3 The cellular components of the blood are: • Red blood cells (erythrocytes) are specialised cells containing the red pigment haemoglobin. They provide most of the oxygen transport from the lungs and much of the return carbon dioxide carriage. They are immotile and serve their function only as the result of being passively circulated around the vascular system. The fraction of blood (by volume) occupied by erythrocytes is called the haematocrit and is in the range of 0.35 to 0.50 in adults. • White blood cells (leucocytes) constitute an important part of the defence and immune systems of the body but undertake these functions mainly in the tissues; leucocytes in circulating blood are in transit or are simply waiting in reserve. • Platelets (thrombocytes) are specialised cells which bind to and coat damaged vessel walls, plug small defects in blood vessel walls and help activate the blood-clotting cascade. They are essential for haemostasis, the system that controls bleeding. In adults these cells are formed in the bone marrow, a process known as haemopoiesis or haematopoiesis. • Basophilia (deep blue) – affinity for the basic dye methylene blue; DNA in nuclei and RNA in ribosomes. • Azurophilia (purple) – affinity for azure dyes; typically lysosomes, one of the granule types in leucocytes. • Eosinophilia (pink/red) – affinity for the acidic dye eosin, thus also described as acidophilia. • Neutrophilia (salmon pink/lilac) – affinity for a dye once erroneously believed to be of neutral pH; characteristic of the specific cytoplasmic granules of neutrophil leucocytes. Five types of leucocytes are normally present in human blood and are classified into two groups: Granulocytes are important components of the innate (non-learned) defences against infection (see Ch. 11); however, this role usually takes place in the tissues, not in blood. All leucocytes carry surface proteins which can bind to receptors on the endothelial cells of blood vessels (see Ch. 8) and, via this binding, granulocytes actively adhere to vessel walls and then migrate into the tissues using pseudopodial movement. FIG. 3.1 Haematopoietic stem cells and progenitors Haematopoiesis is tightly controlled by growth factors and the microenvironment. Growth factors, with the possible exception of erythropoietin, are multifunctional. A single growth factor will influence several different stages of several lineages and promote proliferation and/or differentiation, maturation, egress from bone marrow and survival in tissue. The differences in effect are due to the developmental stage of the cell on which the growth factor is acting, combined with its differentiation, surface receptors and the multitude of other signals it is receiving (see Table 3.1). TABLE 3.1 Major haematopoietic growth factors FIG. 3.2 Haematopoiesis in liver and bone marrow FIG. 3.3 Bone marrow sinusoids FIG. 3.4 Erythropoiesis
Blood, haematopoiesis and bone marrow
Blood Cell Types
Methods Used to Study Blood and Bone Marrow Cells
White Cell Series
Granulocytes
Haematopoiesis
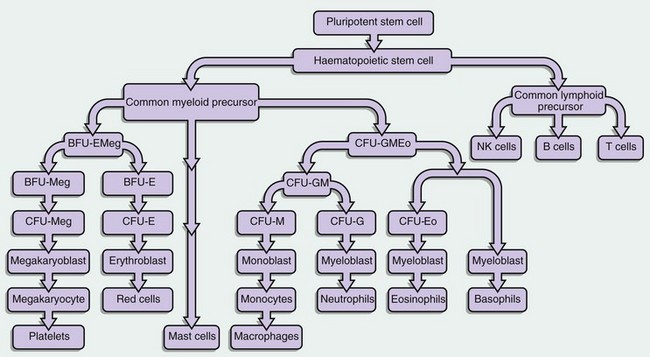
This diagram illustrates some of the identified stem cells and precursors in haematopoiesis. The haematopoietic stem cell (HSC) differentiates into a common lymphoid precursor and a common myeloid precursor (CMP) also known as the CFU-GEMM (CFU–granulocyte, erythroid, monocyte, megakaryocyte).
This common myeloid precursor differentiates into a common erythroid and megakaryocyte progenitor, the BFU-EMeg (BFU–erythroid megakaryocyte) and into a combined granulocyte and monocyte precursor, the CFU-GMEo (CFU–granulocyte, monocyte, eosinophil).
These precursors differentiate as shown in the diagram into a committed precursor type for each individual lineage. BFU- and CFU-GM precursors produce large numbers of progeny (hundreds to thousands), while the individual lineage CFU- precursors may only have 20 to 50 progeny.
Each recognisable blast in a bone marrow smear (other than megakaryocyte lineage) might average 16 offspring when counted as the final differentiated cells.
The mast cell derivation pathway is unclear but is believed to be of early myeloid lineage.
Major haematopoietic growth factors
Factor
Abbreviation
Cells affected
Stem cell factor
SCF
All
Granulocyte-monocyte colony-stimulating factor (CSF)
GM-CSF
Most
Interleukin-3
IL-3
All
Interleukin-11
IL-11
Megakaryocyte production
Interleukin-5
IL-5
Eosinophil production
Granulocyte CSF
G-CSF
Granulocyte production
Monocyte CSF
M-CSF
Monocyte production
Thrombopoietin
TBO
Megakaryocyte and red cell production
Erythropoietin
EPO
Red cell production
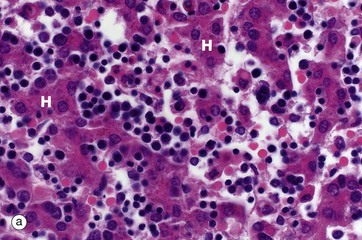
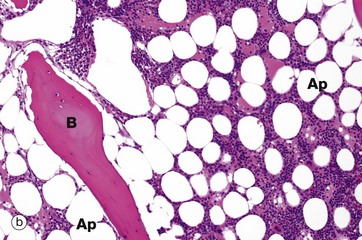
(a) H&E (MP) (b) H&E (LP)
Haematopoiesis begins in early intrauterine life in an embryonic organ, the yolk sac. It soon becomes established in the liver sinusoids. These are the vascular spaces between the hepatocytes H, in micrograph (a), where numerous small, darkly staining haematopoietic cells are seen. This is the dominant site of haematopoiesis from 3 to 7 months gestation. As bones develop, haematopoiesis establishes in the spaces between bone trabeculae B in all bones and, by birth, this provides sufficient space for all the haematopoiesis so that extramedullary haematopoiesis comes to an end. With growth through childhood, bone marrow space increases faster than total body growth and, increasingly, the marrow become occupied by adipocytes Ap (fat cells). Haematopoietic marrow has a macroscopic red colour, while adipocyte-dominated marrow is yellow. By early adulthood, most of the marrow in the limb bones is yellow marrow, while the axial skeleton remains red and haematopoietic, although usually with 30% to 60% of the volume being admixed adipocytes. Micrograph (b) shows adult vertebral marrow with moderate numbers of adipocytes.
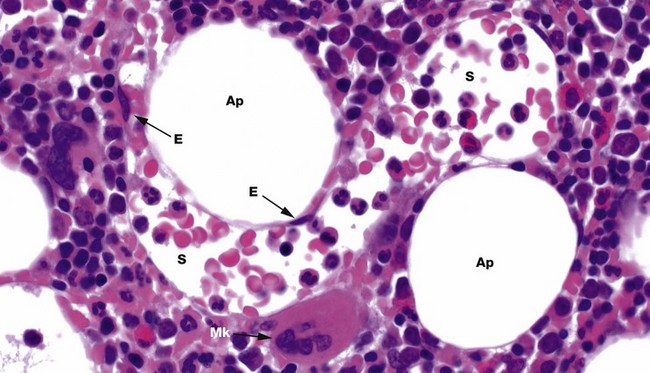
H&E (HP)
Bone marrow has a framework of vascular sinusoids lined by endothelial cells E and intervening spaces called marrow cords, supported by a meshwork of fibroblastic cells with long, branched cytoplasmic processes (reticular cells) and reticulin fibres (type III collagen). Macrophages are numerous and may also have long, branched processes. Adipocytes Ap and plasma cells are present in significant numbers. The result is a microenvironment which supports haematopoiesis and the marrow cords are correspondingly cellular. Megakaryocytes Mk sit next to the sinusoids S so their cytoplasmic processes, proplatelets, can be released into the circulation. Erythroblastic islands also sit next to sinusoids, with red cell precursors adhering to long macrophage processes that direct red cells to, and control egress into, the sinusoids. Granulocyte precursors tend to sit away from the sinusoids, but these are motile cells which can migrate into sinusoids.
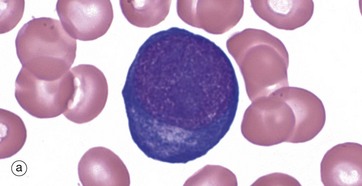
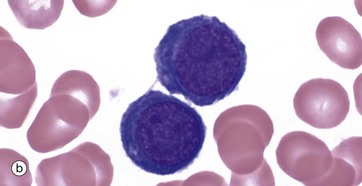
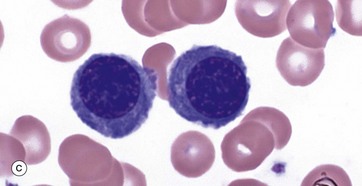
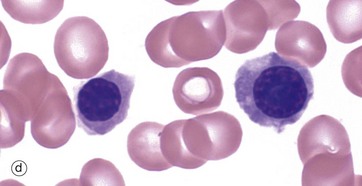
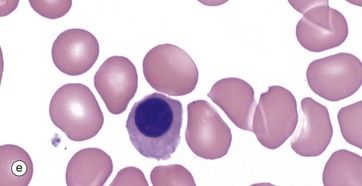
(a–e) Giemsa (HP)
These micrographs from a bone marrow smear illustrate the stages of erythropoiesis. The process of erythropoiesis involves:
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Blood, haematopoiesis and bone marrow
