Structure of the liver
Only about 80% of the cells in the liver are hepatocytes (Figure 13.1); the remainder are endothelial (Kupffer) cells lining the hepatic sinusoids and vascular and supporting tissue cells. The functional unit of each liver acinus consists of the portal tract, surrounded by radiating cords of hepatocytes. Blood enters the acinus via the portal tract and passes along the sinusoids towards the central vein. Hepatocytes in the periportal area, zone 1, receive relatively well-oxygenated blood, whereas the hepatocytes surrounding the central vein, zone 3, receive blood that has lost much of its oxygen and exchanged other substances with the cells of zones 1 and 2. Cells in zone 3 are the most susceptible to anoxia and injury by a wide range of toxic substances.
Cells in zone 1 have relatively high concentrations of the enzymes usually measured in blood for diagnostic purposes (e.g. ALP and the aminotransferases ALT and AST), while cells in zone 3 are relatively deficient in these enzymes. This may help to explain why some patients with centrilobular liver damage may have normal serum enzyme activities.
Liver function tests
Most laboratories perform a standard group of tests (Table 13.1), which do not assess genuine liver function but are useful for:
Figure 13.1 The ultrastructure of the liver. The liver ultrastructure mainly consists of hepatocytes and Kupffer cells (part of the reticuloendothelial system). The bile canaliculus is formed by the plasma membrane of two hepatocytes.

Table 13.1 Routine liver function tests (examples of widely performed groups of serum measurements)
| Standard group of tests | Property being assessed |
| Serum albumin, PT | Protein synthesis |
| Serum bilirubin (total) | Hepatic anion transport |
| Serum enzyme activities | |
| ALT, AST | Hepatocellular integrity |
| ALP, GGT | Presence of cholestasis |
Hepatic anion transport: bilirubin
Measurements of bilirubin in blood and urine are usually used to assess hepatic anion transport, although many other anions, including bile salts, are also transported by the liver. Understanding the mechanisms by which bilirubin is formed and removed is essential for the diagnosis of patients with jaundice or liver disease, since abnormal levels of bilirubin in blood can occur in patients in whom there is no liver disease.
Bilirubin production and metabolism
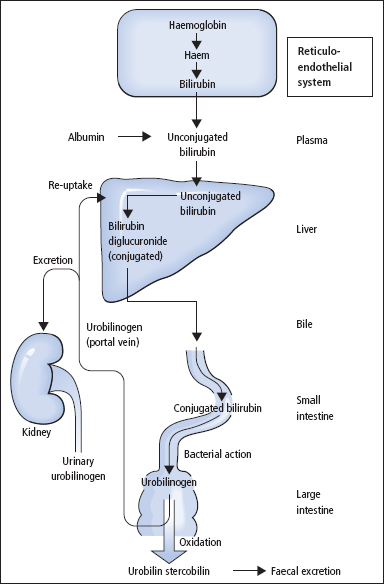
The pathway of bilirubin production and excretion is shown in Figure 13.2.
Production
The body usually produces about 300 mg of bilirubin per day as a breakdown product of haem. About 80% arises from red cells, with the remainder coming from red cell precursors destroyed in the bone marrow (‘ineffective erythropoiesis’), and from other haem proteins such as myoglobin and the cytochromes. Iron is removed from the haem molecule, and the porphyrin ring is opened to form bilirubin.
Transport in plasma and hepatic uptake
Bilirubin is insoluble in water and is carried in plasma bound to albumin, and is thus not filtered at the glomerulus unless there is glomerular proteinuria. On reaching the liver, the bilirubin is taken into the hepatocyte by a specific carrier mechanism.
Conjugation of bilirubin and secretion into bile
In the endoplasmic reticulum of the hepatocyte, the enzyme bilirubin UDP-glucuronyltransferase conjugates bilirubin with glucuronic acid to produce bilirubin glucuronides which are water soluble and readily transported into bile. Secretion of bilirubin glucuronides into bile occurs against a high concentration gradient and is the rate-limiting step in removing bilirubin from the body. Secretion is a carrier-mediated energy-dependent process.
Further metabolism of bilirubin in the gut
Bilirubin glucuronides cannot be reabsorbed from the gut and are degraded by bacterial action, mainly in the colon, to a mixture of colourless, water-soluble compounds collectively termed urobilinogen. These compounds oxidise to brown compounds known as urobilins and stercobilins and are excreted in the faeces. A small percentage of urobilinogen is absorbed and carried to the liver in the portal blood supply, that is, it undergoes an enterohepatic circulation. Most of this urobilinogen is cleared by the liver, but a proportion escapes clearance and is filtered at the kidney and appears in the urine, where it can be detected using point of care urine dipsticks.
Figure 13.2 The formation and metabolism of bilirubin and its excretion into the intestine.
Measurements of serum bilirubin
Normally, more than 95% of bilirubin in serum is unconjugated, but in liver disease the conjugated form may predominate. For most purposes, the measurement of serum [total bilirubin] (i.e. the sum of unconjugated and conjugated forms) is sufficient, especially when results are interpreted in relation to the patient’s history, findings on clinical examination and the results of urine urobilinogen and bilirubin measurements. Occasionally, it may be helpful to measure serum [conjugated bilirubin] and serum [unconjugated bilirubin] separately especially in neonates (p. 292).
Hepatocellular damage: aminotransferase measurements
Soluble cytoplasmic enzymes and, to a lesser extent, mitochondrial enzymes are released into plasma in hepatocellular damage. The measurement of the activity of ALT or AST in serum provides a sensitive index of hepatocellular damage. Serum ALT measurements are more liver specific than those of AST. Cytoplasmic and mitochondrial isoenzymes of AST exist and, in chronic hepatocellular disease (e.g. cirrhosis), serum AST tends to be increased to a greater extent than ALT. The aminotransferases are mainly located in the periportal hepatocytes, and they do not give a reliable indication of centrilobular liver damage. As with all tests based on the release of enzymes from damaged tissue, there is a lag period of some 24 h from the initiation of tissue damage to the first appearance of increased enzyme levels in the plasma.
Cholestasis: alkaline phosphatase and γ-glutamyltransferase
Some enzymes, such as ALP and GGT, are normally attached, or ‘anchored’, to the biliary canalicular and sinusoidal membranes of the hepatocyte. For this reason, ALP and GGT tend to be released into plasma in only small amounts following hepatocellular damage. However, they are released in much greater amounts when there is cholestasis, since their synthesis is induced and they are rendered soluble – due, at least in part, to the presence of high hepatic concentrations of bile acids.
Changes in the activities of GGT and ALP often parallel each other in cholestatic liver disease. Serum GGT has the advantage of being more liver specific, as serum ALP may also be increased due to release from bone in bone disease. However, alcohol and many drugs such as anti-convulsants may induce the expression of GGT without causing cholestasis. An isolated increase in GGT should thus be interpreted with caution.
Hepatic protein synthesis
The measurement of certain plasma proteins provides an index of the liver’s ability to synthesise protein.
Albumin
In chronic hepatocellular damage, there is impaired albumin synthesis with an accompanying fall in serum [albumin]. Albumin measurements provide a fairly good index of the progress of chronic liver disease. In acute liver disease, however, there may be little or no reduction in serum [albumin], as the biological half-life of albumin is about 20 days and the fractional clearance rate is therefore low. Factors other than impaired hepatic synthesis may lead to a decreased serum [albumin]. These include loss of albumin into the extravascular compartment, ascites, increased degradation, poor nutritional status and a fall as part of the acute-phase response.
Ascites
Increased portal venous pressure, a low plasma colloid oncotic pressure and Na+ retention due to secondary hyperaldosteronism combine to cause ascites in cirrhotic patients. This often develops when serum [albumin] falls below 30 g/L.
Coagulation factors
In liver disease, the synthesis of prothrombin and other clotting factors is diminished, leading to an increased PT. This may be one of the earliest abnormalities seen in patients with hepatocellular damage, since prothrombin has a short half-life (~6 h). The PT is often expressed as a ratio to a control value (the INR).
Deficiency of fat-soluble vitamin K, due to failure of absorption of lipids, may also cause a prolonged PT. In vitamin K deficiency, the coagulation defect can often be corrected by parenteral administration of vitamin K, but this has no effect in patients with hepatocellular damage.
Immunoglobulins
Serum Ig measurements are of little value in liver disease because the changes are of low specificity. In most types of cirrhosis, serum [IgA] is often increased, while in primary biliary cirrhosis, serum [IgM] increases greatly. In chronic active hepatitis, serum [IgG] tends to be most increased.
Serological tests
Anti-mitochondrial antibodies are present in over 95% of patients with primary biliary cirrhosis, and anti-smooth muscle and anti-nuclear factor antibodies are found in about 50% of patients with chronic active hepatitis. Viral antigens and antibody measurements are also important in detecting infective causes of liver disease.
Marker of fibrosis
A variety of markers have been described that may be of help in the assessment of hepatic fibrosis. Procollagen type III terminal peptide and hyaluronic acid (hyluronin) are the most commonly used tests.
Other liver function tests
A number of liver function tests have been described that give an indication of the functional liver mass. These tests are not often used but include the aminopyrine breath tests, the galactose elimination test and the monoethylglycinexylidide (MEGX) test. The measurement of bile acids is useful in investigating hepatic dysfunction associated with pregnancy (p. 175) and in the investigation of Gilbert’s syndrome (p. 203).
Disordered metabolism
Patients with severe liver disease may have:
The place of chemical tests in the diagnosis of liver disease
The jaundiced patient
Jaundice is due to hyperbilirubinaemia and becomes clinically apparent when the serum [bilirubin] exceeds about 50 μmol/L, although smaller degrees of hyperbilirubinaemia may be of diagnostic significance.
Measurements of serum [bilirubin] give a quantitative index of the severity of the jaundice, while serum enzyme activity measurements and point of care tests on fresh urine specimens, for bilirubin and urobilinogen, usually allow the cause of jaundice to be defined as pre-hepatic, hepatocellular or cholestatic (Figure 13.3). Further appropriate tests can then be requested (Table 13.2).
Investigations of the jaundiced patient often use the strategy shown in Figure 13.4. Increased serum [bilirubin] can arise as a consequence of increased production, impaired metabolism or decreased biliary excretion.
Pre-hepatic hyperbilirubinaemia
This is due to overproduction of bilirubin causing increased serum [unconjugated bilirubin]. It occurs in
- haemolytic anaemia
- haemolytic disease of the newborn, due to rhesus incompatibility
- ineffective erythropoiesis (e.g. pernicious anaemia)
- rhabdomyolysis.
Figure 13.3 Types and causes of hyperbilirubinaemia.
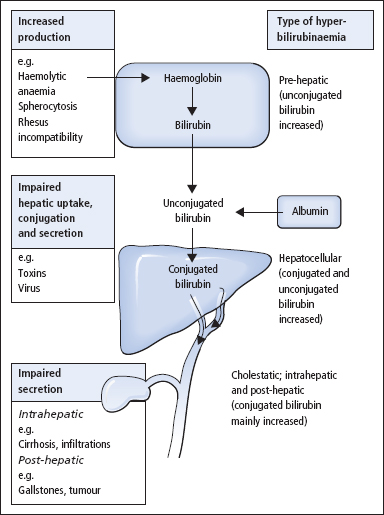
Table 13.2 Bilirubin and urobilinogen measurements (examples of results in various conditions)
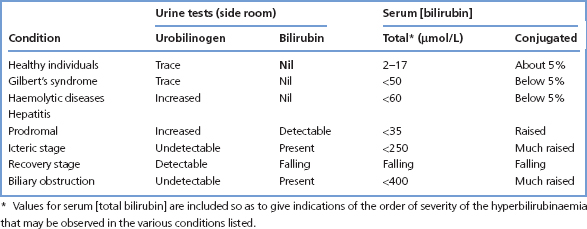
Figure 13.4 The investigation of jaundice. Endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC) may be needed whenever the cause of dilated bile ducts is uncertain.

Hepatocellular hyperbilirubinaemia
This can arise from
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


