompt and predictable laxation, and less use of other laxation techniques (e.g., laxatives and enemas) compared with placebo (Chamberlain, Cross, Winston, et al., 2009) (see Table 19-1). In summary, postoperative ileus has multiple underlying mechanisms and is influenced by a number of factors. Management requires the implementation of a multimodal approach that focuses on prevention. Strategies include continuous thoracic epidural analgesia, opioid-sparing analgesic techniques, peripheral mu opioid antagonists, laparoscopic surgical techniques, and avoidance of routine NG tube, fluid excess, and immobility (see Table 19-1). A variety of antiemetics is available to treat nausea and can be selected based on assumptions concerning the underlying mechanism (see Table 19-1). A dopamine antagonist, such as prochlorperazine, is most often selected, but the occurrence of nausea immediately after eating, or nausea associated with early satiety and bloating, suggests the occurrence of delayed gastric emptying, which in turn, supports a trial of a prokinetic drug, such as metoclopramide (Reglan) (Fine, Portenoy, 2007). A retrospective chart review led researchers to recommend risperidone 1 mg daily for refractory nausea and vomiting in advanced cancer patients (Okamoto, Tsuneto, Matsuda, et al., 2007). When nausea occurs in patients with medical illness and is both severe and presumably determined by several causes, combination therapy should be considered. For example, a prospective, multicenter, phase II clinical trial administered daily IV granisetron (Kytril) (3 mg) and dexamethasone (Decadron) (8 mg) to 24 patients with intestinal obstruction who were refractory to previous antiemetic treatment (Tuca, Roca, Sala, et al., 2009). This regimen controlled nausea and vomiting in 86.9% of the patients. Slow and steady opioid titration helps to reduce nausea. Eliminating nonessential drugs that may be contributing to nausea may be helpful as well (Fine, Portenoy, 2007). Adjustments in diet and activity plus the use of relaxation techniques can also be effective remedies (Coyle, Cherny, Portenoy, 1995). Table 19-1 outlines approaches commonly used to treat opioid-induced nausea and vomiting. Opioids are among a number of factors that increase the incidence of PONV. Box 19-1 lists the primary risk factors. A review of the literature described other factors in addition to this established list, including history of migraine, presence of preoperative anxiety, and the use of longer-acting versus shorter-acting opioids (Gan, 2006). It is important to note that postoperative pain, particularly incident pain, is associated with a higher incidence of postoperative vomiting (Chia, Kuo, Liu, et al., 2002; Ho, Gan, 2009). Despite being listed as a risk factor in accepted guidelines (see Box 19-1), there is controversy about whether or not the type of surgical procedure influences the incidence of PONV (Habib, Gan, 2010; Scuderi, 2010). Researchers conducted a retrospective review of oncology surgeries from their electronic database to evaluate the impact of type of surgery on antiemetic administration within the first 2 hours of PACU admission and found that patients who underwent neurologic, head or neck, and abdominal surgeries received significantly more antiemetic in the PACU than patients who underwent integumetary-musculoskeletal (e.g., puncture procedures of the skin or muscles) and superficial (e.g., breast or axillary, endoscopic) surgeries (Ruiz, Kee, Frenzel, et al., 2010). In a commentary about this research, Habib and Gan (2010) discuss several limitations of this study and problems with methodology, and they conclude that large well-designed studies are needed to firmly establish the type of surgery as a risk factor (Habib, Gan, 2010). Consensus guidelines present a number of recommendations for the management of PONV (Gan, Meyer, Apfel, et al., 2003, 2007). Algorithms that incorporate guideline recommendations are available (American Society of PeriAnesthesia Nurses, 2006; Gan, Meyer, Apfel, et al., 2007), and the December 2006 focus issue of the Journal of PeriAnesthesia Nursing is devoted entirely to content on PONV. Below is a summary of the major guideline recommendations in adults followed by a more detailed discussion of various antiemetics and strategies for treatment of PONV (see Table 19-1). • Identify patients at high risk for PONV (see Box 19-1). There is no consensus on how many risk factors a patient must have to warrant the designation of high risk (Apfel, Kranke, Eberhart, et al., 2002; Gan, Meyer, Apfel, et al., 2003; van den Bosch, Kalkman, Vergouwe, et al., 2005). A simple scoring system (the Apfel risk score) developed by Apfel and colleagues (1999) is widely used and has been shown to be reliable and valid for this purpose. It is based on four predictors: female gender, prior history of motion sickness or PONV, nonsmoking status, and the use of postoperative opioids. If no or only one factor is present, the incidence of PONV varies from 10% to 21%. If two or more are present, the risk rises to 39% to 78% (Apfel, Laara, Koivuranta, et al., 1999). • Reduce baseline risk factors, e.g., implement multimodal analgesic strategies to treat postoperative pain so that no opioid or the lowest effective dose of opioid can be given. This may also include the use of effective nonpharmacologic strategies such as relaxation techniques, acupuncture, and acupressure (Lee, Done, 1999; Nunley, Wakim, Guinn, 2008; Roscoe, Bushunow, Jean-Pierre, et al., 2009). Although nondrug interventions may be helpful, the mainstay of PONV treatment is pharmacologic (Wilhelm, Dehoorne-Smith, Kale-Pradhan, 2007). The use of regional rather than general anesthesia is widely recommended to reduce PONV, although some surgical procedures (e.g., cesarean, some major orthopedic surgeries) are associated with a high incidence of PONV despite using regional anesthesia (Borgeat, Ekatodramis, Schenker, 2003). • Administer PONV prophylaxis using one to two interventions to patients at moderate risk for PONV. For example, administer dexamethasone (Decadron) before anesthesia induction and a serotonin receptor antagonist (e.g., ondansetron [Zofran]) at the end of surgery. It is important to consider that research has shown that single-drug prophylaxis has a high failure rate and is associated with a resultant increased cost of care (Gan, Meyer, Apfel, et al., 2007; Watcha, 2000), so combinations of two antiemetics rather than a single antiemetic prophylactically are preferred. • Administer PONV prophylaxis using two or more interventions (multimodal approach) to patients at high risk for PONV. For example, administer dexamethasone before anesthesia induction, IV total anesthesia (IVTA) propofol (Diprivan), a serotonin receptor antagonist at the end of surgery, and IV propofol rescue doses in the PACU. A prospective study of 376 patients at high risk for PONV revealed that the administration of three or more prophylactic antiemetics produced the largest reduction in PONV, but despite this aggressive treatment, 30% still experienced symptoms severe enough to interfere with function (White, O’Hara, Roberson, et al., 2008). A prospective observational study concluded similarly that compared with lower Apfel risk scores, a high Apfel risk score (see above) was associated with a higher incidence of emetic sequelae in the first 24 hours after surgery despite the prophylactic administration of multiple antiemetics (White, Sacan, Nuangchamnong, et al., 2008). • Do not administer prophylactic antiemetic treatment to low-risk patients as this is not supported by current practice (Apfel, Korttila, Abdalla, et al., 2004; Gan, Meyer, Apfel, et al., 2003, 2007); however, provide antiemetic treatment to patients with PONV who did not receive prophylaxis or in whom prophylaxis failed. There are numerous antiemetic drug options available (Carlisle, Stevenson, 2006), and selection should be based on evidence of efficacy and safety as well as consideration of cost (see Table 19-1). A systematic review concluded that no one antiemetic agent is superior to another (Wilhelm, Dehoorne-Smith, Kale-Pradhan, 2007). A multicenter study of over 4000 patients at high risk for PONV (greater than 40% risk per Apfel risk scoring system [Apfel, Laara, Koivuranta, et al., 1999]) and undergoing a variety of types of surgeries were randomized to receive one of the following interventions: ondansetron (4 mg IV) or no ondansetron; dexamethasone (4 mg IV) or no dexamethasone; droperidol (Inapsine) (1.25 mg IV) or no droperidol; propofol or a volatile anesthetic (i.e., isoflurane, desflurane, or sevoflurane); nitrogen or nitrous oxide; and remifentanil or fentanyl. Because propofol has been shown to reduce PONV (see the paragraphs that follow), twice as many patients were assigned to the propofol group to ensure adequate power to compare treatments. All of the antiemetics were similarly effective; ondansetron, dexamethasone, and droperidol reduced risk by approximately 26%, propofol by 19%, and nitrogen by 12%. Droperidol, which has a “Black Box” warning for potential QTc prolongation and torsades de pointes, was safe (see later in the chapter for more on droperidol). The researchers pointed out that the clinical implication of their findings is that, because the interventions were similarly effective, the safest and least expensive treatment should be used first. The glucocorticoid dexamethasone is an excellent choice antiemetic because numerous studies have shown it to be effective, safe, and inexpensive (Gan, Meyer, Apfel, et al., 2007) (see Table 19-1). It may be given prophylactically before induction of anesthesia as well as for established PONV (Golembiewski, Chernin, Chopra, 2005) and has been shown to have similar efficacy to the serotonin antagonist tropisetron, which is not available in the United States (Wang, Ho, Uen, et al., 2002). Dexamethasone is administered as a single 4 mg to 8 mg IV bolus dose most often and has been combined in multimodal treatment plans with a number of other agents including ondansetron (Zofran) (Paech, Rucklidge, Lain, et al., 2007; Pan, Lee, Harris, 2008), granisetron (Kytril) (Fujii, Saitoh, Tanaka, et al., 1999; Gan, Coop, Philip, et al., 2005), dolasetron (Anzemet) (Coloma, White, Markowitz, et al., 2002; Rusch, Arndt, Martin, et al., 2007), droperidol (Sanchez-Ledesma, Lopez-Olaondo, Pueyo, et al., 2002), metoclopramide (Reglan) (Wallenborn, Gelbrich, Bulst, et al., 2006), and haloperidol (Haldol) (Chu, Shieh, Tzeng, et al., 2008; Rusch, Arndt, Martin, et al., 2007). Adverse effects are rare with short-term use (Gan, Meyer, Apfel, et al., 2007). The serotonin receptor antagonists dolasetron, granisetron, and ondansetron are most effective when administered at the end of surgery (Gan, Meyer, Apfel, et al., 2007) (see Table 19-1). The newest serotonin antagonist palonosetron (Aloxi) is approved for prevention of chemotherapy-induced nausea and has been shown to significantly decrease PONV in a dose-related manner (0.075 mg IV significantly better than 0.025 mg IV) when administered immediately prior to anesthesia induction (Candiotti, Kovac, Melson, et al., 2008). The serotonin receptor antagonists are often combined with other antiemetics in multimodal PONV prophylaxis regimens. This practice is supported by a meta- analysis of 33 randomized controlled trials (3447 patients), which concluded that various combinations of a serotonin antagonist with dexamethasone or droperidol were equally effective with similar adverse effects, and the combinations were more effective than a serotonin antagonist alone (Habib, El-Moalem, Gan, 2004). A 2001 systematic review of the literature found that the serotonin antagonists available at the time were similarly effective for treatment of established PONV and that lower doses were as effective as higher doses, so the lowest dose in the dosing range was recommended (Kazemi-Kjellberg, Henzi, Tramer, 2001). The anticholinergic scopolamine delivered via a transdermal patch (Transderm-Scop, Transderm-V) has been shown to prevent PONV with minimal adverse effects when applied preoperatively (White, Tang, Song, et al., 2007) (see Table 19-1). One patch (1.5 mg) should be applied behind the ear preoperatively, taking into account its 2- to 4-hour onset of action, and it can provide relief for up to 72 hours. A second patch may be applied after the first is removed at 72 hours. Transdermal scopolamine may be combined with other antiemetics in a multimodal treatment plan (Kranke, Morin, Roewer, et al., 2002). A randomized study of 126 patients undergoing cosmetic surgery found that the transdermal scopolamine patch combined with IV ondansetron (4 mg) was more effective in reducing PONV than ondansetron plus a placebo patch (Sah, Ramesh, Kaul, et al., 2009). Another study also found the combination to be more effective than ondansetron alone (Gan, Sinha, Kovac, et al., 2009). Transdermal scopolamine has also been used to reduce nausea and vomiting associated with intrathecal morphine post–cesarean section (Harnett, O’Rourke, Walsh, et al., 2007). Adverse effects include dry mouth, sedation, and visual disturbances; older patients may be more sensitive to CNS adverse effects, such as dizziness and agitation (Golembiewski, Chernin, Chopra, 2005). Research in the 1990s demonstrated that the IV sedative hypnotic propofol at subhypnotic doses (5 to 10 mg IV push q 4 to 6 h or 0.5 to 1 per hour continuous infusion) reduced the overall incidence of PONV in patients at high risk for PONV without untoward sedative or cardiovascular (CV) effects compared with placebo (Ewalenko, Janny, Dejonckheere, et al., 1996). Since then, the drug has gained in popularity as a component of multimodal approaches designed to reduce PONV (Eberhart, Mauch, Morin, et al., 2002; Scudieri, James, Harris, et al., 2000) (see Table 19-1). A randomized controlled study administered 90 patients undergoing laparoscopic cholecystectomy one of the following regimens: (1) a multimodal management strategy that involved the use of TIVA propofol plus droperidol and ondansetron, (2) IV propofol at induction followed by inspired isoflurane/nitrous oxide-based anesthesia, droperidol, and ondansetron, or (3) TIVA with no other antiemetic prophylaxis (Habib, White, Eubanks, et al., 2004). The droperidol (0.625 mg) was administered at induction and ondansetron (4 mg) was administered at the end of surgery in groups 1 and 2. Complete response rate (no PONV and no rescue antiemetic) at 2 hours and 24 hours after surgery was 90% and 80% in group 1, 63% and 63% in group 2, and 66% and 43% in group 3. Patient satisfaction was also higher in group 1. The researchers noted, however, that the higher cost of propofol compared with volatile anesthetics and its short-lived antiemetic effect (limited to early postoperative period) makes it suitable for use only in patients at very high risk for PONV. Since its approval for use in general anesthesia in the 1970s, the butyrophenone droperidol (Inapsine) has been used to effectively and cost-efficiently treat PONV (White, 2002). In 2001, the United States Food and Drug Administration (U.S. FDA) issued a “Black Box” warning that described the drug’s potential to cause prolonged QTc interval and torsade de pointes, a life-threatening cardiac dysrhythmia (Martinez, Moos, Dahlen, 2006). However, citing a lack of documentation of cardiac adverse events, the FDA warning has been widely criticized by anesthesia experts (Gan, White, Scuderi, et al., 2002; White, 2002). A review of the 273 reported adverse events involving the use of droperidol at doses of 1.25 mg or less (customary for treatment of PONV) revealed extensive use of the drug (over 11 million ampules sold in 2001) (Habib, Gan, 2003). Of the 273 adverse events, 74 and 17 were cases of possible cardiac events and torsades de pointes or prolonged QTc interval, respectively. The researchers concluded that there was no evidence of a cause-and-effect relationship between the occurrence of arrhythmias and small-dose droperidol (1.25 mg or less). A later randomized controlled trial of 120 patients undergoing outpatient surgery found no statistically significant increase in QTc interval compared with placebo during general anesthesia and no evidence of any droperidol-induced QTc prolongation after surgery (White, Song, Abrao, et al., 2005). The drug is recommended in doses less than 1.25 mg as a first-line option in evidence-based PONV management guidelines (Gan, Meyer, Apfel, et al., 2003, 2007) (see Table 19-1). Studies have shown that wrist acustimulation/acupressure (acupuncture point P6 [pericardium 6]) (ReliefBand®) reduces PONV (Gan, Jiao, Zenn, et al., 2004; Lee, Fan, 2009; Nunley, Wakim, Guinn, 2008; Roscoe, Bushunow, Jean-Pierre, et al., 2009) and that when combined with ondansetron (4 mg), the response rate to acustimulation is increased and quality of recovery and patient satisfaction are improved (Coloma, White, Ogunnaike, et al., 2002; White, Issioui, Hu, et al., 2002). The optimal time to administer acustimulation for antiemetic prophylaxis is after surgery (White, Hamza, Recart, et al., 2005). Figure 19-2 shows the location of acupuncture point P6. By far, the most effective, safest, and least expensive way to treat PONV is to reduce the opioid dose whenever possible. Postoperative opioid orders should include the expectation that nurses will consider decreasing the opioid dose by 25% prior to or in conjunction with pharmacologic treatment of moderate-to-severe PONV (see following patient example and Form 17-1 on p. 464 for an example of how decreases in opioid dose can be included in opioid order sets). Decreasing the opioid dose is facilitated by adding or increasing a nonopioid, such as an NSAID or acetaminophen, or adding a local anesthetic to the epidural opioid solution to provide additional pain relief. If patients are too nauseated to take oral nonopioids, they may be given rectally (see Section III and Chapter 14 in this section for more on rectal administration). Another commonly used drug is hydroxyzine (Vistaril), but the doses that would be required to produce analgesia create significant risk of respiratory depression that is not reversible by naloxone (Gordon, 1995). IM hydroxyzine is especially irritating to the muscle and soft tissue and can produce sterile abscesses, so this practice is discouraged as well. Other older drugs—dimenhydrinate (Dramamine) (Kothari, Boyd, Bottcher, et al., 2000) and diphenhydramine (Benadryl)—are occasionally used for PONV but can cause significant sedation and dizziness. Antiemetics with better efficacy and safety should be considered before these drugs are used for treatment of PONV (Gan, Meyer, Apfel, et al., 2007) (see Table 19-1). Early research showed morphine increased bile duct pressure in animals (Coelho, Runkel, Herfarth, et al., 1986) and humans (Zsigmond, Vieira, Duarte, et al., 1993). A study of 36 patients without common bile duct stones or anatomic abnormalities who were undergoing cholecystectomy demonstrated that morphine increased the frequency of sphincter of Oddi motility more than meperidine (Thune, Baker, Saccone, et al., 1990); however, an earlier study showed fentanyl, meperidine, morphine, and pentazocine caused a rise in bile duct pressure of 99.5%, 61.3%, 52.7%, and 15.1%, respectively in humans (Radnay, Brodman, Mankikar, et al., 1980). In other words, morphine produced less of a rise than both fentanyl and meperidine. Although pentazocine produced the smallest rise in this study, it causes dysphoria, anxiety, nightmares, depersonalization, and hallucinations, has an analgesic ceiling, and is not recommended for the management of any type of pain (see Chapter 13). The agonist-antagonist opioids (in addition to pentazocine above) have also been researched for their effect on the sphincter of Oddi. One early study showed that fentanyl, morphine, and meperidine increased common bile duct pressure more than butorphanol or placebo in 50 patients undergoing cholecystectomy (Radnay, Duncalf, Novakovic, et al., 1984). Later research found no differences between butorphanol, nalbuphine, and placebo in patients undergoing cholecystectomy (Vieira, Zsigmond, Duarte, et al., 1993); however, a more recent study found that nalbuphine caused a significant stimulatory effect on the sphincter of Oddi in 17 patients with suspected sphincter of Oddi dysfunction when used as a premedication for endoscopy; the researchers recommended against its use for endoscopic diagnosis of this condition (Madacsy, Bertalan, Szepes, et al., 2003). As discussed in Chapter 13, the agonist-antagonists are not recommended as first-line opioid analgesics for the treatment of pain. Although institutional quality improvement initiatives have resulted in a significant decline in the use of meperidine over the years (Gordon, Jones, Goshman, et al., 2000), the drug continues to be a first-choice analgesic of many prescribers (Seifert, Kennedy, 2004). This is particularly true for the management of pain during GI procedures and in patients with pancreatitis or cholecystitis; however, there are many disadvantages to the use of meperidine for pain management, including accumulation of its toxic metabolite with repeated dosing and its inappropriateness in older adults (Latta, Ginsberg, Barkin, 2002) (see Chapter 13).
Management of Opioid-Induced Adverse Effects
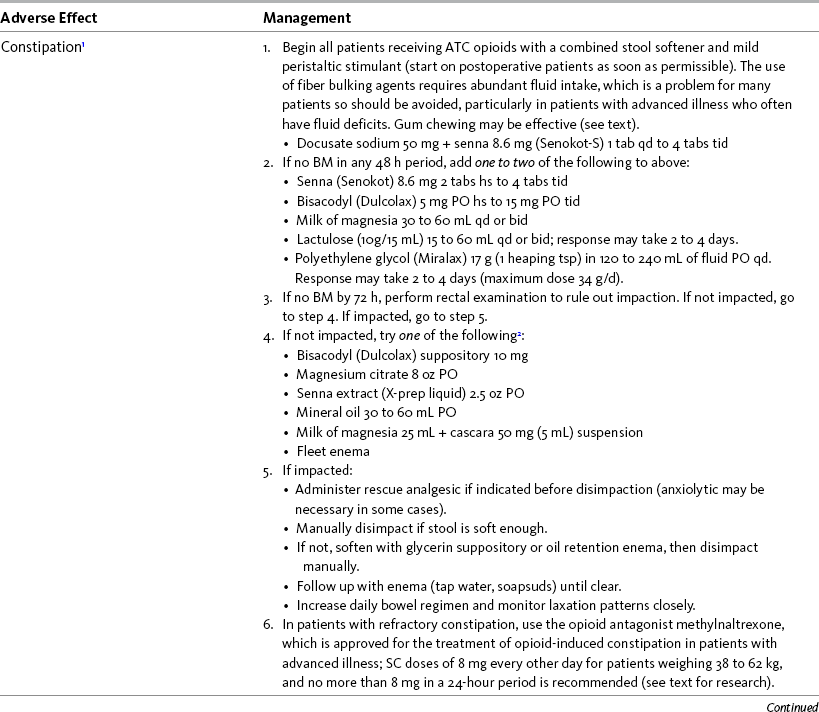
Constipation
Opioid Antagonists for Bowel Dysfunction
Postoperative Ileus
Opioid Antagonists for Management of Ileus
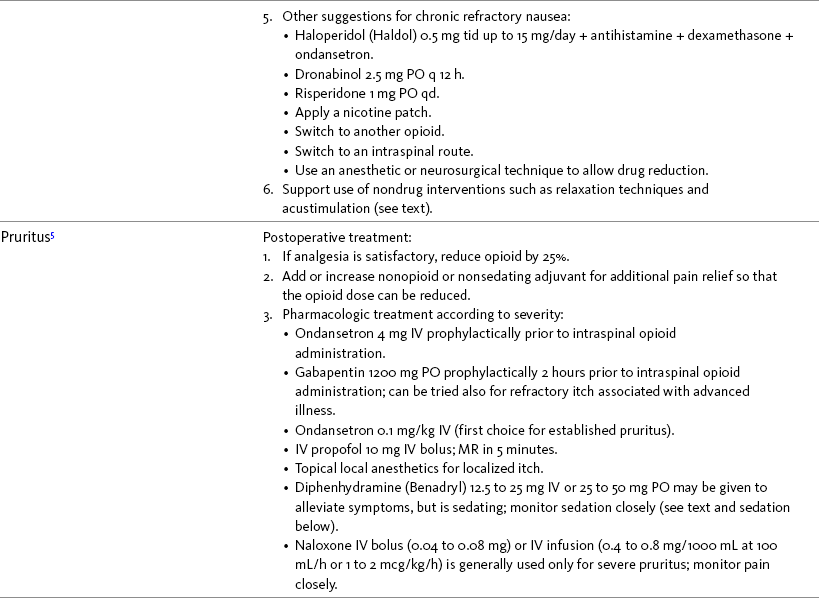
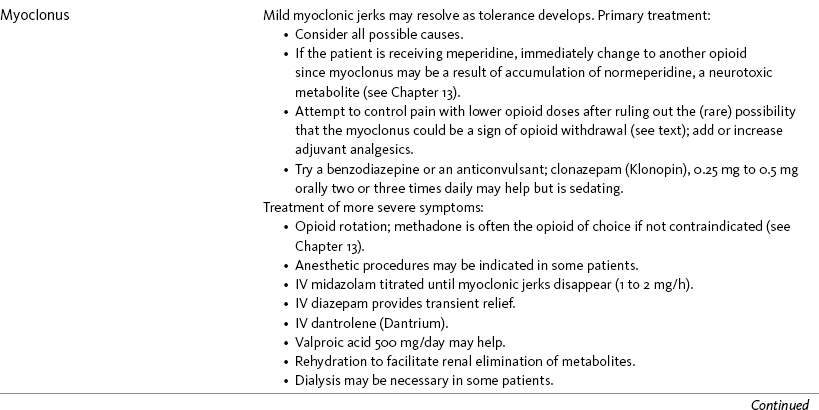
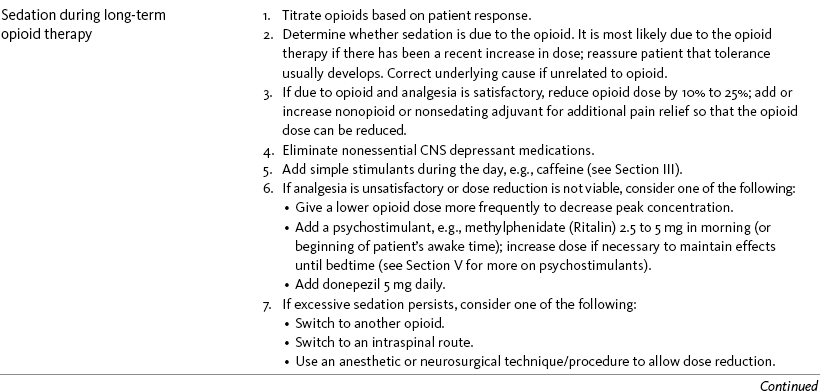
Nausea and Vomiting in Patients Receiving Long-Term Opioid Therapy
Postoperative Nausea and Vomiting (PONV)
Antiemetics
Droperidol and the “Black Box” Warning
Novel Approaches to the Management of PONV
Effective, Safe, and Inexpensive Treatment
Approaches with Little or No Effectiveness
Biliary Spasm
The Meperidine Misconception
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree