Types of Radiation: Basic Theory Explained for the Nonphysicist
Greene Shepherd
THEORETICAL AND SCIENTIFIC BACKGROUND
What are radiation and radioactivity? What are the immediate and long-term threats to life and health that can result from exposure? How is it detected? What safety measures are needed? Where can I find expert help? These are questions that many health care professionals are uncomfortable answering. The next several chapters are devoted to addressing such questions for the clinician. The goal of the current chapter is to provide a clear and user-friendly description of radiological principles.
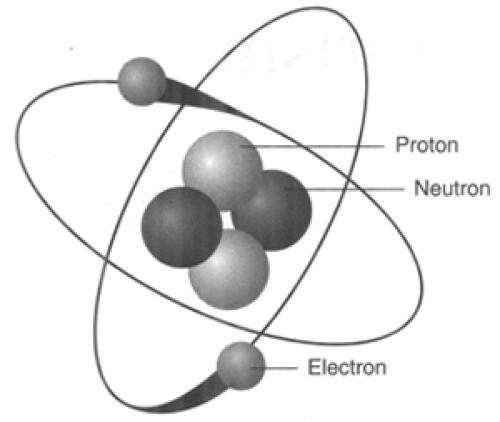
Radiation is simply the release of energy from atoms in the form of particles or waves. To understand how radiation is generated, a basic understanding about the structure of atoms is needed. Atoms are the basic building blocks of all matter (Fig. 13-1). The atom consists of a central nucleus, with shells of electrons orbiting around this nucleus. Electrons are very small relative to the size of the nucleus and are negatively charged. The nucleus carries a positive charge and is made up of protons and neutrons. Each element has a defined number of protons. These protons, which are positively charged, have the tendency to repel each other. Neutrons also exist in the nucleus of atoms, except for hydrogen, which only has one proton. Neutrons are roughly the same size as protons, but they do not carry a charge. Think of neutrons as spacers that keep the positively charged protons from repelling each other. In most atoms there is an average ratio of 1.2 neutrons for every 1 proton. When such a balance exists, the nucleus of the atom is very stable and emits little energy except under very extreme conditions. When there is an imbalance of this ratio of protons to neutrons, an element is radioactive. Often the imbalance is due to an excess of neutrons. An element with an unbalanced nucleus is referred to as an isotope or radionuclide. Different isotopes of the same element are identified based on the difference between the atomic weights. For example the atomic weight of iodine is normally 125.9 g/mol while the most common radioactive isotope weighs 131 g/mol. The extra weight in this case comes from excess neutrons. In order for the nucleus of the atom to return to a stable or balanced condition it will release energy. This release of energy is referred to as radioactive decay or simple decay (Fig. 13-2). This process will continue until enough energy has been released for the nucleus to stabilize.
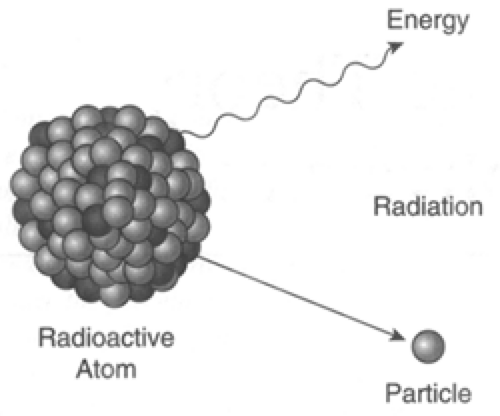 Figure 13-2. Emission of energy from a radioactive atom in the form of a wave (top) and a particle (bottom). (From the Nuclear Regulatory Commission: http://www.nrc.gov/reading-rm/basic-ref/glossary/radioactivity.html.) |
The energy is released in two forms that can be described as particles and waves. The first form of radiation is tiny fast-moving particles that have both energy and mass (weight). The other form of radiation is pure energy with no weight. This kind of radiation is vibrating or pulsating waves of electrical and magnetic energy. It is also referred to as electromagnetic waves or electromagnetic radiation. Another way of describing radiation is in terms of how it interacts with matter; it can be classified as ionizing or nonionizing. Ionization is the process of removing electrons from atoms, leaving two electrically charged particles (ions) behind. Ionizing radiation comes in the form of atomic particles and waves that deliver a large amount of energy. Nonionizing radiation does not have sufficient energy to remove electrons from atoms (1,2).
An unstable nucleus can become stable by changing a neutron into a proton with the ejection of a negative bit of matter (a beta particle or electron). Conversely, a proton can change into a neutron with the ejection of a positively charged bit of matter (a positron or positively charged electron). The nucleus of large unstable atoms can reach stability by ejecting larger particles that consist of two protons and two neutrons (an alpha particle). Since the number of protons can change as an atom reaches stability, the resultant element can be different than the original one.
Because energy is released at predictable rates, we are able to calculate the half-life (t1/2) of the radioactive element. This is simply the amount of time it takes for the amount of a radioactive isotope to decrease by 50% as it loses energy to become a stable element. These half-lives can be very short (technetium 99m, t1/2 = 6 hours) or very long (Uranium 235, t1/2 = 7.1 – 108 years). It is important to remember that even a tiny amount of a substance (say a milligram) will contain millions of individual atoms. When thinking about radioactive decay, we are really dealing with the conversion of individual atoms. When we talk about half-life, we are talking about how many atoms are decaying over a period of time. For example if we started with 100 atoms of a radioactive element, the half-life would indicate how long it would take for 50 of the atoms to decay to a stable configuration. Half-lives will not be the same for different isotopes. Table 13-1 contains a list of common isotopes with half-lives and types of radiation emitted. When all the excess energy and mass is given off, the resultant element finally becomes stable. For all practical purposes, any amount of radioactive material will be completely depleted after 10 half-lives.
The most common types of ionizing radiation are alpha particles, beta particles, protons, gamma rays, and x-rays. Alpha particles usually have high energies (4 million to 8 million electron volts, or MeV), and they consist of two protons and two neutrons. They travel a few centimeters in air and up to 60 micrometers into tissue. The high energy and short path result in a dense track of ionization along the tissues with which the particles interact. Alpha particles will not penetrate the stratum corneum of the skin, and thus they are not an external hazard. However, if alpha-emitting elements are taken into the body by inhalation or ingestion or from open wounds, serious problems such as cancer may develop. Alpha emitters are primarily uranium isotopes and are generally found in nuclear chemistry laboratories and isotope production facilities. They can also be found in hospitals that offer nuclear medical services. They are not likely to be used as a weapon unless combined with a conventional explosive device (Chapter 17). The path length of a proton is somewhat longer than that of an alpha particle of equivalent energy. Beta particles interact much less readily with matter than do alpha particles and will travel up to a few centimeters into tissue or many meters through air. Exposure to external sources of beta particles is potentially hazardous, but internal exposure is more hazardous. Examples of beta-particle emitters are the isotopes carbon-14, gold-198, iodine-131, radium-226, cobalt-60, selenium-75, and chromium-51. Protons with energies of a few million electron volts can be produced by high-energy accelerators and are quite effective in producing tissue ionization. They are similar to alpha particles but have a longer path length and are more likely to produce hazardous effects. Gamma rays are electromagnetic energy emitted from the nucleus. They have a range of many meters in air and many centimeters in tissue and, like beta particles, constitute a biologic hazard both internally and externally. Examples of gamma emitters are cobalt-60, cesium-137, iridium-192, and radium-226. X-rays, like gamma rays, are high-energy electromagnetic energy with short wavelengths. X-rays and gamma rays are sometimes referred to as photons. These waves or rays are very penetrating and can ionize atoms deep within the body. X-rays generally have longer wavelengths and lower frequencies relative to gamma rays; consequently, they have lower energies. The biologic effects of x-rays and gamma rays are better known than any of the other forms of ionizing radiation.
TABLE 13-1 Isotopes Listed Alphabetically* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree