17 • Protein and glycoprotein molecules, e.g. insulin, growth hormone, parathyroid hormone (PTH). • Small peptide molecules, e.g. vasopressin, products of enteroendocrine cells. • Amino acid derivatives, e.g. thyroxine, adrenaline (epinephrine) and noradrenaline (norepinephrine). • Steroids derived from cholesterol, e.g. adrenal cortical hormones, ovarian and testicular hormones. The endocrine system can be divided into three parts: • The major endocrine organs in which the sole or major function of the organ is the synthesis, storage and secretion of hormones (e.g. thyroid and adrenal glands) • Endocrine components within other solid organs, for example, the endocrine components of the pancreas, ovary, testis and kidney, in the form of clusters of endocrine cells within other tissues • The diffuse endocrine system, scattered individual hormone cells (or small clumps), usually within an extensive epithelium (e.g. gastrointestinal and respiratory tracts). The major function of these cells is probably paracrine (i.e. acting on adjacent non-endocrine cells, rather than entering the bloodstream and producing systemic effects). The pituitary hormones fall into two functional groups: • Hormones which act directly on non-endocrine tissues: growth hormone (GH), prolactin, antidiuretic hormone (ADH, vasopressin), oxytocin and melanocyte stimulating hormone (MSH). • Hormones which modulate the secretory activity of other endocrine glands (trophic hormones): thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH) and the gonadotrophic hormones, follicle stimulating hormone (FSH) and luteinising hormone (LH). FIG. 17.1 Pituitary gland FIG. 17.2 Pituitary gland, monkey FIG. 17.3 Anterior pituitary (illustration (d) opposite) In general, one cell produces a single hormone, except for gonadotrophs, which mostly produce both LH and FSH. The different cell types are not evenly distributed throughout the gland, but rather particular cell types tend to congregate in particular zones of the gland. FIG. 17.4 Pituitary, pars intermedia FIG. 17.5 Posterior pituitary • Iodine-containing hormones tri-iodothyronine (T3), and thyroxine (tetra-iodothyronine, T4); T4 is converted to T3 in the general circulation by removal of one iodothyronine unit, although a small amount of T3 is secreted directly. T3 is much more potent than T4 and appears to be the metabolically active form of the hormone. Thyroid hormone regulates the basal metabolic rate and has an important influence on growth and maturation, particularly of nerve tissue. The secretion of these hormones is regulated by TSH secreted by the anterior pituitary. • The polypeptide hormone calcitonin; this hormone regulates blood calcium levels in conjunction with parathyroid hormone. Calcitonin lowers blood calcium levels by inhibiting the rate of decalcification of bone by osteoclastic resorption and by stimulating osteoblastic activity. Control of calcitonin secretion is dependent only on blood calcium levels and is independent of pituitary and parathyroid hormone levels. FIG. 17.6 Thyroid gland
Endocrine system
Introduction
Pituitary Gland
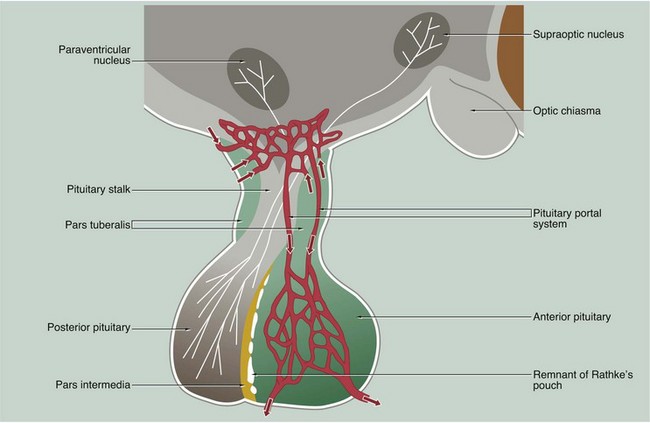
The anterior and posterior parts of the pituitary originate from different embryological sources and this is reflected in their structure and function.
The posterior pituitary, also called the neurohypophysis or pars nervosa, is derived from a downgrowth of nervous tissue from the hypothalamus, to which it remains joined by the pituitary stalk.
The anterior pituitary arises as an epithelial upgrowth from the roof of the primitive oral cavity known as Rathke’s pouch. This specialised glandular epithelium is wrapped around the anterior aspect of the posterior pituitary and is often called the adenohypophysis. The adenohypophysis may contain a cleft or group of cyst-like spaces which represent the vestigial lumen of Rathke’s pouch. This vestigial cleft divides the major part of the anterior pituitary from a thin zone of tissue lying against the posterior pituitary known as the pars intermedia. An extension of the adenohypophysis surrounds the neural stalk and is known as the pars tuberalis.
The type and mode of secretion of the posterior pituitary differs greatly from that of the anterior pituitary. The posterior pituitary secretes two hormones, antidiuretic hormone (ADH), also called vasopressin or arginine vasopressin, and the hormone oxytocin, both of which act directly on non-endocrine tissues. ADH is synthesised in the neurone cell bodies of the supraoptic nucleus, and oxytocin is synthesised in those of the paraventricular nucleus of the hypothalamus. Bound to glycoproteins, the hormones pass down the axons of the hypothalamopituitary tract through the pituitary stalk to the posterior pituitary where they are stored in the distended terminal parts of the axons. Release of posterior pituitary hormones is controlled directly by nervous impulses passing down the axons from the hypothalamus, a process known as neurosecretion.
Hypothalamic control of anterior pituitary secretion is mediated by specific hypothalamic releasing hormones, such as thyroid stimulating hormone releasing hormone (TSHRH); exceptions to this rule are prolactin secretion, which is under the inhibitory control of dopamine, and secretion of growth hormone, which is controlled by both releasing and inhibitory hormones. These releasing and inhibitory hormones are conducted from the median hypothalamic eminence to the anterior pituitary by a unique system of portal veins (pituitary portal system).
The pars intermedia synthesises and secretes melanocyte–stimulating hormone (MSH); in humans, the pars intermedia is rudimentary and the physiological importance of MSH and the control of its secretion are poorly understood.
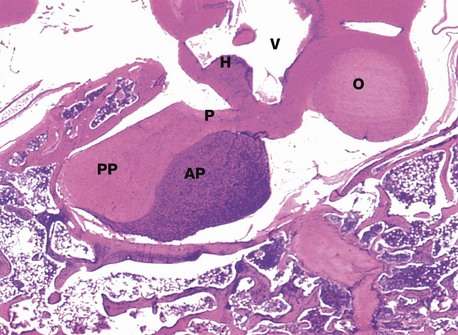
H&E (LP)
This micrograph from a midline section through the brain and cranial floor illustrates the pituitary gland in situ. The pituitary sits in a bony depression in the sphenoid bone called the sella turcica. The two major components of the gland, the anterior pituitary AP and the posterior pituitary PP, are easily seen at this magnification. The posterior pituitary is connected to the hypothalamus H by the pituitary stalk P and, like the hypothalamus, is composed of nervous tissue. Note the close proximity of the third ventricle V above the hypothalamus and the optic chiasma O anteriorly.
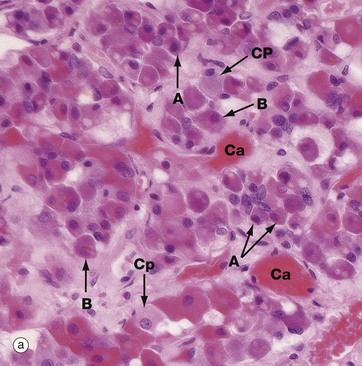
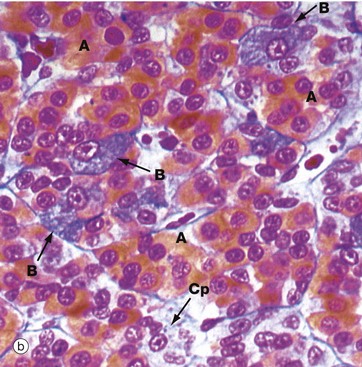
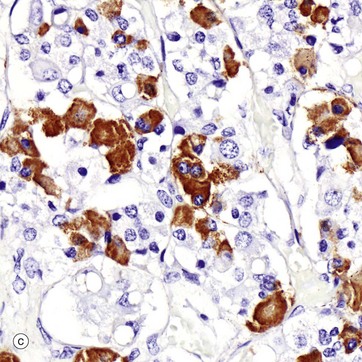
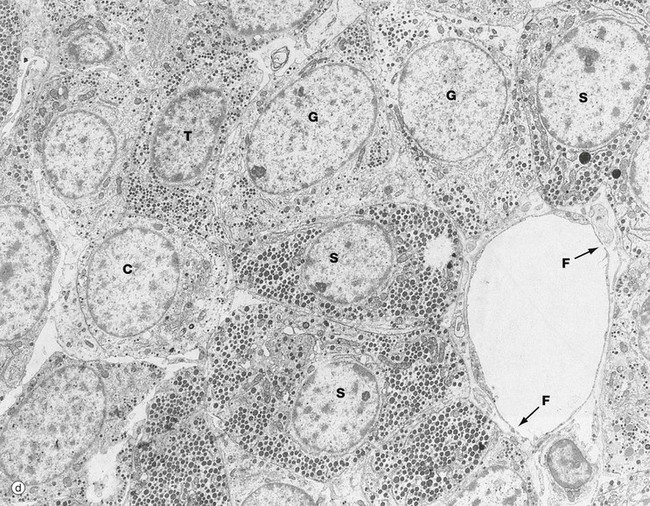
(a) H&E (HP) (b) Azan (HP) (c) Immunohistochemical method for GH (HP) (d) EM ×4270
Micrograph (a) is an H&E-stained preparation of anterior pituitary and shows two main populations of cells, those with strongly staining cytoplasm (chromophils) and those with weakly staining cytoplasm (chromophobes Cp). The chromophils can be separated further into basophils B and acidophils A based on their cytoplasmic staining properties. This is more easily seen in micrograph (b). Note the prominent capillaries Ca lying between clumps of secretory cells. The most accurate identification of cell types is given by immunohistochemical methods and electron microscopy. The number of granules in the cytoplasm of these cells may depend on whether they are in a resting phase or actively secreting. These methods show that chromophobes have very few secretory granules but may produce small amounts of any of the hormones. Chromophobes probably represent cells at the end of a secretory phase, rather than a distinct cell.
Micrograph (c) shows a section of anterior pituitary stained by the immunohistochemical technique for growth hormone (GH). The brown-stained GH-containing cells can be seen scattered at random among the other cell types.
The different cell types are now named as follows:
The secretory granules of each cell type have a characteristic size, shape and electron density by which the different cell types can be recognised with electron microscopy as in micrograph (d). Somatotrophs S are packed with secretory granules of moderate size. Thyrotrophs T have smaller granules which tend to be more peripherally located. Gonadotrophs G are large cells with secretory granules of variable size. Corticotrophs C have sparse secretory granules located at the extreme periphery of the cell.
The clumps and cords of cells have a rich capillary network. The endothelial lining of capillaries in endocrine tissue is characteristically fenestrated (see Fig. 8.16), facilitating the passage of hormones into the sinusoids. Note the fenestrations F in the sinusoid seen in micrograph (d).
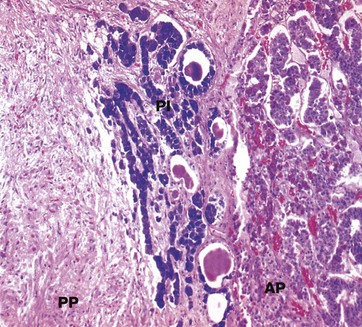
Isamine blue/eosin (MP)
The pars intermedia PI, like the anterior pituitary, is derived embryologically from Rathke’s pouch. The cells are basophilic (stained blue here), lying in irregular clusters between the anterior AP and posterior PP pituitary. The pars intermedia also contains small cystic spaces filled with eosinophilic material.
Ultrastructurally, the cells of the pars intermedia contain secretory granules similar to those of corticotrophs. These cells produce α-MSH from pro-opiomelanocortin, usually at low levels.
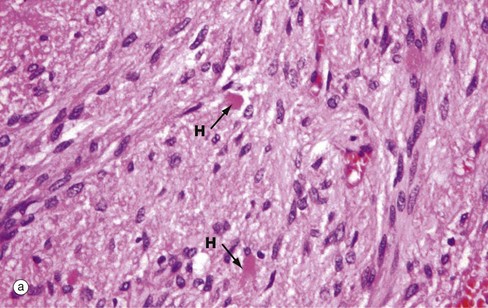
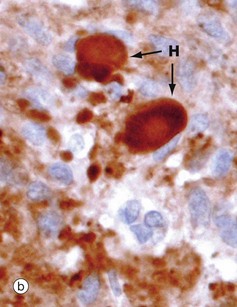
(a) H&E (MP) (b) Immunohistochemical method for synaptophysin (HP)
The posterior pituitary is largely composed of the non-myelinated axons of specialised neurones which have considerable neurosecretory activity. The cell bodies of these neurones are located in the supraoptic and paraventricular nuclei of the hypothalamus and it is here that the posterior pituitary peptide hormones oxytocin and ADH are produced. They are passed down the axons in neurosecretory granules which accumulate in the distended terminations of the axons where they contact capillaries. These distensions are called Herring bodies H. The axons are supported by specialised highly branched glial cells called pituicytes, the cytoplasm of which sometimes contains small amounts of yellowish-brown pigment.
Micrograph (a) shows the structure of posterior pituitary; the fibrillar structures are the axons of the hypothalamic neurones with distended terminal Herring bodies H. The nuclei are those of supporting pituicytes. Micrograph (b) is an immunohistochemical preparation for neurosecretory granules (synaptophysin). Although granules are scattered in the axons, they are particularly concentrated in the round Herring bodies H.
Thyroid Gland
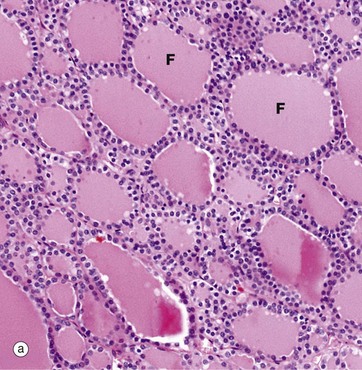
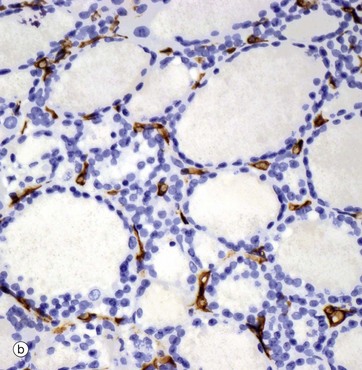
(a) H&E (LP) (b) Immunohistochemical method for CD34
The functional units of the thyroid gland are the thyroid follicles F, spheroidal structures composed of a single layer of cuboidal epithelial cells, bounded by a basement membrane (see also Fig. 5.27). As seen in this micrograph of a normal thyroid, the follicles are variable in size and contain a homogeneous colloid, which is stained pink in this preparation.
The thyroid gland is enveloped by a fibrous capsule from which fine collagenous septa (not shown in this micrograph) extend into the gland, dividing it into lobules. The septa convey a rich blood supply, together with lymphatics and nerves. Tiny capillaries percolate through the thyroid tissue and surround the follicles and, although these are difficult to see in an H&E preparation, they can be highlighted using an immunohistochemical method for an endothelial marker (CD34), as seen in micrograph (b).
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine
