Physiological processes (e.g. nervous conduction, the release of transmitters and hormones, muscle contractility) depend on cell excitability. Therefore, excitability and excitation of cells is tightly regulated.
Resting membrane potential (Vm)
Each cell in the body has an electrical potential difference which is determined by the distribution of ions across the cell membrane (Chapter 2). The potential difference across the membrane is the membrane potential. In the resting state the inside of the cell is negative on the intracellular side with respect to the outside, and this is the resting membrane potential (Figure 18.1). A typical value for a neurone is −70 mV, for skeletal muscle it is −90 mV. The resting membrane potential is determined by ionic balances across the cell membrane. The movement of ions is determined by both their electrical gradients and chemical gradients as driving forces; this is the electrochemical potential gradient. The ionic balance is governed by relative membrane permeability. For example, if we consider K+ and organic anions, both have high intracellular concentrations and the chemical gradient favours their movement out of the cell. However, the membrane is more permeable to K+ compared with the organic anions, and so the net efflux is K+ unaccompanied by the anions. This results in the cell becoming more negative with respect to the outside. The balance of electrical and chemical work for K+ is summarised by the Nernst equation:
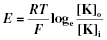
where: E = membrane potential; [K]o = extracellular potassium; R = gas constant; [K]i = intracellular potassium; T = Absolute temperature; F = Faraday’s constant.
At physiological temperatures the equation can be simplified to:
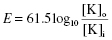
When the electrical and chemical gradients are in balance there is no net flux of ions and E is the Nernst potential for the ion in question (e.g. for K+ it is approximately −90 mV; for Na+ it is approximately +65 mV).
As the membrane potential is governed by a number of ions, the equation can be modified to include each ion (the Goldman field equation), but the contribution of each is determined by membrane permeability:
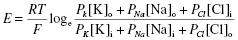
where Pion takes account of the ion’s permeability.
In the resting state, the cell membrane is substantially more permeable to K+, such that the membrane is typically 70- to 100-fold more permeable to K+ compared with Na+. The consequence of this is that the resting membrane potential of cells lies close to the equilibrium potential for K+ (approx. −90 mV). Hence the major determinant of membrane potential is the extracellular K+ concentration. By contrast, as the membrane is relatively impermeable to Na+ in the resting state then changes in extracellular Na+ concentration have little impact on the resting membrane potential.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


