even body position. Urea, a metabolic waste product produced in the liver from the breakdown of protein and amino acids, accounts for almost half of the total dissolved solids in urine. Other organic substances include primarily creatinine and uric acid. The major inorganic solid dissolved in urine is chloride, followed by sodium and potassium. Small or trace amounts of many additional inorganic chemicals are also present in urine. The concentrations of these inorganic compounds are greatly influenced by dietary intake, making it difficult to establish normal levels. Other substances found in urine include hormones, vitamins, and medications. Although they are not a part of the original plasma filtrate, the urine may also contain formed elements such as cells, casts, crystals, mucus, and bacteria. Increased amounts of these formed elements are often indicative of disease.
Urine is a readily available and easily collected specimen.
Urine contains information about many of the body’s major metabolic functions, and this information can be obtained by simple laboratory tests.
Constituent | Filtered (g/24 h) | Reabsorbed (g/24 h) | Excreted (g/24 h) |
Sodium | 540 | 537 | 3.3 |
Chloride | 630 | 625 | 5.3 |
Bicarbonate | 300 | 300 | 0.3 |
Potassium | 28 | 24 | 3.9 |
Glucose | 140 | 140 | 0.0 |
Urea | 53 | 28 | 25 |
Creatinine | 1.4 | 0.0 | 1.4 |
Uric acid | 8.5 | 7.7 | 0.8 |
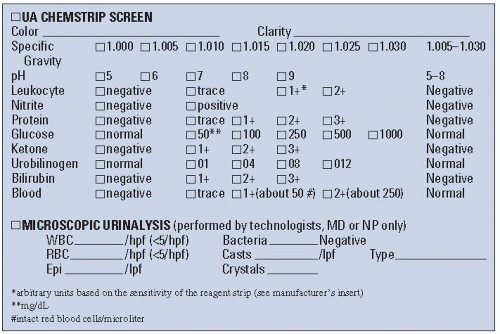
reagent strips, tablets, and treated slides for special determinations such as bacteria, phenylketonuria (PKU), mucopolysaccharides, salicylate, and cystinuria are available for urine analysis.
TABLE 3.1 Urine Testing by Dipstick/Reagent Strip | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||
Use a fresh urine sample within 1 hour of collection or a sample that has been refrigerated; bring to room temperature and mix specimen.
Read or review directions for use of the reagent. Periodically check for changes in procedure.
Dip a reagent strip into well-mixed urine, then remove it, blot, and compare each reagent area on the dipstick with the corresponding color control chart within the established time frame. Correlate color comparisons as closely as possible using good lighting.
If the dipstick is kept in the urine sample too long, the impregnated chemicals in the strip might be dissolved and could produce inaccurate readings and values.
If the reagent chemicals on the impregnated pad become mixed, the readings will be inaccurate. To avoid this, blot off excess urine after withdrawing the dipstick from the sample.
Precise timing is essential. If the test is not timed correctly, color changes may produce invalid or false results.
When not in use, the container of dipsticks should be kept tightly closed and stored in a cool, dry environment. If the reagents absorb moisture from the air before they are used, they will not produce accurate results. A desiccant comes with the reagents and should be kept in the container.
Quality control protocols must be followed:
The expiration date must be honored even if there is no detectable deterioration of strips.
Bottles must be discarded 6 months after opening, regardless of expiration date.
Known positive and negative (abnormal and normal) controls must be run for each new bottle of reagent strips when it is opened and whenever there is a question of deterioration.
Instruct the patient to void directly into a clean, dry container or bedpan. Transfer the specimen directly into an appropriate container. Disposable containers are recommended. Women should always have a clean-catch specimen if a microscopic examination is ordered (see Chapter 7).
Collect specimens from infants and young children into a disposable collection apparatus consisting of a plastic bag with an adhesive backing around the opening that can be fastened to the perineal area or around the penis to permit voiding directly into the bag. The specimen bag is carefully removed, and the urine is transferred to an appropriate specimen container.
Cover all specimens tightly, label properly, and send immediately to the laboratory. Place the label on the cup, not on the lid.
Obtain a clean specimen using the same procedure as for bacteriologic examination (see Chapter 7) if a urine specimen is likely to be contaminated with drainage, vaginal discharge, or menstrual blood.
If a urine specimen is obtained from an indwelling catheter, it may be necessary to clamp off the catheter for about 15 to 30 minutes before obtaining the sample. Clean the specimen port (in the tubing) with antiseptic before aspirating the urine sample with a needle and syringe or a luerlock syringe.
Observe standard precautions when handling urine specimens (see Appendix A).
If the specimen cannot be delivered to the laboratory or tested within 1 hour, it should be refrigerated or have an appropriate preservative added.
Feces, discharges, vaginal secretions, and menstrual blood will contaminate the urine specimen. A clean voided specimen must be obtained.
If the specimen is not refrigerated within 1 hour of collection, the following changes in composition may occur:
Increased pH from the breakdown of urea to ammonia by urease-producing bacteria
Decreased glucose from glycolysis and bacterial utilization
Decreased ketones because of volatilization
Decreased bilirubin from exposure to light
Decreased urobilinogen as a result of its oxidation to urobilin
Increased nitrite from bacterial reduction of nitrate
Increased bacteria from bacterial reproduction
Increased turbidity caused by bacterial growth and possible precipitation of amorphous material
Disintegration of red blood cells (RBCs) and casts, particularly in dilute alkaline urine
Changes in color caused by oxidation or reduction of metabolites
TABLE 3.2 Collection of Urine Specimens* | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ask the patient to void at the beginning of a 24-hour timed urine specimen collection (or any other timed specimen collection). Discard this first specimen, and note the time.
Mark the time the test begins and the time the collection should end on the container. As a reminder, it may be helpful to post a sign above the toilet (e.g., “24-Hour Collection in Progress”), with the beginning and ending times noted.
Collect all urine voided over the next 24 hours into a large container (usually glass or polyethylene) and label it with the patient’s name, the time frame for collection, the test ordered, and other pertinent information. It is not necessary to measure the volume of individual voidings, unless specifically ordered.
Ask the patient to void 24 hours after the first voiding, to conclude the collection. Add urine from this last voiding to the specimen in the container.
Storage
Keep nonrefrigerated samples in a specified area or in the patient’s bathroom.
Refrigerate the collection bottle immediately after the patient has voided or place it into an iced container if refrigeration is necessary.
In a health care facility, responsibility for the collection of urine specimens should be specifically assigned.
When instructing a patient about 24-hour urine collections, make certain the patient understands that the bladder must be emptied just before the 24-hour collection starts and that this preliminary specimen must be discarded; then, all urine voided until the ending time is saved.
Do not predate and pretime requisitions for serial collections. It is difficult for some patients to void at specific times. Instead, mark the actual times of collection on containers.
Documentation of the exact times at which the specimens are obtained is crucial to many urine tests.
Instruct the patient to urinate as near to the end of the collection period as possible.
When a preservative is added to the collection container (e.g., HCl preservative in 24-hour urine collection for vanillylmandelic acid [VMA]), the patient must take precautions against spilling the contents and receiving an acid burn. Instructions regarding spillage need to be provided before the test begins.
The preservative used is determined by the urine substance to be tested for. The laboratory usually provides the container and the proper preservative when the test is ordered. If in doubt, verify this with the laboratory personnel.
TABLE 3.3 24-Hour Collection: Standards for Timed Urine Specimen Collection | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Failure of patient or attending personnel to follow the procedure is the most common source of error.
The patient should be given both verbal and written instructions. If the patient is unable to comprehend these directions, a significant other should be instructed in the process.
If required, the proper preservative must be used.
Instruct the patient to use toilet paper after transferring the urine to the 24-hour collection container. Toilet paper placed in the specimen decreases the actual amount of urine available and contaminates the specimen.
The presence of feces contaminates the specimen. Patients should void first and transfer the urine to the collection receptacle before defecating.
If heavy menstrual flow or other discharges or secretions are present, the test may have to be postponed, or an indwelling catheter may need to be inserted to keep the specimen free of contamination. In some cases, thorough cleansing of the perineal or urethral area before voiding may be sufficient. If in doubt, communicate with laboratory personnel and the patient’s physician.
Empty the bladder completely on awakening and then discard that urine specimen. Record the time the voided specimen is discarded and the time the test is begun.
Save all urine voided during the next 24 hours, including the first specimen voided the next morning.
Add the urine voided the next morning (as close to the ending time as possible) to the collection container. The 24-hour test is then terminated, and the ending time is recorded.
Use a urinal, wide-mouth container, special toilet device, bedpan, or the collection container itself to catch urine. It is probably easier for women to void into another wide-mouth receptacle first and then to transfer the entire specimen carefully to the collection bottle. Men may find it simpler to void directly into the 24-hour collection container.
It is most important that all urine be saved in the 24-hour container. Ideally, the container should be refrigerated or placed on ice.
Test results are calculated on the basis of a 24-hour output. Unless all urine is saved, results will not be accurate. Moreover, these tests are usually expensive, complicated, and necessary for the evaluation and treatment of the patient’s condition.
If the laboratory requests an aliquot, record total amount, mix well, and aliquot the requested amount.
Always check with your laboratory as to the preservative needed—different laboratories may have different requirements.
Interpret test outcome and monitor appropriately.
Follow Chapter 1 guidelines for safe, effective, informed posttest care.
TABLE 3.4 Normal Values in Urinalysis | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Collect a 24-hour urine specimen and keep it refrigerated or on ice.
Record the exact collection starting time and collection ending time on the specimen container and in the patient’s health care record.
Transfer the specimen container to the laboratory refrigerator when the collection is completed. Complete the proper forms and document accordingly.
Ascertain volume by measuring the entire urine amount in a graduated and appropriately calibrated pitcher or other receptacle. The total volume is recorded as urine volume in milliliters (cubic centimeters) per 24 hours.
Polyuria (increased urine output) with elevated blood urea nitrogen (BUN) and creatinine levels
Diabetic ketoacidosis
Partial obstruction of urinary tract
Some types of tubular necrosis (aminoglycoside)
Polyuria with normal BUN and creatinine
Diabetes mellitus and diabetes insipidus
Neurotic states (compulsive water drinking)
Certain tumors of brain and spinal cord
Oliguria (50 to 400 mL in adults, or <15-20 mL/kg in children per 24 hours)
Renal causes
Renal ischemia
Renal disease due to toxic agents (certain drugs are toxic to the renal system)
Glomerulonephritis
Dehydration caused by prolonged vomiting, diarrhea, excessive diaphoresis, or burns
Obstruction (mechanical) of some area of the urinary tract or system
Cardiac insufficiency
Anuria (<50 mL in 24 hours)
Complete urinary tract obstruction
Acute cortical necrosis (cortex of the kidney)
Glomerulonephritis (acute, necrotizing)
Acute tubular necrosis
Hemolytic transfusion reaction
Polyuria
Intravenous glucose or saline
Pharmacologic agents such as thiazides and other diuretics
Coffee, alcohol, tea, caffeine
Oliguria
Water deprivation, dehydration
Excessive salt intake
Explain the purpose and procedure of the test.
Withhold diuretics for 3 days before the test. Check with clinician.
Avoid excessive water (liquid) intake and excessive salt intake. Advise patients to avoid salty foods and added salt in the diet. Eliminate caffeine and alcohol. Determine the patient’s usual liquid intake and request that intake not be increased beyond this daily amount during testing.
Follow guidelines in Chapter 1 for safe, effective, pretest care.
Patient can resume normal fluid and dietary intake and medications, unless specifically ordered otherwise.
Interpret test outcomes and counsel appropriately.
Follow guidelines in Chapter 1 for safe, effective, posttest care.
Test SG with the use of a multiple-test dipstick that has a separate reagent area for SG. An indicator changes color in relation to ionic concentration, and this result is translated into a value for SG.
Determine SG with a refractometer or total solids meter. The refractive index is the ratio of the velocity of light in air to the velocity of light in the test solution. A drop of urine is placed on a clear glass plate of the refractometer, and another plate is pressed on top of the urine sample. The path of light is deviated when it enters the solution, and the degree of deviation (refraction) is directly proportional to the density of the solution.
The urinometer (hydrometer) is the least accurate method. It consists of a bulb-shaped instrument that contains a scale calibrated in SG readings. Urine (10 to 20 mL) is transferred into a small test tube-like cylinder, and the urinometer is floated in the urine. The SG is read off the urinometer at the meniscus level of the urine. This method is becoming obsolete owing to the ease of dipstick testing.
Specimen collection
For regular UA testing, about 20 mL of a random sample is needed (UA including SG).
When a special evaluation of SG is ordered separately from the UA, the patient should fast for 12 hours before specimen collection.
Normal SG: SG values usually vary inversely with the amount of urine excreted (decreased urine volume = increased SG). However, this relationship is not valid in certain conditions, including:
Diabetes—increased urine volume, increased SG
Hypertension—normal volume, decreased SG
Early chronic renal disease—increased volume, decreased SG
Hyposthenuria (low SG, 1.001 to 1.010) occurs in the following conditions:
Diabetes insipidus (low SG with large urine volume). It is caused by absence or decrease of ADH, a hormone that triggers kidney absorption of water. Without ADH, the kidneys produce excessive amounts of urine that are not reabsorbed (sometimes 15-20 L/day).
Glomerulonephritis (kidney inflammation without infection) and pyelonephritis (kidney inflammation with bacterial infection, but not in the acute type of this disease). SG can be low in glomerulonephritis, with decreased urine volume. Tubular damage affects the kidneys’ ability to concentrate urine.
Severe renal damage with disturbance of both concentrating and diluting abilities of urine. The SG is low (1.010) and fixed (varying little from specimen to specimen); this is termed isosthenuria.
Hypersthenuria (increased SG, 1.025 to 1.035) occurs in the following conditions:
Diabetes mellitus
Nephrosis
Excessive water loss (dehydration, fever, vomiting, diarrhea)
Increased secretion of ADH and diuretic effects related to the stress of a surgical procedure
Congestive heart failure
Toxemia of pregnancy
Radiopaque x-ray contrast media, minerals, and dextran may cause falsely high SG readings on the refractometer. The reagent dipstick method is not affected by high-molecular-weight substances. SG > 1.040 suggests radiopaque contrast material is in the urine.
Temperature of urine specimens affects SG; cold specimens produce falsely high values using the hydrometer.
Highly buffered alkaline urine may also cause low readings (with dipsticks only).
Elevated readings may occur in the presence of moderate amounts of protein (100-750 mg/dL) or with patients receiving intravenous albumin.
Detergent residue (on specimen containers) can produce elevated SG results.
Diuretics and antibiotics cause high readings.
See Appendix E for drugs that affect test outcomes.
Explain the purpose and procedure for urine collection.
Follow guidelines in Chapter 1 for safe, effective, informed pretest care.
Interpret test outcomes, counsel, and monitor appropriately for conditions associated with altered SG.
Follow Chapter 1 guidelines for safe, effective, informed posttest care.
serum and urine osmolality tests are run at the same time. The normal ratio between urine and serum osmolality is 3:1. A high urine-to-serum ratio is seen with concentrated urine. With poor concentrating ability, the ratio is low.
Tell patient that this is a 24-hour urine collection test.
For the 24-hour test, the patient first voids at approximately 7:00 a.m. (0700). All of the urine voided is saved in a special 24-hour collection container kept on ice or refrigerated. A high-protein diet may be ordered.
At the end of the test, the specimen is labeled and sent to the laboratory.
Simultaneous determination of serum osmolality may be done. A high urine-to-serum ratio is seen with concentrated urine.
Osmolality is increased in:
Prerenal azotemia
Congestive heart failure
Addison’s disease
Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
Dehydration
Amyloidosis
Hyponatremia
Osmolality is decreased in:
Acute renal failure
Diabetes insipidus
Hypokalemia
Hypernatremia
Primary polydipsia
Hypercalcemia
Compulsive water drinking (increased fluid intake)
Urine-to-serum ratio is:
Increased in prerenal azotemia
Decreased in acute tubular necrosis
Intravenous sodium administration
Intravenous dextrose and water administration
Explain purpose and procedure of the test to the patient.
A normal diet is prescribed for 3 days before testing.
To increase sensitivity of the osmolality test, a high-protein diet may be ordered for 3 days before the test. No liquids should be taken with the evening meal, and no food or liquids should be taken after the evening meal until collection. Check with your laboratory if the patient has diabetes.
Follow guidelines in Chapter 1 for safe, effective, informed pretest care.
Provide the patient with foods and fluids as soon as the last urine sample is obtained.
Interpret test outcomes and monitor appropriately.
Follow guidelines in Chapter 1 for safe, effective, posttest care.
Observe the clarity of a fresh urine sample by visually examining a well-mixed specimen in front of a light source.
Use common terms to report appearance, including the following: clear, hazy, slightly cloudy, cloudy, turbid, and milky.
Document results. The degree of turbidity should correspond to the amount of material observed under the microscope.
Pathologic urines are often turbid or cloudy; however, normal urine can also appear cloudy.
Urine turbidity may result from urinary tract infections (UTIs).
Urine may be cloudy because of the presence of RBCs, WBCs, epithelial cells, or bacteria.
After ingestion of food, urates, carbonates, or phosphates may produce cloudiness in normal urine on standing.
Semen or vaginal discharges mixed with urine are common causes of turbidity.
Fecal contamination causes turbidity.
Extraneous contamination (e.g., talcum, vaginal creams, radiographic contrast media) can cause turbidity.
“Greasy” cloudiness may be caused by large amounts of fat.
Often, normal urine develops a haze or turbidity after refrigeration or standing at room temperature because of precipitation of crystals of calcium oxalate or uric acid.
Almost colorless (straw-colored) urine:
Large fluid intake
Chronic interstitial nephritis
Untreated diabetes mellitus
Diabetes insipidus
Alcohol and caffeine ingestion
Diuretic therapy
Nervousness
Orange-colored (amber) urine:
Concentrated urine caused by fever, sweating, reduced fluid intake, or first morning specimen
Bilirubin (yellow foam when shaken)
Carrots or vitamin A ingestion (large amounts)
Certain urinary tract medications (e.g., phenazopyridine [Pyridium], nitrofurantoin)
Brownish yellow or greenish yellow urine may indicate bilirubin in the urine that has been oxidized to biliverdin (greenish foam when shaken).
Green urine:
Pseudomonal infection
Indican
Chlorophyll
Pink to red urine:
RBCs
Hemoglobin, methemoglobin, oxyhemoglobin
Myoglobin
Porphyrins
Brown-black urine:
RBCs oxidized to methemoglobin
Methemoglobin
Homogentisic acid (alkaptonuria)
Melanin or melanogen
Phenol poisoning (Lysol)
Smoky urine may be caused by RBCs.
Milky urine is associated with fat, cystinuria, many WBCs, or phosphates (not pathologic).
Normal urine color darkens on standing because of the oxidation of urobilinogen to urobilin. This decomposition process starts about 30 minutes after voiding.
Some foods cause changes in urine color:
Beets turn the urine red.
Rhubarb can cause brown urine.
Many drugs alter the color of urine:
Cascara and senna laxatives in the presence of acid urine turn the urine reddish brown; in the presence of alkaline urine, they turn the urine red.
Bright-yellow color in alkaline urine may be a result of riboflavin or phenazopyridine.
Urine that darkens on standing may indicate antiparkinsonian agents such as levodopa (Sinemet).
Black urine may be caused by cascara, chloroquine, iron salts (ferrous sulfate, ferrous fumarate, ferrous gluconate), metronidazole, nitrofurantoin, quinine, or senna.
Blue urine may be caused by triamterene.
Blue-green urine may be caused by amitriptyline, methylene blue, or mitoxantrone.
Orange urine may be caused by heparin, phenazopyridine, rifampin, sulfasalazine, or warfarin.
Red-pink urine may be caused by chlorzoxazone, daunorubicin, doxorubicin, heparin, ibuprofen, methyldopa, phenytoin, rifampin, or senna.
Pink to brown urine may be caused by laxatives.
Brown urine may be caused by chloroquine, furazolidone, or primaquine.
Green urine may be caused by indomethacin.
If the urine is a red color, do not assume drug causation. Check the urine for hemoglobin. Question the patient regarding hematuria and recent activity, injury, or infection. Sometimes, vigorous exercise can bring on hematuria.
Red urine that is negative for occult blood is an indication that porphyria may be present. Report at once and document test results.
Other grossly abnormal colors (e.g., black, brown) should be documented and reported.
Interpret abnormal urine colors and counsel appropriately.
Explain that follow-up testing may be needed.
The urine of patients with diabetes mellitus may have a fruity (acetone) odor because of ketosis.
UTIs result in foul-smelling urine because of the presence of bacteria, which split urea to form ammonia.
The urine of infants with an inherited disorder of amino acid metabolism known as maple syrup urine disease smells like maple or burnt sugar.
Cystinuria and homocystinuria result in a sulfurous odor.
Oasthouse urine (Smith-Strang) disease, methionine malabsorption syndrome, causes an odor associated with the smell of a brewery (yeasts, hops).
In phenylketonuria, a musty, mousy smell may be evident.
Tyrosinemia is characterized by a cabbage-like or “fishy” urine odor.
Butyric/hexanoic acidemia produces a urine odor resembling that of sweaty feet.
Some foods, such as asparagus, produce characteristic urine odors.
Bacterial activity produces ammonia from the decomposition of urea, with its characteristic pungent odor.
Renal calculi
Renal stone formation partially depends on the pH of urine. Patients being treated for renal calculi are frequently given diets or medication to change the pH of the urine so that kidney stones will not form.
Calcium phosphate, calcium carbonate, and magnesium phosphate stones develop in alkaline urine. In such instances, the urine must be kept acidic (see Diet, number 4, below).
Uric acid, cystine, and calcium oxalate stones precipitate in acid urines. Therefore, as part of treatment, the urine should be kept alkaline (see Diet, number 4, below).
Drug treatment
Streptomycin, neomycin, and kanamycin are effective for treating genitourinary tract infections, provided the urine is alkaline.
During sulfa therapy, alkaline urine should help prevent formation of sulfonamide crystals.
Urine should also be kept persistently alkaline in the presence of salicylate intoxication (to enhance excretion) and during blood transfusions.
Clinical conditions
The urine should be kept acidic during treatment of UTI or persistent bacteriuria and during management of urinary calculi that develop in alkaline urine.
An accurate measurement of urinary pH can be made only on a freshly voided specimen. If the urine must be kept for any length of time before analysis, it must be refrigerated.
Highly concentrated urine, such as that formed in hot, dry environments, is strongly acidic and may produce irritation.
During sleep, decreased pulmonary ventilation causes respiratory acidosis; as a result, urine becomes more acidic.
Chlorothiazide diuretic administration causes acid urine to be excreted.
Bacteria from a UTI or from bacterial contamination of the specimen produce alkaline urine. Urea is converted to ammonia.
Diet
A vegetarian diet that emphasizes citrus fruits and most vegetables, particularly legumes, helps keep the urine alkaline. Alkaline urine after meals is a normal response to the secretion of hydrochloric acid in gastric juice (“alkaline tide”).
A diet high in meat and protein keeps the urine acidic.
Cranberry and prune juices will maintain an acidic urine and will help reduce UTIs.
Use reagent strips for a dipstick measurement. They produce a spectrum of color changes from orange to green-blue to identify pH ranges from 5.0 to 9.0.
Dip the reagent strip into a freshly voided urine specimen, and compare the color change with the standardized color chart on the bottle that correlates color results with pH values.
Maintenance of the urine at a consistent pH requires frequent urine pH testing.
Acidic urine (pH < 7.0) occurs in:
Metabolic acidosis, diabetic ketosis, diarrhea, starvation, uremia
UTIs caused by Escherichia coli
Respiratory acidosis (carbon dioxide retention)
Renal tuberculosis
Pyrexia
Alkaline urine (pH >7.0) occurs in:
UTIs caused by urea-splitting bacteria (Proteus and Pseudomonas)
Renal tubular acidosis, chronic renal failure
Metabolic acidosis (vomiting)
Respiratory alkalosis involving hyperventilation (“blowing off” carbon dioxide)
Potassium depletion
With prolonged “standing,” the pH of a urine specimen becomes alkaline because bacteria split urea and produce ammonia. Note: A urine pH of >9.0 is indicative of prolonged standing.
Ammonium chloride and mandelic acid may produce acid urines.
Runover between the pH testing area and the highly acidic protein area on the dipsticks may cause alkaline urine to give an acidic reading.
Sodium bicarbonate, potassium citrate, and acetazolamide may produce alkaline urine.
Urine becomes alkaline after eating because of excretion of stomach acid; this is known as the “alkaline tide.”
The pH tends to be low following overnight fasting and high following ingestion of a meal.
Explain test purpose and specimen collection procedure.
Follow guidelines in Chapter 1 for safe, effective, informed pretest care.
Interpret test outcomes and monitor patient appropriately (see Control of Urine pH).
Follow guidelines in Chapter 1 for safe, effective, informed posttest care.
cannot metabolize or store the excess free hemoglobin. The hemoglobin is then filtered through the glomerulus. Hemoglobinuria may also occur as a result of lysis of RBCs in the urinary tract.
Collect a fresh, random urine specimen.
Hemoglobinuria (hemoglobin in urine)
Dip reagent sticks into the urine; the color change on the dipstick correlates with a standardized color chart specifically used with that particular type of dipstick.
The color chart indicates color gradients for negative, moderate, and large amounts of hemoglobin.
Hematuria (RBCs in urine)
This dipstick method allows detection of intact RBCs when the number is greater than 10 cells/m L of urine. The color change appears stippled on the dipstick.
The degree of hematuria can be estimated by the intensity of the speckled pattern.
Centrifuge the urine sample and examine the sediment microscopically (see Microscopic Examination of Urine Sediment) to verify the presence of RBCs.
Hemoglobinuria is suspected when no RBCs are seen or the number seen does not correspond to the degree of color on the dipstick.
Myoglobinemia may be suspected if the urine is cherry-red, no RBCs are seen, and blood serum enzymes for muscle destruction are elevated.
Hematuria is found in:
Acute UTI (cystitis)
Lupus nephritis
Urinary tract or renal tumors
Urinary calculi (intermittent hematuria)
Malignant hypertension
Glomerulonephritis (acute or chronic)
Pyelonephritis
Trauma to kidneys
Polycystic kidney disease
Leukemia
Thrombocytopenia
Strenuous exercise
Benign familial or recurrent hematuria (asymptomatic hematuria without proteinuria; other clinical and laboratory data are normal)
Heavy smokers
Hemoglobinuria is found in:
Extensive burns
Transfusion reactions (incompatible blood products)
Febrile intoxication
Certain chemical agents and alkaloids (poisonous mushrooms, snake venom)
Malaria
Bleeding resulting from operative procedures on the prostate (can be difficult to control, especially in the presence of malignancies)
Hemolytic disorders such as sickle cell anemia, thalassemia, and glucose-6-phosphate dehydrogenase deficiency
Paroxysmal hemoglobinuria (large quantities of hemoglobin appear in urine at irregular intervals)
Kidney infarction
Hemolysis occurring while the urine is in the urinary tract (RBC lysis from hypotonic urine or alkaline urine)
Fava bean (or broad bean, member of the legume family) sensitivity (causes severe hemolytic anemia)
Disseminated intravascular coagulation (DIC)
Strenuous exercise (“march hemoglobinuria”)
Drugs causing a positive result for blood or hemoglobin include:
Drugs toxic to the kidneys (e.g., bacitracin, amphotericin)
Drugs that alter blood clotting (warfarin [Coumadin])
Drugs that cause hemolysis of RBCs (aspirin)
Drugs that may give a false-positive result (e.g., bromides, copper, iodides, oxidizing agents)
High doses of ascorbic acid or vitamin C may cause a false-negative result.
High SG or elevated protein reduces sensitivity.
Myoglobin produces a false-positive result.
Hypochlorites or bleach used to clean urine containers causes false-positive results.
Menstrual blood may contaminate the specimen and alter results.
Prostatic infections may cause false-positive results.
See Appendix E for a complete list of drugs that affect test outcomes.
Explain test purpose and procedure for urine specimen collection.
Follow guidelines in Chapter 1 for safe, effective, informed pretest care.
Interpret test outcomes and explain possible need for follow-up testing.
Follow guidelines in Chapter 1 for safe, effective, informed posttest care.
Collect a random urine sample in a clean container and test it as soon as possible.
Use a protein reagent dipstick and compare the test result color with the color comparison chart provided on the reagent strip bottle. Protein can also be detected by turbidimetric methods using sulfosalicylic acid.
Test a new second specimen and investigate any interfering factors if one of these methods produces positive results. A 24-hour urine test may then be ordered for a quantitative measurement of protein.
Label a 24-hour urine container with the name of the patient, the test, and the date and time the test is started.
Refrigerate the specimen as it is being collected.
See general instructions for 24-hour urine collection listed (see Long-Term, Timed Urine Specimen).
Record the exact starting and ending times for the 24-hour collection on the specimen container and on the patient’s record. (The usual starting and ending times are 0700 to 0700.)
Proteinuria occurs by two main mechanisms: damage to the glomeruli or a defect in the reabsorption process that occurs in the tubules.
Glomerular damage
Glomerulonephritis, acute and chronic
Systemic lupus erythematosus (SLE)
Malignant hypertension
Amyloidosis
Diabetes mellitus
Nephrotic syndrome
Polycystic kidney disease
Diminished tubular reabsorption
Renal tubular disease
Pyelonephritis, acute and chronic
Cystinosis
Wilson’s disease
Fanconi’s syndrome (defect of proximal tubular function)
Interstitial nephritis
In pathologic states, the level of proteinuria is rarely constant, so not every sample of urine is abnormal in patients with renal disease, and the concentration of protein in the urine is not necessarily indicative of the severity of renal disease.
Proteinuria may result from glomerular blood flow changes without the presence of a structural abnormality, as in congestive heart failure.
Proteinuria may be caused by increased serum protein levels.
Multiple myeloma (Bence Jones protein)
Waldenström’s macroglobulinemia
Malignant lymphoma
Proteinuria can occur in other nonrenal disease (“functional proteinuria”).
Acute infection, septicemia
Trauma, stress
Leukemia, hematologic disorders
Toxemia, preeclampsia of pregnancy
Hyperthyroidism
Vascular disease (hypertension), cardiac disease
Renal transplant rejection
Central nervous system lesions
Poisoning from turpentine, phosphorus, mercury, gold, lead, phenol, opiates, or other drugs
Hereditary, sickle cell, oxalosis
Large numbers of leukocytes accompanying proteinuria usually indicate infection at some level in the urinary tract. Large numbers of both leukocytes and erythrocytes indicate a noninfectious inflammatory disease of the glomerulus. Proteinuria associated with pyelonephritis may have as many RBCs as WBCs.
Proteinuria does not always accompany renal disease.
Pyelonephritis
Urinary tract obstructions
Nephrolithiasis
Tumors
Congenital malformations
Renal artery stenosis
Proteinuria is often associated with the finding of casts on sediment examination because protein is necessary for cast formation.
Postural proteinuria results from the excretion of protein by some patients when they stand or move about. This type of proteinuria is intermittent and disappears when the patient lies down. Postural proteinuria occurs in 3% to 15% of healthy young adults. It is also known as orthostatic proteinuria.
The patient is instructed to void at bedtime and to discard this urine.
The next morning, a urine specimen is collected immediately after the patient awakens and before the patient has been in an upright position for longer than 1 minute. This may involve the use of a bedpan or urinal.
A second specimen is collected after the patient has been standing or walking for at least 2 hours.
With postural proteinuria, the first specimen contains no protein, but the second one is positive for protein.
The urine looks microscopically normal; no RBCs or WBCs are apparent. Orthostatic proteinuria is considered a benign condition and slowly disappears with time. Progressive renal impairment usually does not occur.
Proteinuria of >2000 mg/24 hours in an adult or ≥40 mg/24 hours in a child usually indicates a glomerular cause.
Proteinuria of >3500 mg/24 hours points to a nephrotic syndrome.
Because of renal vasoconstriction, the presence of a functional, mild, and transitory proteinuria is associated with:
Strenuous exercise leading to urine protein values of up to 300 mg/24 hours
Severe emotional stress, seizures
Cold baths, exposure to very cold temperatures
Increased protein in urine occurs in these benign states:
Fever and dehydration (salt depletion)
Non-immunoglobulin E food allergies
Salicylate therapy
In the premenstrual period and immediately after delivery
False or accidental proteinuria may occur because of a mixture of pus and RBCs in the urinary tract related to infections, menstrual or vaginal discharge, mucus, or semen.
False-positive results can occur from incorrect use and interpretation of the color reagent strip test.
Alkaline, highly buffered urine can produce false-positive results on the dipstick test.
Very dilute urine may give a falsely low protein value.
Certain drugs may cause false-positive or false-negative urine protein tests (see Appendix E).
Radiographic contrast agents may produce false-positive results with turbidimetric measurements.
Instruct the patient about the purpose and procedure for collection of the 24-hour urine specimen. Emphasize the importance of compliance with the procedure.
Food and fluids are permitted; however, fluids should not be forced because very dilute urine can produce false-negative values.
Follow guidelines in Chapter 1 for safe, effective, informed pretest care.
24-hour: Same as for total urine protein
10-hour: Overnight collection
Last voiding before sleep (10:00 p.m. or 2200)
Collect all urine at first morning voiding (8:00 a.m. or 0800)
These results approximate 24-hour collection.
Diabetes with early diabetic nephropathy
Hypertension—heart disease
Generalized vascular disease
Preeclampsia
Strenuous exercise
Hematuria (menses)
High-protein diet or high salt levels
Albumin excretion of >30 mg/24 hours or >20 mg/L/10 hours indicates an abnormal excretion.
Patient management should be reviewed.
Patient compliance can be checked by glycosylated hemoglobin to determine further control.
Patients with borderline results should be assessed on more than one occasion before the significance of a given urine measurement is finally judged.
The posttest care is the same as for 24-hour total protein.
Collect a 24-hour urine specimen or a serum sample (approximately 5 mL).
Keep the pH neutral or alkaline (pH >6.0).
Freeze specimen if not analyzed immediately. Not stable at room temperature.
Increased urine β2-microglobulin occurs in:
Renal tubular disorders (>50 mg/24 hours or >50 mg/day)
Heavy metal poisoning (mercury, cadmium)
Drug toxicity (aminoglycosides, cyclosporine)
Fanconi’s syndrome, Wilson’s disease
Pyelonephritis
Renal allograft rejection
Lymphoid malignancies associated with B-lymphocyte lineage
Acquired immunodeficiency syndrome (AIDS) (can be used as a predictor of the progression to AIDS)
Increased serum β2-microglobulin occurs in:
Multiple myeloma (associated with a poor survival prognosis)
Renal dialysis
Amyloidosis
Viral infection
Acid urine—not stable, pH <6.0
Certain antibiotics (e.g., gentamicin, tobramycin)
Recent nuclear medicine scan
Increased synthesis in certain diseases (e.g., Crohn’s disease, hepatitis, sarcoidosis) decreases the usefulness of the blood serum test.
Random specimens are not recommended.
Instruct patient regarding the purpose of and procedure for test.
See instructions for 24-hour urine collection (see Long-Term, Timed Urine Specimen).
See Chapter 1 guidelines for safe, effective, informed pretest care.
Interpret test outcomes and monitor appropriately.
See Chapter 1 guidelines for safe, effective, informed posttest care.
Reduction tests (Clinitest)
These are based on reduction of cupric ions by glucose. When the compounds are added to urine, a heat reaction takes place. This results in precipitation and a change in the color of the urine if glucose is present.
These tests are nonspecific for glucose because the reaction can also be caused by other reducing substances in the urine, including:
Creatinine, uric acid, ascorbic acid
Other sugars, such as galactose, lactose, fructose, pentose, and maltose
These tests have a lower sensitivity than enzyme tests.
Enzyme tests (Clinistix, Diastix, Tes-Tape)
These tests are based on interaction between glucose oxidase (an enzyme) and glucose. When dipped into urine, the enzyme-impregnated strip changes color according to the amount of glucose in the urine. The manufacturer’s color chart provides a basis for comparison of colors between the sample and the manufacturer’s control.
These tests are specific for glucose only.
Use a freshly voided specimen.
Follow directions on the test container exactly. Timing must be exact; the color reaction must be compared with the closest matching control color on the manufacturer’s color chart to ascertain accurate results.
Record the results on the patient’s record.
Refrigerate or ice the entire urine sample during collection if a 24-hour urine specimen is also ordered. See Table 3.3 on pages 187-191 for proper preservative.
Urine glucose >1000 mg/dL (>55 mmol/L) (4+) is a critical value.
Determine exactly what drugs the patient is taking and whether the metabolites of these drugs can affect the urine glucose results. Frequent updating in regard to the effects of drugs on blood glucose levels is necessary in light of the many new drugs introduced and prescribed.
Test results may be reported as “plus” (+) or as percentages. Percentages are more accurate.
When screening for galactose (galactosuria) in infants, the reduction test must be used. The enzyme tests do not react with galactose.
Newborns should always be tested by both methods (reduction and enzymatic).
Increased glucose occurs in:
Diabetes mellitus
Endocrine disorders (thyrotoxicosis, Cushing’s syndrome, acromegaly)
Liver and pancreatic disease
Central nervous system disorders (brain injury, stroke)
Impaired tubular reabsorption
Fanconi’s syndrome
Advanced renal tubular disease
Pregnancy with possible latent diabetes (gestational diabetes)
Increase of other sugars (react only with reduction tests, not dipstick tests):
Lactose—pregnancy, lactation, lactose intolerance
Galactose—hereditary galactosuria (severe enzyme deficiency in infants; must be treated promptly)
Xylose—excessive ingestion of fruit
Fructose—hereditary fructose intolerance, hepatic disorders
Pentose—certain drug therapies and rare hereditary conditions
Interfering factors for reduction test (false-positive results):
Presence of non-sugar-reducing substances such as ascorbic acid, homogentisic acid, creatinine
Tyrosine
Nalidixic acid, cephalosporins, probenecid, and penicillin
Large amounts of urine protein (slows reaction)
Interfering factors for dipstick enzyme tests:
Ascorbic acid (in large amounts) may cause a false-negative result.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree