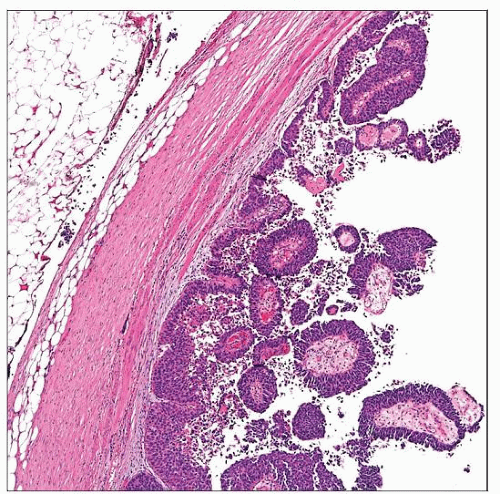
Urothelial Carcinoma of the Renal Pelvis
Satish K. Tickoo, MD
Mahesha Vankalakunti, MD
Victor E. Reuter, MD
Key Facts
Terminology
Malignant neoplasm of urothelial (transitional cell) origin involving renal pelvicalyceal system
Urothelial carcinoma of renal pelvis (UCP)
Etiology/Pathogenesis
Tobacco smoking is important risk factor
Long-term use of analgesics, especially phenacetin, also implicated as independent risk factor
Clinical Issues
4-5% of all urothelial tumors
Pathologic stage is single most important prognostic factor for urothelial carcinomas of upper urinary tract
Macroscopic Features
Either predominantly papillary or polypoid, or infiltrative mass with thickening of pelvic wall
Microscopic Pathology
Papillary urothelial neoplasms of low malignant potential (PUNLMP) extremely uncommon in upper tract
Low-grade carcinoma relatively less common, compared to that in bladder
Overall lymph node involvement reported to be approximately 10%
Top Differential Diagnoses
Collecting duct carcinoma (CDC)/RCC, unclassified
Metastatic carcinoma
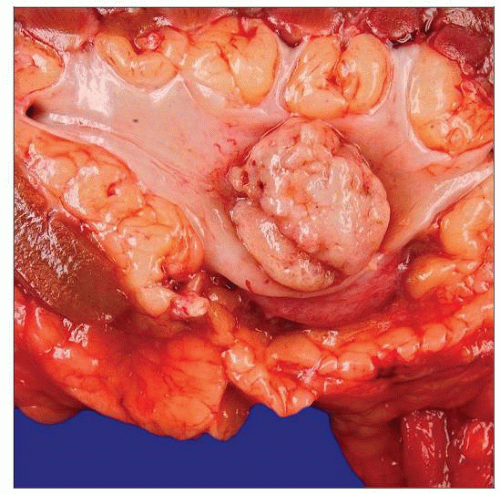 This gross specimen of a urothelial papillary carcinoma of the renal pelvis shows a polypoid lesion with solid, smooth surface, indicative of a histologically high-grade tumor. |
TERMINOLOGY
Abbreviations
Urothelial carcinoma of renal pelvis (UCP)
Definitions
Malignant neoplasm of urothelial (transitional cell) origin involving renal pelvicalyceal system
ETIOLOGY/PATHOGENESIS
Risk Factors
Tobacco smoking is important risk factor
Lifetime risk increases with increased consumption and intensity of smoking
Long-term use of analgesics, especially phenacetin, is also implicated as independent risk factor
Increases risk of renal pelvis tumors 4-8x in men and 10-13x in women
With decrease in usage of phenacetin, it is a less significant risk factor
Other risk factors include Balkan nephropathy and occupational exposures
Petrochemicals, plastic materials, coal, asphalt, tar, and thorium-containing contrast media
History of previous lower urinary tract carcinoma is also well-known predisposing factor
> 2/3 have prior, concurrent, or subsequent bladder carcinoma
Molecular Features
Similar to that of urothelial carcinomas of bladder
Deletions of part or all of chromosome 9; common event in urothelial carcinoma
Occurs early in tumorigenesis
Present in most cases of urothelial carcinoma, both papillary and nonpapillary
Fibroblastic growth factor receptor 3 (FGFR3) gene mutations
Occur in > 80% of noninvasive papillary urothelial carcinomas (stage Ta)
Also found in 20% of lamina propria invasive (stage T1) and 15% of muscle invasive tumors
No such mutations in carcinoma in situ
Relative incidences of FGFR3 mutations suggest that noninvasive papillary tumors do progress, although infrequently
Papillary tumors appear to progress along pathway that is different than carcinoma in situ (CIS) in most cases
Tumors with FGFR3 mutations have lower risk for recurrence than those without
Increased gene expression of HRAS is found in CIS and high-grade tumors
Often associated with allelic loss of p53, which might contribute to its up-regulation
Mutations in p53 are found at high rate in CIS (> 70% cases)
Microsatellite instability and loss of mismatch repair proteins MSH2, MLH1, or MSH6 present in upper urinary tract tumors
Seen in up to 20-30% tumors of upper urinary tract
Incidence in upper tract is many times more common than in bladder tumors
More commonly observed in females or patients with low tumor stage, grade, or inverted tumor growth pattern
Upper urinary tract tumors form 3rd most common tumor with microsatellite instability
Colon and endometrium are 2 most common sites within hereditary nonpolyposis colorectal cancer (HNPCC) related tumors
CLINICAL ISSUES
Epidemiology
Presentation
Flank pain
Hematuria
Treatment
Surgical approaches
Nephroureterectomy, ± removal of bladder cuff in high-grade or high-stage lesions
Segmental ureterectomy coupled with ureteral reimplantation in distal uretal tumors, generally of lower grade and stage
Renal-sparing surgery, including segmental ureterectomy and endoscopic therapy
Prognosis
Pathologic stage is single most important prognostic factor for urothelial carcinomas of upper urinary tract
On univariate analysis, significant prognostic indicators include
Size
Tumor grade
Pathologic stage
pTa: Papillary noninvasive carcinoma
pT1: Tumor invades subepithelial connective tissue
pT2: Tumor invades muscularis
pT3: Tumor invades (for renal pelvis): Beyond muscularis in peripelvic fat/renal parenchyma; (for ureter): Beyond muscularis in periureteric fat
pT4: Tumor invades adjacent organs or through kidney to perinephric fat
Lymphovascular invasion
However, on multivariate analysis, stage is only significant prognostic factor for survival
Based on multiple studies, 5-year survivals > 99% for pTa, 91% for pT1, 72% for pT2, 40% for pT3, and 16% for patients with metastasis
IMAGE FINDINGS
Radiographic Findings
Filling defect, obstructive mass associated with hydronephrosis, hydroureter, renal stones
MACROSCOPIC FEATURES
General Features
Either predominantly papillary or polypoid, or infiltrative mass with thickening of pelvic wall
Tumors that primarily appear as papillary or polypoid
May expand and fill pelvicalyceal system
Tend to be noninvasive or are associated with limited invasion
Systematic sampling after fixation and maintaining relationship to underlying structures important for accurate staging
Infiltrative mass may sometimes extensively involve renal parenchyma, mimicking primary renal parenchymal tumor
Occasionally may arise from minor calyx and grossly appear cortical in location
Equivocal radiographic localization may warrant intraoperative assessment of urothelial vs. renal parenchymal origin
Surgical approaches quite different in these 2 situations
Radical nephroureterectomy for urothelial vs. partial, total, or radical nephrectomy for renal cortical tumors
MICROSCOPIC PATHOLOGY
Histologic Features
Histopathological features of upper tract urothelial tumors similar to those in urinary bladder
However, papillary urothelial neoplasms of low malignant potential (PUNLMP) extremely uncommon in upper tract
Low-grade carcinoma relatively less common, compared to that in bladder
High-grade tumors are most common and invasion should be diligently looked for, if not obvious
Histopathologic diversity with morphologic variants/aberrant differentiations similar to that in bladder
Variant morphologies seen, among others, include
Micropapillary variant
Lymphoepithelioma-like carcinoma
Squamous differentiation and squamous cell carcinoma
Sarcomatoid differentiation
Signet ring or plasmacytoid features
Small cell carcinomatous features
Renal parenchymal invasion requires destructive invasive beyond renal tubules
Tumors often extend inside kidney within tubules
May, at times, form grossly identified expansile nodules
For staging purposes of renal parenchymal invasion, tumor cells have to invade out of well-defined tubular structures
Lymph Nodes
Overall lymph node involvement reported to be approximately 10%
Reported incidence is not based on cases where lymph nodes were removed at time of nephroureterectomy
Rates of lymph node metastasis close to 25% among cases where lymph nodes were removed at surgery
Predominant Pattern/Injury Type
Neoplastic
Predominant Cell/Compartment Type
Epithelial, transitional
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree