Fig. 6.1
Diagram of pathogenesis of deforming osteoarthrosis
Most researchers believe that a disorder of metabolism of chondrocytes when catabolic processes dominate over anabolic processes starts cartilage degeneration in osteoarthrosis [11–13, 15]. Appearance of the products of the altered cellular synthesis subject to fast decay and elimination favors leakage of proteoglycans from the cartilage matrix. Proteolytic enzymes released alongside play en essential role in maintaining the process of degradation of proteoglycans and secondary damage of ultrastructures of chondrocytes [11].
As a result, the dynamic equilibrium between the synthesis and degradation of the cartilage tissue is upset. Therefore, in case of a deficit of normal synthesis and excessive pathologically altered synthesis the chondrocytes become unable to compensate its lacking matrix in sulfonated mycopolysaccharides. The damage appearing in response morphologically cause proliferative responses of chondrocytes and intensify their functional activity so that their deficient reparative potential cannot stop the already started process [6, 12].
These facts have been convincingly proven in experimental works. At present, a number of models exist of the osteoarthrosis induced by changes of external and internal factors of the joint: immobilization, a trauma of the cartilage, the meniscus and ligaments, penetration of foreign bodies, enzymes etc. into the joint cavity.
The cartilage is known to swell and its elastic properties depend on the concentration of mucopolysaccharides in the cartilage tissue. E.g., any change in the mean molecular mass and osmosis of glucose aminoglycans under the effect of hyaluronidase destroys the elastiviscous characteristics of the cartilage [8, 9, 15–17]. The most pronounced physico-chemical damage of the matrix appears initially in the intermediate cartilage zone. Microfractures of the collagenous skeleton and depletion of the matrix by proteoglycans lead to its irregular hiperhydration proportional to the residual concentration of glucose aminoglycans [8, 9, 15, 17]. This process is accompanied by the thinning and splitting of collagenous fibers.
As a result of this change in the optimal structural and quantitative interrelations between the matrix components the biomechanical and tribological cartilage properties are inevitably disordered followed by further development of the process of mechanodestruction of the cartilaginous tissue.
In case of the idiopathic osteoarthrosis, this damage has secondary nature in respect to the preceding change in the ultrastructure and resistance of collagenous fibers to loading. However, a primary rupture of collagenous fibrils is possible in the healthy cartilage in case a tolerable physiological load is exceeded significantly, e.g., in case of the impact traumatic mechanism.
In the physiological conditions, the joint functions properly like in an isolated system. During osteoarthrosis, any damage of the collagenous skeleton and reduction of the barrier role of the cartilage matrix open up unrestricted two-way transport of larger protein molecules. Therefore, the pathological changes and the processes of mechanodestruction fetch masses of products of wear and degradation of the collagen, proteoglycans, and cellular membranes into the synovial fluid. These proteins, absent in the normal synovial fluid, acquire autoantigenic properties, and induce immunal mechanisms of cartilage destruction. The immunopathological process like a reaction of retarded hypersensitivity develops parallel both in the cartilage and in the synovial membrane [15].
Its activation is strongly influenced by the appearance of a significant quantity of the so-called inflammation mediators and modulators (histamine, kinins, prostaglandins, chemotactic and other substances), intensification of free-radical oxidation. The bone tissue is believed to influence this process in a certain manner. Remodeling is observed and consolidation of the subchondrial bone plate with disorders of the blood flow and cartilage trophism. Any rise of specific loads is responded by a peculiar protective reaction of articular bone surfaces as an intensification of reparative processes over the periphery and expansion of the contact area, i.e. osteophytes appear (Fig. 6.2). These zones reveal deposition of crystals of calcium dihydropyrophosphate and hydroxyapatite [6, 15]. Obduced by the protein in the synovial fluid they activate the complementary system.
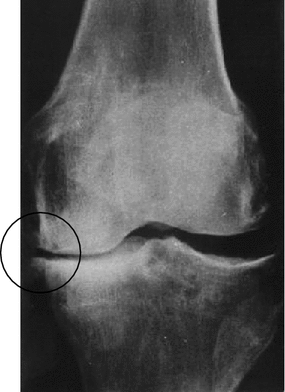

Fig. 6.2
X-ray pattern of deforming osteoarthrosis of knee joint demonstrating appearance of osteophytes over edges of the tibial plateau
A complex of cellular and vascular reactions appears in the synovial membrane typical for the hyperplastic inflammatory process. The developing synovitis and disorder of the functions of the synovial membrane are accompanied by accumulation of inflammatory or effusion in the joint cavity. Its qualitative and quantitative characteristics are strongly different from those of the normal synovial fluid. The effusion typically changes the pH of the medium, leads to accumulation of protein components and products of degradation of proteoglycans, activation of destructive enzymes, and reduction of concentration of the hyaluronic acid [15]. As a result, the rheological, trophic, and lubricating functions of the synovial fluid become strongly impaired.
On the other hand, when the inflammation mediators cause liberation of proteolytic and lysosomal enzymes macrophages, lymphocytes and plasmocytes in the composition of cellular infiltrates of the synovial membrane, the latter facilitate turning the synovitis into chronic and the processes of destruction of joint elements [6].
The neglected osteoarthrosis stages manifest how the synovial membrane gets sclerosed, secretion of the synovial lubricating fluid becomes deficient [8]. Cracks, fragmentation portions appear in the cartilage, fibers become loose and hydrophily drops (Fig. 6.3a). The process of destruction of the cartilage ends in its mechanodestruction (Fig. 6.3b).
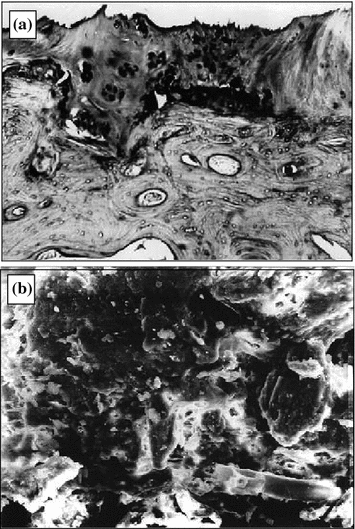

Fig. 6.3
Deforming osteoarthrosis: a pathomorphological changes in joint cartilage (1.5 × 1.2 mm, dyed with hematoxyl and eosin); b joint cartilage scan (300 × 250 µm)
The rheumatoid arthritis occupies a dominating place in the group of inflammatory disorders in joints. The rheumatoid arthritis is a systemic immunal inflammatory disease of the connective tissue ; its main pathological manifestation is affliction of the locomotorium. It features fast evolution of osseal and cartilaginous destruction of joints among predominantly young patients (20–40 years) that leads to early and stable disability. Fifty percent of them become invalids 2–3 years after the rheumatoid arthritis begins [2, 6].
According to modern ideas, the inflammatory process during rheumatoid arthritis relates to immunopathological disorders of cellular and humoral immunity. However, its etiology and many pathogenetic mechanisms of development remain obscure. A triggering role of exogenic or endogenic antigens in this process is debated (the structurally damaged collagen, immunoglobulin, fragments of bacteria, micoplasmas, and viruses) [18]. Using these concepts, the experiment yielded a number of chronic arthritis among animals resulting from various immunological effects, but they reflect just individual links of the pathogenesis of the diseases [6, 18]. There is an adequate arthritis model identical to the rheumatoid process in man. That is why it seems impossible to formulate a single scheme of the rheumatoid arthritis pathogenesis and the like disorders at present.
The cartilage destruction in rheumatoid arthritis seems an intricate and multifactor process. The leading role belongs to the cellular and humoral immunopathological reactions that evolve in the so-called “locus minoris resistencia” [6]. These are borderline zones between the cartilage and the synovial membrane. Active destruction of the cartilage and then of the subchondrial bone develops through invasion by the degenerative pannus which is the vascular and fibrous tissue of the synovial membrane perichondrial portion. Degradation of the collagen and proteoglycans in the matrix occurs under the effect of free-radical oxidation of tissues, collagenolytic and lysosomal enzymes, such as macrophages and neutrophils, chondrocytes and synovicytes. A similar effect is produced in the cartilage by synovial effusion that the same causes turn into a medium “aggressive” to it. This process is maintained by persistence of antigens in all synovial tissues, including the cartilage. The products of degradation of proteoglycans released by the cartilage and acquiring antigenicity play apparently a definite role in evolution of immunopathological reactions.
A disorder of the hematosynovial barrier permeability and pathochemical processes in the synovial fluid change drastically its basic biophysical properties. It is facilitated by dystrophic changes in the cartilage, profound impairment of its biomechanical characteristics. Under these conditions, the cartilage easily undergoes both enzymic and mechanical destruction. Joint stiffness, one of early symptoms of the rheumatoid arthritis, is attributed to the disorder of lubricating parameters of tissues in articular pairs. The friction coefficient of a sick joint cartilage is supposed to increase sharply to 0.4 [19]. Therefore, the process terminates in premature wear of the cartilage, severe deformation of joints, appearance of a fibrous and an osseous anchylosis.
The known stereotype of development of inflammatory changes in articular tissues, irrespective of etiological factors, makes all chronic arthritis alike because of commonness of many link of the pathogenesis of destruction of joints. Proceeding from this fact a certain similarity of the synovitis during the osteoarthrosis and rheumatoid arthritis has been remarked.
Notwithstanding the variety of forms and features of evolution of rheumatic disorders, the articular syndrome a number of similar clinical symptoms; it is a combination of pain, symptoms of synovitis, joint stiffness and limited functions of joints.
Sensations of pain have a complicated and variegated genesis. A degenerative damage of the joint cartilage in the deforming osteoarthrosis manifests itself clinically by dull, aching pain of the “mechanical” type. It appears during ambulation and after exertion in the afternoon, as a rule. Friction and crackling in joints, restriction of motions due to pain appear in the late stages of osteoarthrosis. It is due to deficient lubrication in the joint, cracking and fissuring of contacting cartilage surfaces frequently reaching the subchondrial bone plate (Fig. 6.4).
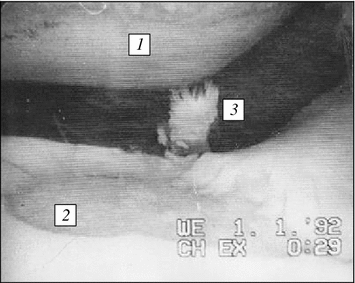

Fig. 6.4
Arthroscopic pattern of mechanodestruction of joint cartilage in knee joint medial portion during post-traumatic deforming osteoarthrosis: 1 pronounced cartilage thinning on sliding contact of hip condyle reaching subchondrial bone plate; 2 deep extensive cartilage erosion stopping at subchondrial bone plate contacting with hip shin-bone condyle; 3 products of wear of joint cartilage in joint cavity
The rheumatoid arthritis and similar inflammatory disorders have the pattern of «inflammatory rhythm» of articular pain coinciding with arthritic aggravation, it is permanent and pain intensifies in ambulation. Accumulation of excessive effusion, inflammation mediators, and products of the upset tissue metabolism, higher intraarticular pressure and appearance of deep and extensive erosion of the cartilage integument play large role in pain induction. Pain receptors of vessels, the periosteum, the fibrous capsule , ligaments, and adipose bodies in the joint are irritated mechanically and chemically.
6.2 Basic Directions of Treating Joint Rheumatic Deceases
Treatment of rheumatic diseases is an intricate task because due to the above reasons no etiotrophic therapy is feasible so far. The therapeutic schemes are based on the known ideas about partial links of the pathogenesis of the disorders. Widely employed means and methods of therapy of rheumatic diseases are aimed predominantly at achieving the counterinflammatory and analgesic effect. That is why this symptomatic treatment achieves just a temporary inhibition of the process without eliminating its causes, let alone development and progress of cartilage destruction.
From this standpoint, one of the main principles of therapy should be effective protection of the cartilage against enzymatic destruction and premature wear. This trend of arthrology acquired the name of “chondroprotection ” [20]. The main task for the next decade is to find means stimulating the reparative functions, correcting metabolic disorders and preventing cartilage destruction [20, 21].
Since rheumatic diseases evolve in a chronic relapsing manner, the known means of their therapy are palliative, hence their treatment should be combinatory and long-term. It implies combined administration of medicamentous drugs, physiotherapy, preventive, and corrective orthopedics, physical exercises, massage, sanatoria, and spas. The main principles and features of the therapy of individual nosological forms of rheumatic diseases are quite well disclosed in the recent publications [6, 12, 22]. Therefore, it seems expedient to consider the most general means of treatment of rheumatic diseases from the viewpoint of evaluation of their chondroprotective effectiveness.
At present steroid and non-steroid counterinflammatory drugs are widely administered. Among them glucocorticoids are administered more often as natural hormones of the adrenal cortex or their synthetic analogs. They possess a broad spectrum of pharmacological effects; their mechanism is dictated by the physico-chemical properties, high trophicity, and their classification as lipids. The main therapeutical effect of the steroids is a pronounced counterinflammatory, antiallergic, and immunodepressive action [23].
The main way of administration of corticosteroids in rheumatoid arthritis is enteral. However, log administration of the drugs may cause complications due to immunity depression, disorders of the adrenal cortex functions and water—electrolyte metabolism. Moreover, glucocorticoids can inhibit the biosynthetic function of chondrocytes suppressing production of collagen and proteoglycans [23–25]. Clinical observations of the progress of cartilage destruction after prolonged steroid therapy confirm that these drugs produce no chondroprotective effect.
Another large group of medicamentous means is non-steroid counterinflammatory drugs (NCID). Their non-specific pharmacological effect favors their administration in arthrosis arthritis of any etiology. The NCID produce no hormonal effect of glucocorticoids and feature relatively good tolerance. They produced pronounced counterinflammatory, pain and temperature relieving effect by direct inhibition of the synthesis of prostaglandins, mono- and lymphoquins, histamine, etc. [6, 22]. These drugs can reduce the permeability of capillaries and restrain exhudative manifestations of inflammatory reactions; they produce a cytotoxic effect and depress proliferative processes tissues [26]. Some of them are capable to stabilize release of destructive enzymes.
Still, since the NCID are derivatives of various acids, they may cause side effects or provoke aggravation of chronic gastrointestinal disorders [27]. It has been demonstrated that prolonged administration of the NCID produces a clinical effect, but they impair metabolism in the cartilage and reduce the synthesis of proteoglycans [21, 28, 29]. Hence, it has been concluded that these drugs fail to prevent cartilage destruction during rheumatic afflictions [30].
An alternative approach to solving the problem was discovered in the early 60 s. Adoption of the so-called “true chondroprotectors” into the clinical practice (rumalonum, arteparonum and their analogs) containing proteoglycan complexes as the main biologically active substance has become a definite achievement of modern pharmacotherapy of rheumatic diseases.
Long-term clinical and experimental studies revealed that the drugs, due to their biochemical affinity with the sulfated proteoglycan matrix, possess special trophism to the arthrosis-altered cartilage structure and a capability to propagate in it. Destruction of the main substance of the cartilage matrix is inhibited by rivaling inhibition of the enzymic system of lysosomes and activation of the synthetic functions of chondro- and synovicytes [20, 21, 25, 31, 32]. As a result, the trophism and metabolism of the cartilage normalize, the biosynthesis of mucopolysaccharides is stimulated, the processes of their depolymerization and elimination are inhibited. The drugs of these groups have shown correlation between the number of injections and higher concentration of the HYA in the synovial fluid [20, 30]. This fact favors both improvement of the tribomechanical properties of the cartilage and the synovial fluid and the functional abilities of the joint in general.
Prolonged rumalonum therapy of osteoarthrotic patients stabilizes annual workday losses, significantly reduces disablement cases compared with the patients’ control group [20, 21, 30]. The most optimum is to treat early stages of disorders when the process of leakage of proteoglycans is still reversible. In such cases the positive results of administration of the drugs is observed in 63–70 % cases [21].
These data permitted to administer for a long period rumalonum and arteparum as basic therapy of the deforming osteoarthrosis. However, notwithstanding the relatively good tolerance of the drugs, side effects and complications can be observed. Since the therapeutical drugs contain biologically active parts of the cartilage matrix (peptides, glicoaminoglycans, nucleotides and nucleosides, chondrocytes, bone marrow cells, etc.) possessing antigenic properties, a severe complication is possible, such as an allergic reaction. That is why it is difficult to administer these therapeutical drugs in case of the rheumatoid arthritis [25]. Because the anticoagulation effect is similar to that of reparinum, they are counter indicated in case of stronger risk of a hemorrhage (hemorrhagic diatheses), diabetes mellitus, chronic afflictions of the cardiovascular system (hypertension, endocarditis, cardiosclerosis), ulcer diseases of the gastrointestinal tract, hepatic and renal pathology [20, 30].
In recent years, new therapeutical drugs appeared containing the cartilage matrix components and improving the cartilaginous tissue metabolism. In this connection, a new term emerged—the “drugs structurally modifying the cartilage”. These therapeutical drugs include, e.g., structum or its analogs. The effective component of the drug is the sodium salt of chondroitin sulfate or the basic component of proteoglycans in the cartilage matrix. Now the chondroitin sulfate designates a group of structurally similar polysaccharides, as a rule, consisting of the sulfonated and unsulfonated remnants of glucuronic acid and N-acetylglucosamine. The chondroitin sulfate mechanism of effect is obscure. It is assumed that it is most probably due to the physico-chemical properties of the drug rather than to the biological ones. Experimentally it is established that the structum therapeutic effect is due to substitution of the chondroitin sulfate destroyed by catabolism in the cartilage and activation of the synthetic functions of chondrocytes inhibiting the destructive enzymes and further development inflammatory reactions in articular tissues. Experiments in vitro revealed that chondroitin sulfate is combined by chondrocytes and is included into the cartilage matrix composition. Chondroitin sulfate in the cultures of chondrocytes is capable to prevent the destructive effect of interleukin-1 in the cartilage [33]. Nevertheless, it has yet been proven if incorporation of chondroitin sulfate can restore normal sulfonation of glucose aminoglycans in osteoarthritis [34]. The mechanism of counterinflammatory effect of chondroitin sulfate observed experimentally as protease inhibition of пpoтeaз and reduction of decay of the cartilage matrix has not been explained so far [35, 36].
The true bioavailability of chondroitin sulfate is unknown. It is believed that in case of peroral administration the absorption of the intact low-molecular chondroitin sulfate is about 5 % and does not exceed 10–13 % [37–39]. According to the meta-analyses of over 20 studies the chondroitin sulfate drug has moderate effectiveness of pain relief and improvement of functions of joints in osteoarthritis among approximately 65 % of patients during 4 months of administration [40–42]. No convincing proof of structurally modifying properties of chondroitin sulfate has yet been obtained.
Structum as a therapeutic drug is produce in pills and intended for ingestion. It has been established that the drug can accumulate in the articular cartilage and synovial fluid preserving residual therapeutic effectiveness for 2 months after it is withdrawn. The structum is a harmless drug that practically produces no side effects. Therefore, it can be administered for prolonged therapy of osteoarthroses. Clinical research has revealed that administration of structum during 4 months reduces substantially the pain syndrome and improves functions of injured joints among most patients. Glucosamine is becoming attractive in recent years. A number of randomized studies have revealed that this compound produces a structure modifying effect in respect to the articular cartilage and symptomatic effects during therapy of osteoarthritis [40]. Most of the research of glucosamine dealt with its sulfate salt, some used glucosamine hydrochloride. The problem of the sulfate role in the pathogenesis of osteoarthritis has been discussed in publications. It is established that chondrocytes are very sensitive even to the least reduction of sulfate concentration, in this case the synthesis of glucose aminoglycans is upset [43, 44]. A positive effect of exogenic sulfate cannot be fully excluded.
Glucosamine is a monoaminosaccharide. It is synthesized in the body from glucose [45, 46]. The glucosamine molecular weight is 179.17, that of glucosamine hydrochloride is 215.63. Chemical concern Sigma-Aldrich produces glucosamine drugs as both hydrochloride and sulfate 98–99 % pure. The commercially produced crystalline drugs of glucosamine sulfate contain 78.5 % glucosamine on the average.
Unlike chondroitin sulfate, the mechanisms of effect of glucosamine sulfate are better known. It is believed that exogenically administered glucosamine sulfate intensifies the synthesis of proteoglycans being a substrate for the synthesis of chondroitin sulfate [47]. Glucosamine is a competitive inhibitor of glucose in the synthesis of chondroitin sulfate [48]. Glucosamine possesses immunity modulating properties. It prevents activation of T-lymphocytes in vitro [49]. Glucosamine produces a counterinflammatory effect on neutrophils, inhibits appearance of active forms of oxygen, hemotaxis and phagocytosis [50]. Thus, a certain progress of studies of the mechanism of effect of glucosamine sulfate has enabled to explain both the symptomatic and structure modifying effect of the drug.
According to the detailed review of pharmakinetics of glucosamine the bioavailability of glucosamine sulfate during peroral administration is 26 %, it approaches to 95 % after intramuscular administration [51]. It is believed that high doses of glucosamine are safe. It has been established that the therapeutic doses of the drug do not produce any significant effect on the metabolism of glucose [52, 53]. In the majority of clinical studies, the incidence of side effects during administration of glucosamine sulfate did not exceed that induced by a placebo [40].
Administration of glucosamine began in Europe in the 60s of the last century. A number of reviews dealing the clinical application of glucosamine report that the drug is an effective pain reliever and improves functions of knee joints in osteoarthrosis [41, 54–56]. The extent of improvement is comparable with that of non-steroid counterinflammatory therapeutical drugs with fewer side effects. It has been established in a number of recent studies that glucosamine sulfate inhibits the progress of osteoarthrosis, prevents restriction of articulation of the knee fissure [57–60]. This result is commonly achieved during administration of glucosamine sulfate in a dose 1,500 mg/day during 3 years [40, 57, 58]. Nevertheless, some researchers remark absence of any symptomatic effectiveness of glucosamine during prolonged administration [61].
Local therapy of the joint process is another necessary component of treatment of rheumatic diseases. In this connection, the effective component of ointments and gels administered for this purpose (butadionum, indometicinum, bruphenum, ketaprophenum, etc.) is attributed to the presence of NCID that both produces analgesic and counterinflammatory effects [6, 30].
A selection of drugs for intraarticular treatment of rheumatic diseases is highly limited and fails to satisfy the present-day requirements. Administration of corticosteroids is indicated primarily for stopping the rheumatoid or similar synovitis attacks and unacceptable for “dry” joints.
In case of the rheumatoid arthritis the drugs are administered intraarticularly that produce the immunodepressive effect (chlorineochinum, resochinum, delagilum) and cytostatics (cyclophosphanum) that perform the synoviortesis too. In the first case, the exhudative proliferative changes in the joint are reduced due to the cytotoxic and cytolytic effect on the synovial membrane. Synoviortesis (chemical synovectomy) relates to bloodless methods of destruction of the pathologically altered synovial membrane by intraarticular administration of radioactive colloids of gold, yttrium, radium, osmium oxides, etc. [62]. The arthritis is stopped by coagulating the synovial tissues followed b their restoration and partial sclerotherapy. Recently this method of treatment has lost its popularity because it has shown a significant destructive effect on the articular cartilage and a high incidence of arthritis relapses [63, 64].
Thus, at present there is a deficit of means and methods of general and local treatment of rheumatic diseases that would effectively protect the cartilage from destruction. Nevertheless, the features of enzymatic injury of the cartilage in rheumatic diseases are better studied and ways of overcoming this injury have been determined, meanwhile the processes of mechanodestruction of the cartilaginous tissue and possibilities of its prevention have been studied insufficiently. From this viewpoint there are worthwhile efforts to create artificial synovial fluids (lubricants) intended to produce effect in this link of pathogenesis disorders.
6.3 Problems of Developing Artificial Lubricants for Local Therapy of Joint Deceases
The study of the problems of therapeutic correction of the articular tribological parameters in case of pathologies has a comparatively short background. The first efforts to develop artificial lubricants can be referred to the late 60s, the period of intensive studies of the mechanism of friction and lubrication of joints. By this time, the idea existed already that the lubricating effect of the synovial fluid was due to the hyaluronic acid [65, 66]. In case of rheumatic diseases, because of enzymatic destruction of the latter, the rheological characteristics of the synovial fluid become strongly impaired and its secretion becomes deficient [67]. Therefore, in order to compensate temporarily the lacking synovial fluid and to protect the cartilage against mechanodestruction, it was proposed to test several biocompatible synthetic materials simulating some properties of the natural synovial fluid, in particular silicon organic (silicone) fluids and water-soluble polymers [68–71].
The experimental study of the silicon organic fluid as artificial lubrication of joints yielded no significant results because it was discovered that there is no statistically significant difference between its friction coefficient and that of the saline solution [68, 70]. It can be explained by its extremely high (300 cSt) and constant viscosity that would cause considerable shear forces during relative motion of cartilages. It is quite apparent that such behavior of the silicon organic fluid affected negatively the process of friction itself and was intolerable during osteoarthrosis when the muscular apparatus is incapable to overcome any extra exertion of joints.
A possibility was also considered of using polymers with the characteristics resembling those of the compounds of HUA with protein, such as polyethylene oxide (Fig. 6.5) [69]. The latter dissolves in water easily, it has a high molecular weight (100,000–6,000,000 c.u.) that permit to obtain rather viscous solutions with small concentrations. A microscopic study of the polymer frozen on thee slide under loading revealed that its structure is similar to the structure of the natural synovial fluid under the same conditions. However, the poor lubricity and thermomechanodestruction in the tribological tests did not permit to use polyethylene oxide as an artificial lubricant for treatment of joint pathologies. The experiments demonstrated a sharp viscosity drop leading to impairment of the antifriction behavior of this substance. Sterilization of the polymer also presented certain difficulties.
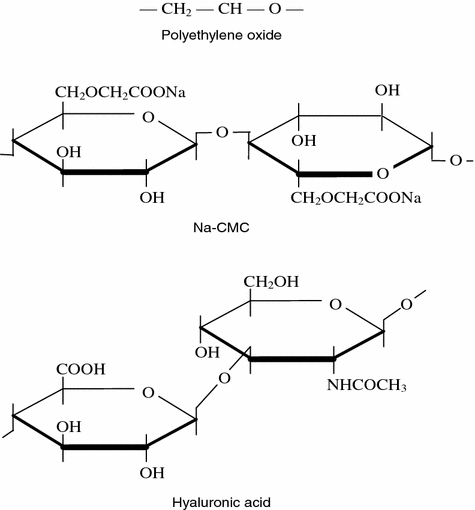

Fig. 6.5
Chemical structure of polyethylene oxide, sodium salts of carboxymethylcellulose and hyaluronic acid
Since the assumption that it would be possible to use polyethylene oxide, as an artificial lubricating material for joints did not prove true experimentally, researchers looked at another more complex compound. It was a sodium salt of carboxymethylcellulose (Na-CMC). It dissolves readily in water, biologically inert and low toxic, after a certain quality of purification it serves as an additive into foodstuffs. Aqueous solutions of Na-CMC have the alkaline medium and viscosity similar to the synovial fluid. Like all other compounds containing pyranose cycles, the structure of this natural polymer is similar to the HUA chemical structures (Fig. 6.5). Moreover, it was shown experimentally that Na-CMC yields insignificantly to mechanodestruction in friction in contrast to polyethylene oxide. As a result, the Na-CMC aqueous solutions served to develop a pseudosynovial fluid that, according to the patent [72], contained additionally saline and other components intrinsic for the blood plasma. However, the assumption of the researchers that it would be possible to use the latter expensively in the clinical practice as an artificial lubricant was not implemented because of its rather poor antifriction properties.
There had also been an effort of clinical application of polyvinylpyrrolidone (PVP) as a lubricating medium for therapeutic correction of articulation parameters in rheumatic diseases. It is a polymer of N-vinylpyrrolidone (γ-vinyllactam of N-aminobutyric acid). The PVP in the practical medicine serves as a blood substitute because of its low toxicity and absence of antigenic properties. This drug is known to be an agent prolonging the effect of antibiotics and hormones, a good desintoxicating and counterinflammatory means, a preservative of blood cells. Yet, they are not solely these properties that pointed to the possibility of PVP application for a new purpose.
It was shown experimentally that a 15 % PVP solution could simulate to some extent the rheological properties of the synovial fluid. It is believed that the latter can be improved significantly through joint application of this drug with HUA solutions. In this case other positive PVP properties are apparently implemented, such as the action of HUA as a protector against enzymic destruction in the pathological joint cavity. Whence followed an assumption that this drug can be used effectively as a lubricant and a means of suppression of negative effects of corticosteroids and an immunosuppressant during intraarticular administration.
However, the PP-containing drugs have negative in addition to positive properties. Their friction coefficient is an order of magnitude larger (0.05–0.08) than the extreme ultimate values typical for healthy joints. Besides, the PP-containing drugs, while having constant rheological characteristics, belong to Newtonian fluids; their viscosity does not depend in the shear rate, meanwhile natural synovial fluids belong to the thyrotrophic fluids; their viscosity, on the contrary, changes as the shear rate varies. Therefore, while the viscosity of the 15 % aqueous PVP solution is 1.8 MPa c and is determined exceptionally by the PVP concentration, the rheological characteristics of the natural synovial fluid depends typically both on the HUA concentration and the shear rate; therefore, the viscosity of the synovial fluid can vary within a rather broad range, vis. from 5–7 MPa s to several hundreds. Some difference is noteworthy between the pH values of PVP solutions (pH = 5.2…7.0) and the synovial fluid (pH = 7.4…8.2).
Hence, it can be assumed that the obtained experimental data about inhibition of cartilage degeneration in osteoarthrosis among animals are due rather to the counterinflammatory and immunoregulatory effect than the PVP lubricating properties. It is confirmed too in the work showing with the same experimental model a similar effect of PVP and dimethyl sulfoxide (DMSO) suppressing inflammation and inhibiting cartilage degeneration. The results of clinical tests that PVP intraarticular administration yields temporary improvement of functions of joints among 70–80 % osteoarthrotic patients, while it is less effective in rheumatoid arthritis.
The clinical practice has adopted in recent years the drugs based on the derivatives of hyaluronan (hyalhan, hyalart, synvisc, orthovisc) [73–79]. Hyaluronan is a polysaccharide (glycane) the molecules of which are long chains containing up to several hundreds or thousands of alternating disaccharides of two types: N-acetyl glycosamine glycane and glycouronate. Each glycouronate subunit has a negatively charged carboxyl group neutralized by Na+, K+, Ca++, and Mg++ ions [74]. The term ‘hyaluronan’ was introduced into biology and medicine for more precise definition of the complex compound that is called hyaluronic acid in chemical scientific publications.
The drug hyalhan was among the first to be tested; its biologically active substance is a derivative of hyaluronan—hylan (a sodium salt of hyaluronic acid) with a moderate molecular weight (0.5–1.0 mln) [76]. The representatives of the new generation of these drugs are synvisc and orthovisc. The drugs contain in the saline solution (pH = 7.2) liquid hylan A and gel-like hylan B with a high molecular weight (6 mln.), hence the elasticity and viscosity excel these characteristics of the natural synovial fluid.
The drugs are intended for intraarticular administration in order to substitute and replenish the synovial fluid in osteoarthrosis. In fact, it is hard to refer them to truly therapeutical means because they are just biological analogs of those substances with the inherent properties of the synovial fluid of a healthy person. After injections, the hyaluronan derivatives cannot stay in the joint cavity for a long time and can hardly maintain the optimal rheological properties synovial fluid independently for a long term. Moreover, in case of osteoarthrosis they can be vulnerable to enzymic and free radical degradation in the joint cavity [74]. Therefore, the positive clinical effect of the drugs is significantly attributed to the response of synovicytes to their administration and production of a fuller proper hyaluronan [74, 75, 78–80]. The pharmacological activity of the hyaluronan derivatives is explained also by the fact that many cells have receptors of these substances maintaining interrelations between and protection of cellular membranes from inflammation mediators and free radicals [74]. Hyaluronan in experiments inhibits hemotaxis and migration of leukocytes, reactions of phagocytosis, proliferation of lymphocytes and adhesion of monocytes, reduces the synthesis of prostaglandin E2 and bradykinin, modulates secretion of cytokines and inflammation mediators [75].
The therapeutical effect of the drugs based on hyaluronan derivatives is attributed to improvement of the rheological properties of articular lubrication and the physiological status of the intraarticular tissues, their capability to protect the articular cartilage from mechanical and enzymic destruction. The effect is observed after administration of five doses of hyalhan or three doses of synvisc with a 1-week interval. The positive clinical effect of the drugs persists up to 6–8 months. It is observed as reduction of the intensive pain syndrome and improvement of the joint functional status among 76–77 % patients. Better results are achieved at early stages of osteoarthrosis [76].
The drugs are capable to induce allergic reactions. Therefore, they should not be administered during aggravations of osteoarthrosis accompanied by the intraarticular effusion, in case of the arthritis of the inflammatory genesis, or injected into the circumarticular tissue, to administer to the patients with allergic reactions to proteins in the anamnesis, to pregnants or children. Cases are known when short-term pain syndromes, discomfort in joints and intraarticular exudation occurred after injection of the drugs.
Thus, the following facts characterize the early stages of development of pseudosynovial fluids to protect cartilages from destruction by rheumatic diseases.
All the proposed models of the synovial fluid replicate just in part its chemical (the pH of the medium) or physical (rheological and structural) properties. They can appear only under certain specific conditions (loads, shear rates, etc.); it is not enough to achieve low friction during a dynamic contact of cartilages in the joint where these conditions are variable within a rather broad range. The artificial lubricants are known that contain none of the components inherent to the natural synovial fluid; they are, in the first place, those that are responsible for the antifriction characteristics . So they can be considered only as temporary materials in the interface between rubbing cartilage surfaces devoid of the natural lubricating properties. Therefore, the only more realistic way of development of effective artificial lubricants is to create them on the basis of comprehensive analysis of the synovial medium in joints in order to identify and later adopt exactly those components that are truly capable to affect actively the biological mechanisms of reduction of the intraarticular friction and processes of mechanical and enzymatic destruction of the joint cartilage.
From this standpoint, it should be acknowledged a significant achievement of modern pharmacology and medicine that the drugs have been developed and comprehensively studied that are based on the hyaluronan derivatives similar to the natural synovial fluid. This trend of studies coincides with our trend and they both enable to expand the arsenal of means containing biologically and functionally active components of the synovial fluid for effective local therapy of disorders of joints [80, 81].
6.4 Liquid-Crystal Lubricants for Treating Joint Deceases and Similar Pathologies
Now it is certain that similarity of the tribological, rheological and structural properties of artificial lubricants with the natural synovial fluid is the most essential provision of successful replacement of the fluid in case of joint pathologies [82, 83]. This similarity of the properties when the deficit of the synovial fluid is to be replenished is capable both to ensure the same pattern of artificial and natural lubrication of joint cartilage surfaces in static and dynamic conditions and to influence actively the tribomechanical parameters of joints in rheumatic diseases. In this connection, using the accumulated experimental material and its theoretical explanation, it has been assumed that introduction of medicamentous means based on aqueous solutions of high-molecular polymers with thixotropic properties into the zone of friction of cartilages when articular lubrication is lacking, i.e. such that resemble the rheological properties of the natural synovial fluid and liquid crystalline cholesterol compounds capable of planar orientation in the joint cartilage contact, producing the mesophase within the range of physiological temperatures, the friction of cartilaginous surfaces will be identical to that under natural physiological conditions. This assumption was validated experimentally.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


