Classic (Medullary Epicenter)
Osteosarcoma is one of the most common malignant tumors of adolescence, exceeded by only leukemias, brain tumors, and lymphomas (
39). There are roughly 8.2 cases per million population, with less than 1,000 new cases each year in the United States. It is a highly malignant tumor, which, by definition, produces neoplastic osteoid or bone or both. Osteoid is type I collagen, which, in the normal sequence of events, being bathed in bone-forming proteins, will form bone. Osteosarcoma characteristically arises within the metaphysis of the long bones that are the sites of the most rapid growth and greatest blood flow: the distal femur, proximal tibia, and proximal humus. It grows circumferentially through the cortex into the soft tissue raising the periosteum. It rarely invades the joint space. Fifty-six percent of all osteogenic sarcomas occur at the knee, resulting in its being the most common primary osseous knee tumor reported in the literature (32 percent) (
Fig. 9.15). Of osteogenic sarcomas of the knee, 64 percent occur in the distal femur, 32 percent in the proximal tibia, 4 percent in the proximal fibula, and less than 1 percent in the patella.
Given the predilection of osteosarcoma occurring around the knee, the initial misdiagnosis of an athletic injury such as a meniscal lesion at this site has been shown in several studies (
40).
Osteogenic sarcoma, for which there is an animal model in the Great Dane, has a seeming predilection for areas of rapid growth (
41). It peaks, as mentioned, in the adolescent growth spurt, and is also seen with increased incidence in bone affected by Paget’s disease.
Osteogenic sarcoma characteristically occurs at the adolescent growth spurt, with the peak age of occurrence between 10 and 20 years of age; 75 percent of all cases occur between 10 and 30 years of age. There is a male predominance of 1.5:1. In the immature skeleton with an intact growth plate, the epiphysis may act as a relative barrier to its growth. Microscopic and MRI evidence of transepiphyseal spread is more common than appreciated on routine roentgenograms. Osteogenic sarcoma typically presents with pain, which is often mild and intermittent initially, but more continuous and exacerbated by deep palpation later. Pain is the most common presenting symptom occurring in about 80 percent of patients. It is usually exacerbated by activity with less than one quarter of patients showing pain at night. A mass or a swelling may be felt. Patient may present with a pathologic fracture. In general, patients are symptomatic for several weeks before coming to clinical attention. On examination, a palpable mass may be felt. Large lesions may lead to overlying venous engorgement or edema.
The laboratory workup of osteosarcoma would include alkaline phosphatase (almost always elevated) and lactate dehydrogenase (may be elevated). imaging workup of osteosarcoma includes biplanar radiographs, CT, and Prebiopsy MRI of the involved site. A whole-body three-phase bone scan should also be done.
A postbiopsy chest CT is employed to look for metastatic disease.
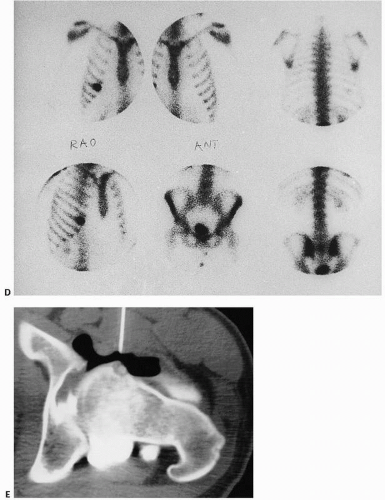
The characteristic radiograph reveals a radiodense or mixed radiolucent and radiodense lesion over the metaphysis with indistinct borders and periosteal elevation (
Fig. 9.16). The raised periosteum creates a triangle (referred to as Codman triangle), whose borders are the intact cortex, the tumor, and the periosteum proper. CT scans may be helpful in defining soft tissue or joint penetration, with MRI defining the exact extent of involvement of the cancellous and medullary bone and soft tissue.
By definition, osteosarcoma is bone forming. However, bone production may not be roentgenographically evident in all cases. Although blastic lesions predominate, mixed lesions with patchy lysis and sclerosis admixed may be seen. Telangiectatic osteosarcomas (TOSs) are lytic.
The MRI pattern of a classic osteosarcoma is low signal onT1-weighted image.
Tumor bone formation is seen as dense areas in the metaphyses. Usually, these areas of increased density are not well defined. The tumor bone as it breaks through the cortex of the bone will project close to or over the new periosteal reactive bone. The description of “cloud formation” has been applied to the tumor bone seen in osteogenic sarcomas.
Osteogenic sarcomas are always seen as areas of markedly increased concentration of radioactivity on radionuclide bone scans regardless of their sclerotic, lytic, or mixed radiographic pattern.
CT and MRI scans can be helpful in demonstrating the soft tissue component, which is often present in cases of osteogenic sarcomas. The MRI scan is also helpful in demonstrating “skip lesions” in the marrow cavity of the bone. These are seen as areas of diminished signal intensity because the normal marrow fat has been replaced by the neoplasm. CT scans can also be used for this purpose, however, with less sensitivity than MRI scans. Skip lesions can also be demonstrated with radionuclide bone scans.
Imaging techniques are useful in assessing medullary tumor spread in osteosarcoma to precisely plan surgical resection. In this regard, MRI is more sensitive than CT scan (
42).
PET scans have recently been employed to help identify metastatic disease (especially in Ewing), and to help differentiate benign from malignant tumors and benign versus malignant pathologic fractures based on maximum standardized uptake values (SUVmax). Although overlapping values are noted, cutoffs for
a malignant pathologic fracture (SUVmax 12.0, range 4 to 45 for malignant) versus benign (SUVmax 2.9, range 0.6 to 5.5) has been used (
43). For distinguishing benign tumors from malignant ones, a SUVmax of 6.8 ± 4.7 for malignant tumors and 4.5 ± 3.3 for benign lesions have been used, notably not statistically significant findings (
44). PET scanning has also been used to evaluate tumor necrosis after chemotherapy with high SUVmax values in one study correlated with poor survival (
45).
The periosteal reaction surrounding an osteogenic sarcoma can be linear, multilayered, or dense, or have a “sunburst” appearance. New bone formation may appear as spicules radiating perpendicularly from the involved bone. The “onion-skin” appearance often seen in Ewing sarcoma is unusual in osteogenic sarcomas.
Pathologic fractures through osteogenic sarcomas are seen at times, though not as commonly as with other skeletal lucent lesions. In these cases, the radiographic and histologic diagnosis can be difficult because of the overlap of tumor bone with callus.
At times, ABCs are superimposed on osteogenic sarcomas. These cysts are demonstrated as expansile lesions, often with septations. The differentiation between an ABC and the underlying osteogenic sarcoma (especially the lytic type) can be difficult. ABCs are often seen as areas of increased signal intensity on the T2-weighted images of MRI scans. Increased signal intensity, however, can also be seen in areas of tumoral necrosis.
The laboratory hallmark of an osteogenic sarcoma is an elevated alkaline phosphatase, seen in more than 50 percent of children, usually in excess of that noted during pediatric growth. Some have argued that pretreatment serum alkaline phosphatase measurements are of prognostic significance, those with higher values tending to relapse (
46). Other esoteric lab tests, such as monoclonal antibodies to recombinant bone morphogenic protein (BMP) are still experimental in nature. In one study, as expected, over half of the osteosarcomas studied were positive for BMP, whereas chondrosarcomas and Ewing sarcomas were negative (
47). Clohisy et al. (
48) have assessed the nucleolar organizer regions (large loops of DNA located in the nucleolus that contain RNA genes) and found higher values in malignant tumors, but could not differentiate those that metastasize. Scotlandi et al. (
49) have found Ki-67 monoclonal antibody (a specific nuclear antigen marker for proliferative cells) to predict biologic aggressiveness in high-grade osteosarcomas.
The hallmark in diagnosing osteosarcoma is by histologic examination of biopsied tissue, a procedure not without risk. Recurrence of osteosarcoma can occur in a needle biopsy tract (
50) and may compromise definitive surgery if not planned properly (
51). In addition, the dissemination of tumor cells through the venous circulation after biopsy of the femur has been documented experimentally (
52).
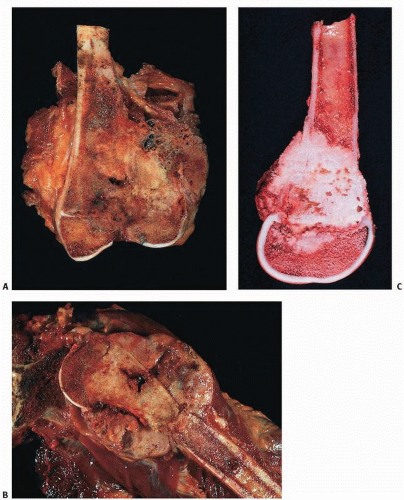
Grossly, the osteogenic sarcoma is generally a hard, compact tumor that has penetrated the cortex, raised the periosteum, and invaded the soft tissue (
Fig. 9.17). There is a pluripotentiality of the proliferating mesenchymal tissue. Although predominantly osteoblastic and bone forming, there may be fibrous or cartilaginous foci. Grossly, the tissue may be rock hard or soft and gritty, depending on the degrees of bone formation, hemorrhage, and necrosis. Codman triangle may be appreciated grossly (
Fig. 9.18). A “pseudocapsule” is frequently observed enveloping the soft tissue component at the periphery of osteosarcoma. Miura et al. (
53) found significantly better survival rates in patients whose tumors had thick encapsulation.
Histologically, osteogenic sarcoma is characterized by the presence of sarcomatous osteoblast cells producing a disorganized maze of calcified tissue including osteoid and bone (
Fig. 9.19). Since the amount of osteoid can be limited in extent and hyalinized or fibrous collagen can mimic osteoid, markers of osteoblastic differentiation, such as SATB2, can be useful in diagnosis (
54). The lesion may vary from one that is very cellular with little osteoid or bone production to those that are sparsely cellular with abundant calcified matrix being produced. Masses of osteoid without accompanying groups of cells are highly suspicious for osteosarcoma. Bizarre and undifferentiated tumor cells are commonplace. There may be exuberant foci of neoplastic cartilage or fibrous tissue, and patterns similar to malignant fibrous histiocytoma (MFH) are well described. Because of the polymorphism of the tumor, mistaken diagnoses of chondrosarcoma and fibrosarcoma can be made if poorly sampled microscopy alone is used. Osteoid often predominates in well-vascularized osteosarcoma, and malignant cartilage in poorly vascularized osteosarcoma.
Although cellularity varies, cytologic characteristics of malignancy such as pleomorphism, hyperchromatism, and atypical mitoses are usually noted. Predominantly osteoblastic lesions may be sparsely cellular. In these cases, the bizarre appearance of the aberrant mineralized matrix and its indiscriminate juxtaposition on native trabecular and cortical bone is diagnostic (
Fig. 9.19). Bone production is almost always woven, with no discrete lining of malignant bone by discrete osteoblasts. Rather, sheets of malignant cells appear pushed against malignant bone. Giant cells, usually benign looking, may be abundant. Necrosis is often identified and, in general, the greater the necrosis, the worse the prognosis.
Because exuberant fracture callus can mimic osteosarcoma, careful evaluation of an adequate sample is important (
Fig. 9.20).
Recently, epithelial-appearing cells in osteosarcoma have been shown to contain epithelial markers (cytokeratin and epithelial membrane antigen), strongly suggesting some osteosarcomas are the neoplastic manifestation of a primitive pluripotential uncommitted stem cell (
55).
Osteosarcomas with predominantly cartilaginous giant-cell tumor-like, rhabdomyosarcomatous, lymphomatous, and even malignant fibrous histiocytomatous (
56) appearances have been described. Osteogenic sarcoma grows by relatively rapid local expansion with a doubling time of 34 days (
57). Hematogenous spread is the most common route of metastatic disease occurring early and usually to the lungs or other bones.
Most patients with osteosarcoma have metastatic disease at the time of diagnosis, with 15 percent having one or more lesions identified by chest CT scan and 65 percent only subclinical microscopic disease (
51). Patients with advanced age, a tumor in an axial location, a larger tumor size, and residing in less-affluent regions are more likely to have metastatic disease at presentation (
58).
Some studies have shown PET/CT scans to be more sensitive and accurate than bone scans for the detection of bone metastases in osteosarcoma (
59).
Systemic neoadjuvant chemotherapy can treat micrometastases with surgical resection usually utilized for discernible metastases.
Lymph node metastases, far rarer, have been reported in up to 28 percent of cases at postmortem (
60), most frequently in hilar, mediastinal, mesenteric, abdominal, and even inguinal regions. Surgical resection of pulmonary metastases appears to improve outcome (
61).
Transarticular spread of malignant bone tumors, a relatively unusual phenomenon, may occur more frequently across joints that lack mobility (
62).
The diagnosis, staging, and treatment of classic osteosarcoma should follow a careful sequence of steps (
Fig. 9.21).
In the 1980s, use of aggressive multimodality therapy in conjunction with improved imaging techniques to detect pulmonary
metastases has improved the outlook considerably in children with osteosarcoma and synchronous pulmonary metastases. Since 1982, there has been a 50 percent probability of survival at 3 years, compared with no survival prior to that time (
63).
Prognostic indicators include the size and extent of cortical and soft tissue penetration and weight loss of greater than 10 pounds.
John Healey, at Memorial Sloan-Kettering Center, has reported 80 percent survival rates for Stage IIB lower limb lesions, and 90 percent for Stage IIA. Tibial lesions had a better survival than distal femoral lesions. He found metastases a bad prognostic factor, with only 15 percent of patients with distant metastases cured (
51).
In recent years, the standard treatment has evolved into chemotherapeutic regimens consisting predominantly of
doxorubicin,
cisplatin, and
high-dose metotrexate.





 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access