11 The innate immune response provides a rapid reaction to infections and, characteristically, the same magnitude of response each time the same pathogen is encountered (i.e. there is no learning in the innate system). The cells, proteins and peptides involved circulate in the blood of healthy individuals in sufficient amounts to overcome many trivial infections and contain more serious infections until an adaptive immune response can develop. The cellular components include neutrophils, eosinophils, basophils and macrophages, as well as tissue resident cells such as histiocytes and mast cells. The proteins and peptides of the innate response include complement, acute–phase proteins, chemokines and interleukins. The major functions of the most important components of the innate immune system are outlined in Table 11.1. The innate immune response causes a pathological condition known as inflammation, familiar to anyone who has ever had a cut finger. Acute inflammation is characterised by vascular changes including dilatation, enhanced permeability of capillaries and increased blood flow, resulting in the production of a fibrin-rich inflammatory exudate, thus bringing the proteins and cells required for early defence to the site of infection. Many of the cells and signalling molecules of the innate immune system are vital to the functioning of the adaptive immune system. TABLE 11.1 Major components of innate immunity FIG. 11.1 The organs of the immune system The thymus and bone marrow, where immature lymphocytes acquire the receptors to recognise antigen, are known as primary lymphoid organs. The spleen, lymph nodes and organised lymphoid tissues of MALT, where lymphocytes are activated in response to antigen, are the secondary lymphoid organs. Lymphocytes comprise some 20% to 50% of white cells in the circulation. Most circulating lymphocytes measure 6 to 9 µm (i.e. about the same size as erythrocytes) and are called small lymphocytes. About 3% are large lymphocytes, measuring 9 to 20 µm. The light and electron microscopic features of lymphocytes are described in Fig. 3.17. Briefly, small lymphocytes have a round to ovoid nucleus occupying about 90% of the cell volume, with a thin rim of basophilic (bluish) cytoplasm. The effectiveness of the adaptive immune system in recognising the huge range of pathogenic organisms found in nature depends upon the unique ability of lymphocytes to produce an equally huge range of antigen receptors i.e. the B cell receptor (BCR), comprising surface immunoglobulin (sIg) plus accessory molecules for B cells and the T cell receptor (TCR) for T cells. The ability of antibody to bind to antigen is determined by the physico-chemical properties of the antibody. Put simply, the shape and electrical charge of the binding site of the antibody must be complementary to the antigen, and the closer the fit of binding site to antigen, the stronger the bond formed and the greater the likelihood of the lymphocyte being stimulated. The TCR binds to antigen by similar reciprocity of shape and charge but it must also bind to the major histocompatibility complex (MHC) (see Figs 11.2 and 11.3). During maturation of lymphocytes, alternate components of the antigen-binding part of the antigen receptor genes are spliced together (rearranged) in a random fashion. Thus a huge range of possible antigen specificities are generated before the lymphocytes have a chance to meet external antigen. The best known subsets of T cells include: • T helper cells (TH cells). These T lymphocytes ‘help’ other cells to perform their effector functions by secreting a variety of mediators known as interleukins. TH cells thus provide ‘help’ to B cells, cytotoxic T cells (see below) and macrophages. TH cells can be subdivided into subgroups with different functions. TH1 cells tend to promote a cell-mediated reaction, important for defence against viruses and intracellular pathogens. TH2 cells are important for humoral (antibody mediated) responses and TH17 modify and augment certain types of acute inflammation. TH cells express the surface markers CD2, CD3 and CD4. • Cytotoxic T cells (TC cells). These lymphocytes are able to kill virus-infected and some cancer cells. They require interaction with TH cells to become activated and proliferate to form clones of effector cells. TC cells express CD2, CD3 and CD8. • Regulatory T cells (TREG). These cells suppress immune responsiveness to self-antigens (autoimmunity) and switch off the response when antigen is removed. These cells usually express CD4 and FOXP3. • Memory T cells develop from activated T cells to provide a ‘rapid reaction force’ for a subsequent encounter with the same antigen. This is the basis of persisting immunity after infection with some organisms and also the basis of vaccination. • γδ T cells are a subset of T cells where the TCR is a heterodimer consisting of one γ chain and one δ chain, rather than the usual heterodimer of one α and one β chain. These cells populate the epithelium of the gastrointestinal tract and are CD8 positive. Once activated, the B cell undergoes mitotic division to produce a clone of cells able to synthesise immunoglobulin of the same antigen specificity. Most of the B cells of such a clone mature into plasma cells. When an antigen is encountered for the first time, this is described as the primary immune response. A few cells from the same clone mature to become memory B cells, small long-lived circulating lymphocytes that are able to respond quickly to any subsequent challenge with the same antigen. Antibody production during this secondary immune response occurs much more rapidly, is of much greater magnitude and produces IgG rather than IgM. This phenomenon explains the lifetime immunity that follows many common infections; it is also the general principle on which vaccination is based. Antibodies neutralise or destroy invading organisms by a number of methods (see Fig. 11.2). FIG. 11.2 The basics of the immune response (illustration opposite) 1. Activation of T cells is dependent on antigen presenting cells APC. The antigen is taken up by an APC (e.g. macrophage, B lymphocyte, dendritic cell, Langerhans cell of skin) and broken down to short peptides (see Fig. 11.3). Processed antigen PA is then bound to a major histocompatibility complex molecule MHC, and the MHC-peptide complex is incorporated into the cell membrane so that the bound antigenic peptide is exposed to the extracellular fluid. Contact with a mature T cell bearing a T cell receptor with appropriate specificity activates the T cell. The type of response depends on whether the peptide is presented bound to MHC class I or II. Antigenic peptides bound to class II MHC molecules induce a T helper cell TH response needed to activate B cells B and cytotoxic T cells TC. B cell receptors (sIg) or TC receptor must also bind to the antigen for activation to occur. TH cells secrete a variety of interleukins IL that mediate activation, clonal expansion and maturation of the B or cytotoxic T cell response. Generation of effector mechanisms • Antibody is essential for antibody-dependent cell cytotoxicity (ADCC) (see below). • Antibody bound to toxins inactivates them and facilitates their removal by phagocytic cells. Termination of the immune response FIG. 11.3 Lymphocytes and antigen presenting cells (illustration (b) opposite) The functions of the thymus include: • Development of immunocompetent T lymphocytes from bone marrow–derived T cell precursors to produce mature TH and TC cells. • Proliferation of clones of mature naïve T cells to supply the circulating lymphocyte pool and peripheral tissues. • Development of immunological self-tolerance. More than 98% of maturing cells die by apoptosis within the thymus, and many of these are self-reactive. • The thymus secretes various polypeptides with hormonal characteristics, including thymulin, thymopoietin and various thymosins. These hormones regulate T cell maturation, proliferation and function within the thymus and peripheral lymphoid tissues. They also interact with other endocrine systems in the regulation of inflammation. FIG. 11.4 Thymus
Immune system
Introduction
The innate immune system
Component
Actions
Neutrophil polymorphs
Phagocytosis and killing of pathogenic organisms
Secretion of cytokines and extracellular antimicrobial molecules, including neutrophil extracellular traps (NETs) and pattern recognition molecules (PRMs)
Macrophages
Phagocytosis and killing of pathogenic organisms, removal of foreign material and dead cells
Secretion of a wide range of cytokines and interleukins
Present antigen to lymphocytes (adaptive immune system)
Eosinophils
Destroy larger multicellular pathogens
Modulate allergic responses
Natural killer (NK) cells
Recognise and kill virus-infected and cancerous cells
Complement
Opsonises organisms to facilitate phagocytosis
Chemoattractant for various cells
Membrane attack complex (MAC) kills cells by puncturing plasma membrane
Acute-phase proteins
Plasma proteins which are increased during inflammation e.g. C-reactive protein (CRP)
Wide range of actions that promote defense against pathogens
Chemokines
Recruit cells to specific sites and activate cells of innate and adaptive immune systems
Induce differentiation of cells to more active and effective subtypes
Wound healing and angiogenesis
Interleukins
Signalling molecules produced by many cell types, including macrophages, dendritic cells, lymphocytes
Regulate the immune system
The adaptive immune system
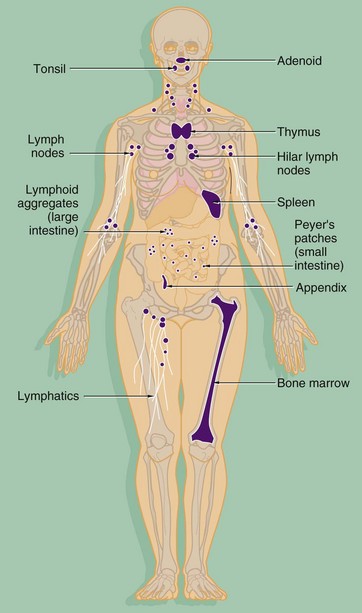
The components of both the innate and adaptive systems are found throughout the body. The lymphocytes of the adaptive immune system are produced in the bone marrow from haematopoietic stem cells along with the cells of the innate system (see Ch. 3). As well as circulating in the blood, the cells of the adaptive immune system form specialised lymphoid tissues and also constitute a significant component of other tissues such as the gastrointestinal tract. The major lymphoid organs include:
Lymphocytes
The role of T lymphocytes
The role of B lymphocytes
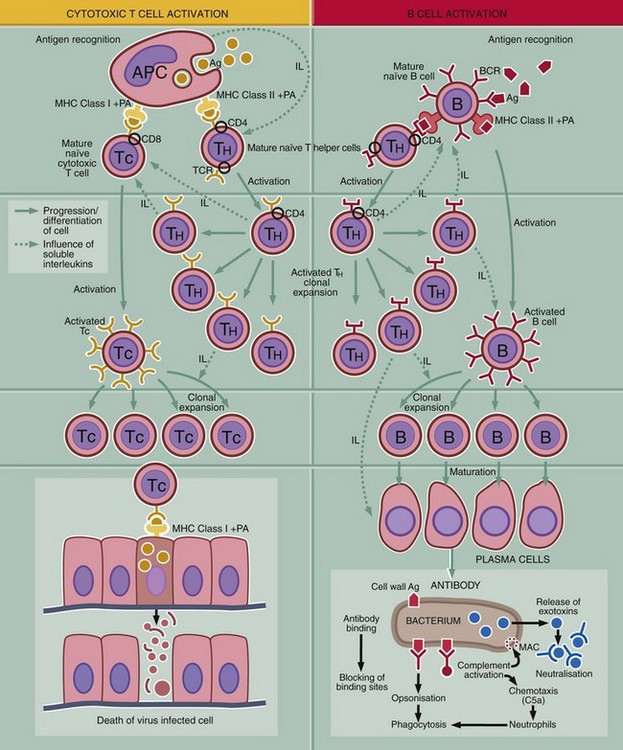
This diagram outlines the key steps in the adaptive immune response, i.e. recognition of antigen, activation of the response, generation of effector mechanism and destruction or inactivation of the antigen.
Recognition of antigen
T and B cells carry antigen receptors on their surface, the T cell receptor (TCR) and B cell receptor (BCR). The BRC consists of surface immunoglobulin plus certain accessory molecules. Random rearrangement of the genes for the variable region of the receptor molecules gives rise to receptors with a truly staggering range of antigen binding sites. Each individual T or B cell has specificity for only one antigen, but the entire population is very varied.
Activation of the immune system
Initiation of an immune response first requires contact between antigen Ag and surface receptors on mature lymphocytes. There are several mechanisms of activation:
There are a number of mechanisms for switching off the immune response when the need for it has been removed. These include removal of antigen, the short life span of plasma cells, the activities of regulatory T cells and a variety of other mechanisms that downregulate the activity of T and B cells. It is vital that the immune response is terminated when no longer needed to prevent damage to normal tissue from an overenthusiastic immune response. These mechanisms are also important in the prevention of autoimmunity.
Immunological memory
When activated lymphocytes undergo clonal expansion during an immune response, some of the cells so generated mature to become memory T and B cells. These lymphocytes have a similar appearance to naïve lymphocytes but are able to produce a faster and more effective response to a smaller quantity of antigen. This is known as a secondary immune response and is the basis of lifelong immunity after certain infections and of vaccination.
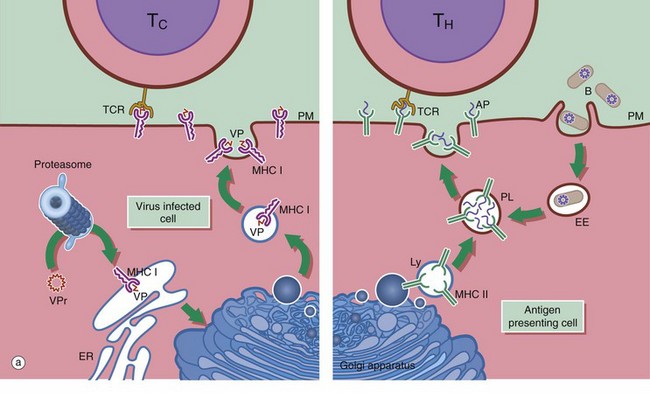
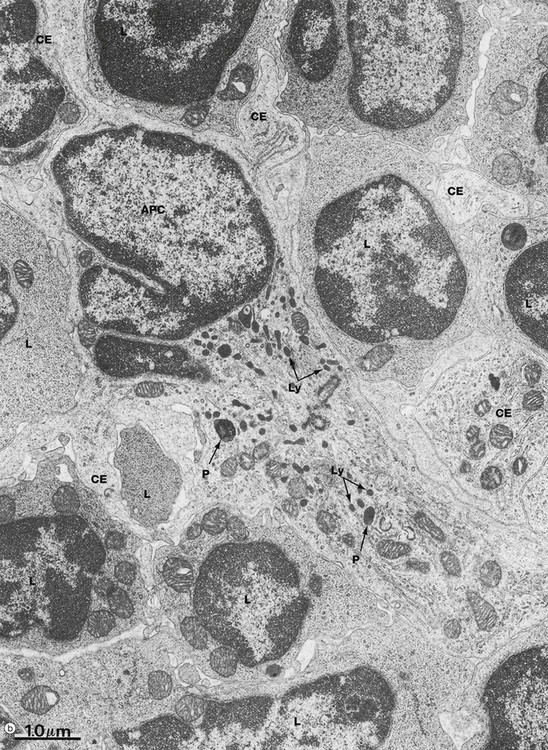
(a) Schematic diagram (b) EM ×18 000
Antigen presenting cells APC are vital for the activation of lymphocytes to produce an adaptive immune response. They include macrophages, dendritic cells and B lymphocytes. Dendritic cells patrol the body surfaces and phagocytose invading pathogens. Dendritic cells are versatile and potent APCs. Some appear to be resident in the lymph node while others, carrying antigen from peripheral tissues, migrate in the lymph to the regional lymph nodes. Dendritic cells are found in the paracortical area of lymph nodes. This group of cells also includes interdigitating cells of the thymus and Langerhans cells of the skin. Follicular dendritic cells are accessible to B cells in the germinal centres of lymph nodes. They are similar cells which are able to bind antibody-antigen complexes to their surface without prior processing.
APC function is shown on the right side of diagram (a). Antigen (e.g. a bacterium B) is taken up by APCs into an early endosome EE that fuses with a lysosome containing major histocompatibility complex class II molecules MHC II. The antigen is broken down into short antigenic peptides AP that bind to MHC II and the peptide-MHC II complex is transported to the plasma membrane. After fusion of the phagolysosome PL with the plasma membrane PM, the MHC II-peptide complex is exposed on the cell surface where it may come into contact with helper T cells TH. If the T cell receptor TCR on the TH cell can bind to that particular peptide-MHC II complex, activation will occur and the adaptive immune response will proceed. Obviously, processing of a bacterium will generate many different antigenic peptides, but only one peptide and one TH cell is shown here for simplicity.
In general, TH cells recognise peptide bound to MHC II and cytotoxic T cells TC recognise antigen bound to MHC class I MHC I. On the left of diagram (a), processing of intrinsic viral antigen in a virus-infected cell is shown. The viral protein VPr is chopped into short peptides VP by a proteasome (an organelle that breaks down abnormal proteins). The peptides bind to MHC I and are presented on the cell surface for interaction with a TC. Almost all body cells express MHC I but usually only APCs express MHC II.
Micrograph (b) illustrates several lymphocytes and an APC in a lymph node. Lymphocytes and APCs exhibit similar features in other lymphoid tissues. The lymphocytes L are relatively small with round nuclei and condensed chromatin that tends to be clumped around the periphery of the nucleus. Cell outlines are fairly regular with occasional surface projections. The scanty cytoplasm contains plentiful free ribosomes and a few mitochondria but little endoplasmic reticulum, lysosomes or secretory granules.
The centre of the field is occupied by the large cell body of an antigen presenting cell APC, in this case a dendritic cell. These have numerous long branched cytoplasmic extensions CE reaching out between the surrounding lymphocytes so that a single dendritic cell can be in contact with many different lymphocytes. Its nucleus is deeply indented with dispersed chromatin; in this example, the plane of section has resulted in a small nuclear extension appearing to be separate from the main part of the nucleus. Typically, the APC cytoplasm contains numerous small lysosomes Ly and larger phagosomes P.
Thymus
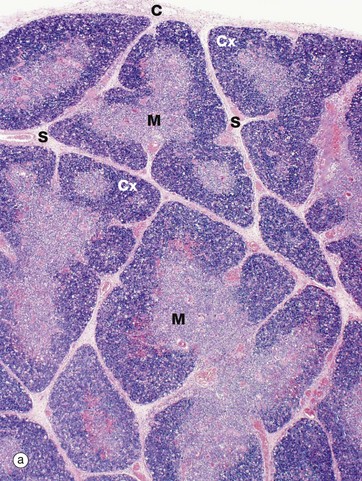
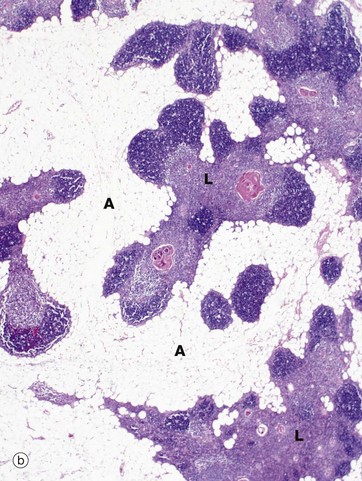
(a) Infant, H&E (LP) (b) Adult, H&E (LP)
The infant thymus (a) is a lobulated organ invested by a loose collagenous capsule C from which interlobular septa S containing blood vessels radiate into the substance of the organ. The thymic tissue is divided into two distinct zones, a deeply basophilic outer cortex Cx and an inner eosinophilic medulla M; distinction between the two is most marked in early childhood, as in this specimen.
In the adult (mid-30s in this case), the thymus (b) is already well into the process of involution, which involves two distinct processes, fatty infiltration and lymphocyte depletion. Fat cells (adipocytes) first begin to appear at birth, their numbers slowly rising until puberty when the rate of fatty infiltration increases markedly. Fatty infiltration of the interlobular septa occurs first, spreading out into the cortex and later the medulla. Thus, in the mature thymus islands of lymphoid tissue L are separated by areas of adipose tissue A. At this age, the cortex and medulla can still be differentiated. In the elderly, the thymus can be very difficult to detect both macroscopically and microscopically, with only small islands of lymphoid tissue lost in a sea of adipose tissue. Lymphocyte numbers begin to fall from about 1 year of age, the process continuing thereafter at a constant rate. Despite this, the thymus continues to provide a supply of mature T lymphocytes to the circulating pool and peripheral tissues. Lymphocyte depletion results in collapse of the epithelial framework. However, cords of epithelial cells persist and continue to secrete thymic hormones throughout life.
The normal process of slow thymic involution associated with aging should be distinguished from acute thymic involution, which may occur in response to severe disease and metabolic stress associated with pregnancy, lactation, infection, surgery, malnutrition, malignancy and other systemic insults. Stress involution is characterised by greatly increased lymphocyte death and is probably mediated by high levels of corticosteroids; thus the size and activity of the adult thymus are often underestimated if examined after prolonged illness.
Numerous small branches of the internal thoracic and inferior thyroid arteries enter the thymus via the interlobular septa, branching at the corticomedullary junction to supply the cortex and medulla. Postcapillary venules in the corticomedullary region have a specialised cuboidal endothelium similar to that of the high endothelial venules of the lymph node (see Fig. 11.11), which allows passage of lymphocytes into and out of the thymus. The venous and lymphatic drainage follow the course of the arterial supply; there are no afferent lymphatics. Sympathetic and parasympathetic nerve fibres derived from the sympathetic chain and phrenic nerves, respectively, accompany the blood vessels into the thymus.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine
