KEY POINTS
Trauma remains the most common cause of death for all individuals between the ages of 1 and 44 years and is the third most common cause of death regardless of age.
The initial management of seriously injured patients consists of performing the primary survey (the “ABCs”—Airway with cervical spine protection, Breathing, and Circulation); the goals of the primary survey are to identify and treat conditions that constitute an immediate threat to life.
All patients with blunt injury should be assumed to have unstable cervical spine injuries until proven otherwise; one must maintain cervical spine precautions and in-line stabilization.
Patients with ongoing hemodynamic instability, whether “nonresponders” or “transient responders,” require prompt intervention; one must consider the four categories of shock that may represent the underlying pathophysiology: hemorrhagic, cardiogenic, neurogenic, and septic.
Indications for immediate operative intervention for penetrating cervical injury include hemodynamic instability and significant external arterial hemorrhage; the management algorithm for hemodynamically stable patients is based on the presenting symptoms and anatomic location of injury, with the neck being divided into three distinct zones.
The gold standard for determining if there is a blunt descending torn aorta injury is CT scanning; indications are primarily based on injury mechanisms.
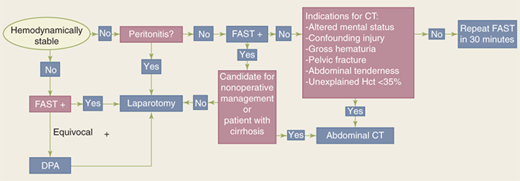
The abdomen is a diagnostic black box. However, physical examination and ultrasound can rapidly identify patients requiring emergent laparotomy. Computed tomographic (CT) scanning is the mainstay of evaluation in the remaining patients to more precisely identify the site and magnitude of injury.
Manifestation of the “bloody vicious cycle” (the lethal combination of coagulopathy, hypothermia, and metabolic acidosis) is the most common indication for damage control surgery. The primary objectives of damage control laparotomy are to control bleeding and limit GI spillage.
Blunt injuries to the carotid and vertebral arteries are usually managed with systemic antithrombotic therapy.
The abdominal compartment syndrome may be primary (i.e., due to the injury of abdominal organs, bleeding, and packing) or secondary (i.e., due to reperfusion visceral edema, retroperitoneal edema, and ascites).
INTRODUCTION
Trauma, or injury, is defined as cellular disruption caused by an exchange with environmental energy that is beyond the body’s resilience which is compounded by cell death due to ischemia/reperfusion. Trauma remains the most common cause of death for all individuals between the ages of 1 and 44 years and is the third most common cause of death regardless of age.1 It is also the leading cause of years of productive life lost. Unintentional injuries account for over 110,000 deaths per year, with motor vehicle collisions accounting for over 40%. Homicides, suicides, and other causes are responsible for another 50,000 deaths each year. However, death rate underestimates the magnitude of the societal toll. For example, in 2004 there were approximately 167,000 injury-related deaths, but 29.6 million injured patients treated in emergency departments (EDs). Injury-related medical expenditures are estimated to be $117 billion each year in the United States.2 The aggregate lifetime cost for all injured patients is estimated to be in excess of $260 trillion. For these reasons, trauma must be considered a major public health issue. The American College of Surgeons Committee on Trauma addresses this issue by assisting in the development of trauma centers and systems. The organization of trauma systems has had a significant favorable impact on patient outcomes.3,4,5
INITIAL EVALUATION AND RESUSCITATION OF THE INJURED PATIENT
The Advanced Trauma Life Support (ATLS) course of the American College of Surgeons Committee on Trauma was developed in the late 1970s, based on the premise that appropriate and timely care can significantly improve the outcome for the injured patient.6 ATLS provides a structured approach to the trauma patient with standard algorithms of care; it emphasizes the “golden hour” concept that timely, prioritized interventions are necessary to prevent death and disability. The ATLS format and basic tenets are followed throughout this chapter, with some modifications. The initial management of seriously injured patients consists of phases that include the primary survey/ concurrent resuscitation, the secondary survey/diagnostic evaluation, definitive care, and the tertiary survey. The first step in patient management is performing the primary survey, the goal of which is to identify and treat conditions that constitute an immediate threat to life. The ATLS course refers to the primary survey as assessment of the “ABCs” (Airway with cervical spine protection, Breathing, and Circulation). Although the concepts within the primary survey are presented in a sequential fashion, in reality they are pursued simultaneously in coordinated team resuscitation. Life-threatening injuries must be identified (Table 7-1) and treated before being distracted by the secondary survey.
Airway Airway obstruction Airway injury Breathing Tension pneumothorax Open pneumothorax Massive air leak Flail chest with underlying pulmonary contusion Circulation Hemorrhagic shock Massive hemothorax Massive hemoperitoneum Mechanically unstable pelvis fracture with bleeding Extremity blood loss Cardiogenic shock Cardiac tamponade Neurogenic shock Disability Intracranial hemorrhage/mass lesion Cervical spine injury |
Ensuring a patent airway is the first priority in the primary survey. This is essential, because efforts to restore cardiovascular integrity will be futile unless the oxygen content of the blood is adequate. Simultaneously, all patients with blunt trauma require cervical spine immobilization until injury is excluded. This is typically accomplished by applying a hard collar or placing sandbags on both sides of the head with the patient’s forehead taped across the bags to the backboard. Soft collars do not effectively immobilize the cervical spine. For penetrating neck wounds, however, cervical collars are not believed useful because they provide no benefit, but may interfere with assessment and treatment. 7,8
In general, patients who are conscious, without tachypnea, and have a normal voice are unlikely to require early airway intervention. Exceptions are penetrating injuries to the neck with an expanding hematoma; evidence of chemical or thermal injury to the mouth, nares, or hypopharynx; extensive subcutaneous air in the neck; complex maxillofacial trauma; or airway bleeding. Although these patients may initially have an adequate airway, it may become obstructed if soft tissue swelling, hematoma formation, or edema progresses. In these cases, pre-emptive intubation should be performed before airway access becomes challenging.
Patients who have an abnormal voice, abnormal breathing sounds, tachypnea, or altered mental status require further airway evaluation. Blood, vomit, the tongue, foreign objects, and soft tissue swelling can cause airway obstruction; suctioning affords immediate relief in many patients. In the comatose patient, the tongue may fall backward and obstruct the hypopharynx; this can be relieved by either a chin lift or jaw thrust. An oral airway or a nasal trumpet is also helpful in maintaining airway patency, although the former is not usually tolerated by an awake patient. Establishing a definitive airway (i.e., endotracheal intubation) is indicated in patients with apnea; inability to protect the airway due to altered mental status; impending airway compromise due to inhalation injury, hematoma, facial bleeding, soft tissue swelling, or aspiration; and inability to maintain oxygenation. Altered mental status is the most common indication for intubation. Agitation or obtundation, often attributed to intoxication or drug use, may actually be due to hypoxia.
Options for endotracheal intubation include nasotracheal, orotracheal, or operative routes. Nasotracheal intubation can be accomplished only in patients who are breathing spontaneously. Although nasotracheal intubation is frequently used by prehospital providers, the application for this technique in the ED is limited to those patients requiring emergent airway support in whom chemical paralysis cannot be used. Orotracheal intubation is the preferred technique used to establish a definitive airway. Because all patients are presumed to have cervical spine injuries, manual in-line cervical immobilization is essential.6 Correct endotracheal placement is verified with direct laryngoscopy, capnography, audible bilateral breath sounds, and finally a chest film. The GlideScope¯, a video laryngoscope that uses fiber optics to visualize the vocal cords, is being employed more frequently.9 Advantages of orotracheal intubation include the direct visualization of the vocal cords, ability to use larger-diameter endotracheal tubes, and applicability to apneic patients. The disadvantage of orotracheal intubation is that conscious patients usually require neuromuscular blockade, which may result in inability to intubate, aspiration, or medication complications. Those who attempt rapid-sequence induction must be thoroughly familiar with the procedure (see Chap. 13).
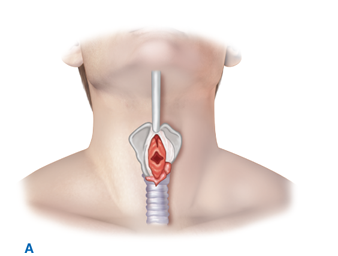
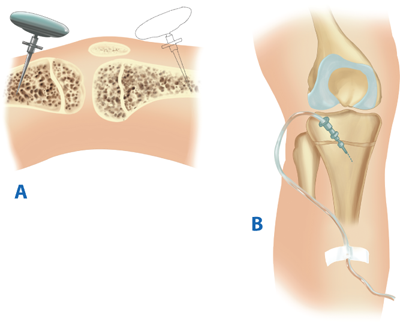
Patients in whom attempts at intubation have failed or who are precluded from intubation due to extensive facial injuries require operative establishment of an airway. Cricothyroidotomy (Fig. 7-1) is performed through a generous vertical incision, with sharp division of the subcutaneous tissues. Visualization may be improved by having an assistant retract laterally on the neck incision using army-navy retractors. The cricothyroid membrane is verified by digital palpation and opened in a horizontal direction. The airway may be stabilized before incision of the membrane using a tracheostomy hook; the hook should be placed under the thyroid cartilage to elevate the airway. A 6.0 endotracheal tube (maximum diameter in adults) is then advanced through the cricothyroid opening and sutured into place. In patients under the age of 11, cricothyroidotomy is relatively contraindicated due to the risk of subglottic stenosis, and tracheostomy should be performed.
Figure 7-1.
Cricothyroidotomy is recommended for emergent surgical establishment of a patent airway. A vertical skin incision avoids injury to the anterior jugular veins, which are located just lateral to the midline. Hemorrhage from these vessels obscures vision and prolongs the procedure. When a transverse incision is made in the cricothyroid membrane, the blade of the knife should be angled inferiorly to avoid injury to the vocal cords. A. Use of a tracheostomy hook stabilizes the thyroid cartilage and facilitates tube insertion. B. A 6.0 endotracheal tube is inserted after digital confirmation of airway access.
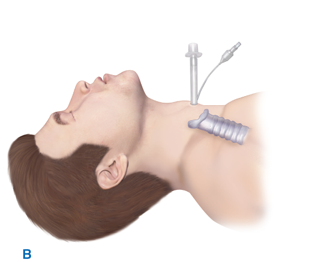
Emergent tracheostomy is indicated in patients with laryngotracheal separation or laryngeal fractures, in whom cricothyroidotomy may cause further damage or result in complete loss of the airway. This procedure is best performed in the OR where there is optimal lighting and availability of more equipment (e.g., sternal saw). In these cases, often after a “clothesline” injury, direct visualization and instrumentation of the trachea usually is done through the traumatic anterior neck defect or after a generous collar skin incision (Fig. 7-2). If the trachea is completely transected, a nonpenetrating clamp should be placed on the distal aspect to prevent tracheal retraction into the mediastinum; this is particularly important before placement of the endotracheal tube.
Once a secure airway is obtained, adequate oxygenation and ventilation must be ensured. All injured patients should receive supplemental oxygen and be monitored by pulse oximetry. The following conditions constitute an immediate threat to life due to inadequate ventilation and should be recognized during the primary survey: tension pneumothorax, open pneumothorax, flail chest with underlying pulmonary contusion, and massive air leak. All of these diagnoses should be made during the initial physical examination.
The diagnosis of tension pneumothorax is presumed in any patient manifesting respiratory distress and hypotension in combination with any of the following physical signs: tracheal deviation away from the affected side, lack of or decreased breath sounds on the affected side, and subcutaneous emphysema on the affected side. Patients may have distended neck veins due to impedance of venous return, but the neck veins may be flat due to concurrent systemic hypovolemia. Tension pneumothorax and simple pneumothorax have similar signs, symptoms, and examination findings, but hypotension qualifies the pneumothorax as a tension pneumothorax. Although immediate needle thoracostomy decompression with a 14-gauge angiocatheter in the second intercostal space in the midclavicular line may be indicated in the field, tube thoracostomy should be performed immediately in the ED before a chest radiograph is obtained (Fig. 7-3). Recent studies suggest the preferred location for needle decompression may be the 5th intercostal space in the anterior axillary line due to body habitus.10 In cases of tension pneumothorax, the parenchymal tear in the lung acts as a one-way valve, with each inhalation allowing additional air to accumulate in the pleural space. The normally negative intrapleural pressure becomes positive, which depresses the ipsilateral hemidiaphragm and shifts the mediastinal structures into the contralateral chest. Subsequently, the contralateral lung is compressed and the heart rotates about the superior and inferior vena cava; this decreases venous return and ultimately cardiac output, which culminates in cardiovascular collapse.
Figure 7-3.
A. Tube thoracostomy is performed in the midaxillary line at the fourth or fifth intercostal space (inframammary crease) to avoid iatrogenic injury to the liver or spleen. B. Heavy scissors are used to cut through the intercostal muscle into the pleural space. This is done on top of the rib to avoid injury to the intercostal bundle located just beneath the rib. C. The incision is digitally explored to confirm intrathoracic location and identify pleural adhesions. D. A 28F chest tube is directed superiorly and posteriorly with the aid of a large clamp.
An open pneumothorax or “sucking chest wound” occurs with full-thickness loss of the chest wall, permitting free communication between the pleural space and the atmosphere (Fig. 7-4). This compromises ventilation due to equilibration of atmospheric and pleural pressures, which prevents lung inflation and alveolar ventilation, and results in hypoxia and hypercarbia. Complete occlusion of the chest wall defect without a tube thoracostomy may convert an open pneumothorax to a tension pneumothorax. Temporary management of this injury includes covering the wound with an occlusive dressing that is taped on three sides. This acts as a flutter valve, permitting effective ventilation on inspiration while allowing accumulated air to escape from the pleural space on the untaped side, so that a tension pneumothorax is prevented. Definitive treatment requires closure of the chest wall defect and tube thoracostomy remote from the wound.
Figure 7-4.
A. Full-thickness loss of the chest wall results in an open pneumothorax. B. The defect is temporarily managed with an occlusive dressing that is taped on three sides, which allows accumulated air to escape from the pleural space and thus prevents a tension pneumothorax. Repair of the chest wall defect and tube thoracostomy remote from the wound is definitive treatment.
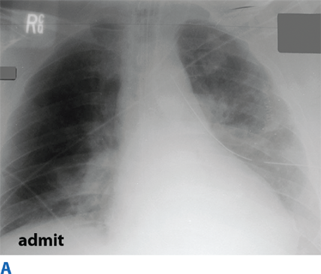
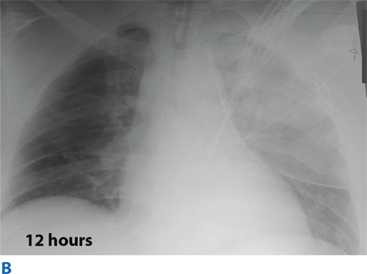
Flail chest occurs when three or more contiguous ribs are fractured in at least two locations. Paradoxical movement of this free-floating segment of chest wall is usually evident in patients with spontaneous ventilation, due to the negative intrapleural pressure of inspiration. However, the additional work of breathing and chest wall pain caused by the flail segment is rarely sufficient to compromise ventilation. Instead, it is the decreased compliance and increased shunt fraction caused by the associated pulmonary contusion that is the source of acute respiratory failure. Pulmonary contusion often progresses during the first 12 hours. Resultant hypoventilation and hypoxemia may require intubation and mechanical ventilation. The patient’s initial chest radiograph often underestimates the extent of the pulmonary parenchymal damage (Fig. 7-5); close monitoring and frequent clinical re-evaluation are warranted.
Massive air leak occurs from major tracheobronchial injuries. Type I injuries are those occurring within 2 cm of the carina.11,12 These are often not associated with a pneumothorax due to the envelopment in the mediastinal pleura. Type II injuries are more distal injuries within the tracheobronchial tree and manifest with pneumothorax. Bronchoscopy confirms diagnosis and directs management.
With a secure airway and adequate ventilation established, circulatory status is the next priority. An initial approximation of the patient’s cardiovascular status can be obtained by palpating peripheral pulses. In general, systolic blood pressure (SBP) must be 60 mm Hg for the carotid pulse to be palpable, 70 mm Hg for the femoral pulse, and 80 mm Hg for the radial pulse. Any episode of hypotension (defined as a SBP <90 mm Hg) is assumed to be caused by hemorrhage until proven otherwise. Patients with acute massive blood loss may have paradoxical bradycardia.13 Blood pressure and pulse should be measured at least every 5 minutes in patients with significant blood loss until normal vital sign values are restored. High energy auto-pedestrian victims should have their pelvis wrapped with a sheet until radiography can be done.
IV access for fluid resuscitation is obtained with two peripheral catheters, 16-gauge or larger in adults. For patients in whom peripheral angiocatheter access is difficult, intraosseous (IO) needles can be rapidly placed in the proximal tibia of the lower extremity (Fig. 7-6).14,15 All medications administered IV may be administered in a similar dosage intraosseously. Although safe for emergent use, the needle should be removed once alternative access is established to prevent osteomyelitis. Blood should be drawn simultaneously for a bedside hemoglobin level and routine trauma laboratory tests. In the seriously injured patient arriving in shock, an arterial blood gas, cross-matching for possible red blood cell (RBC) transfusion, and a coagulation panel should be obtained. In these patients, secondary large bore cannulae should be obtained via the femoral or subclavian veins, or saphenous vein cutdown; Cordis introducer catheters are preferred over triple-lumen catheters. In general, initial access in trauma patients is best secured in the groin or ankle, so that the catheter will not interfere with the performance of other diagnostic and therapeutic thoracic procedures. Saphenous vein cutdowns at the ankle provide excellent access (Fig. 7-7). The saphenous vein is reliably found 1 cm anterior and 1 cm superior to the medial malleolus. Standard 14-gauge catheters can be quickly placed, even in an exsanguinating patient with collapsed veins. If IV access cannot be achieved readily, the IO route is very useful, particularly for drug administration.14,15 Additional venous access often is obtained through the femoral or subclavian veins with Cordis introducer catheters. A rule of thumb to consider for secondary access is placement of femoral access for thoracic trauma and jugular or subclavian access for abdominal trauma. However, jugular or subclavian catheters provide a more reliable measurement of central venous pressure (CVP), which may be helpful in determining the volume status of the patient and in excluding cardiac tamponade. In severely injured children < 6 years of age, the preferred venous access is peripheral intravenous catheters followed by an IO needle. Central venous catheter placement or saphenous vein cutdown may be considered as the third choice of access based upon provider experience. Inadvertent femoral artery cannulation, however, may result in limb-threatening distal arterial spasm.
Figure 7-6.
Intraosseous infusions are indicated for children <6 years of age in whom one or two attempts at IV access have failed. A. The proximal tibia is the preferred location. Alternatively, the distal femur can be used if the tibia is fractured. B. The needle should be directed away from the epiphyseal plate to avoid injury. The position is satisfactory if bone marrow can be aspirated and saline can be easily infused without evidence of extravasation.
Figure 7-7.
Saphenous vein cutdowns are excellent sites for fluid resuscitation access. A. The vein is consistently found 1 cm anterior and 1 cm superior to the medial malleolus. B. Proximal and distal traction sutures are placed with the distal suture ligated. C. A 14-gauge IV catheter is introduced and secured with sutures and tape to prevent dislodgment.
External control of any visible hemorrhage should be achieved promptly while circulating volume is restored. Manual compression of open wounds with ongoing bleeding should be done with a single 4 × 4 gauze and a gloved hand. Covering the wound with excessive dressings may permit ongoing unrecognized blood loss that is hidden underneath the dressing. Blind clamping of bleeding vessels should be avoided because of the risk to adjacent structures, including nerves. This is particularly true for penetrating injuries of the neck, thoracic outlet, and groin, where bleeding may be torrential and arising deep within the wound. In these situations, a gloved finger is placed through the wound directly onto the bleeding vessel and enough pressure is applied to control active bleeding. The surgeon performing this maneuver must then walk with the patient to the OR for definitive treatment. For bleeding of the extremities it is tempting to apply tourniquets for hemorrhage control, but digital occlusion will usually control the bleeding, and complete vascular occlusion risks permanent neuromuscular impairment. Patients in shock have a lower tolerance to warm ischemia, and an occluded extremity is prone to small vessel thrombosis. For patients with open fractures, fracture reduction with stabilization via splints will limit bleeding both externally and into the subcutaneous tissues. Scalp lacerations through the galea aponeurotica tend to bleed profusely; these can be temporarily controlled with skin staples, Raney clips, or a large full-thickness continuous running nylon stitch.
During the circulation section of the primary survey, four life-threatening injuries must be identified promptly: (a) massive hemothorax, (b) cardiac tamponade, (c) massive hemoperitoneum, and (d) mechanically unstable pelvic fractures with bleeding. Massive hemoperitoneum and mechanically unstable pelvic fractures are discussed in “Emergent Abdominal Exploration” and “Pelvic Fractures and Emergent Hemorrhage Control,” respectively. Three critical tools used to differentiate these in the multisystem trauma patient are chest radiograph, pelvis radiograph, and focused abdominal sonography for trauma (FAST) (see “Regional Assessment and Special Diagnostic Tests”). A massive hemothorax (life-threatening injury number one) is defined as >1500 mL of blood or, in the pediatric population, >25% of the patient’s blood volume in the pleural space (Fig. 7-8). Although it may be estimated on chest radiograph, tube thoracostomy is the only reliable means to quantify the amount of hemothorax. After blunt trauma, a major hemothorax usually is due to multiple rib fractures with severed intercostal arteries, but occasionally bleeding is from lacerated lung parenchyma which is usually associated with an air leak. After penetrating trauma, a great vessel or pulmonary hilar vessel injury should be presumed. In either scenario, a massive hemothorax is an indication for operative intervention, but tube thoracostomy is critical to facilitate lung re-expansion, which may improve oxygenation and cardiac performance as well as tamponade venous bleeding.
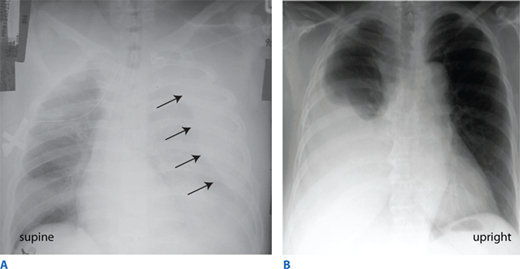
Figure 7-8.
More than 1500 mL of blood in the pleural space is considered a massive hemothorax. Chest film findings reflect the positioning of the patient. A. In the supine position, blood tracks along the entire posterior section of the chest and is most notable pushing the lung away from the chest wall. B. In the upright position, blood is visible dependently in the right pleural space.
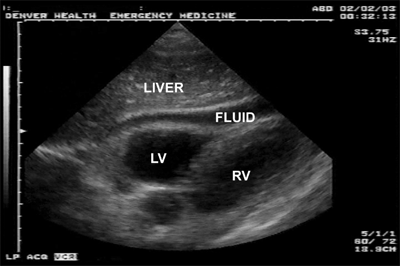
Cardiac tamponade (life-threatening injury number two) occurs most commonly after penetrating thoracic wounds, although occasionally blunt rupture of the heart, particularly the atrial appendage, is seen. Acutely, <100 mL of pericardial blood may cause pericardial tamponade.16 The classic Beck’s triad—dilated neck veins, muffled heart tones, and a decline in arterial pressure—is usually not appreciated in the trauma bay because of the noisy environment and associated hypovolemia. Because the pericardium is not acutely distensible, the pressure in the pericardial sac will rise to match that of the injured chamber. When this pressure exceeds that of the right atrium, right atrial filling is impaired and right ventricular preload is reduced. This ultimately leads to decreased right ventricular output. Additionally, increased intrapericardial pressure impedes myocardial blood flow, which leads to subendocardial ischemia and a further reduction in cardiac output.
Diagnosis of hemopericardium is best achieved by bedside ultrasound of the pericardium (Fig. 7-9). Early in the course of tamponade, blood pressure and cardiac output will transiently improve with fluid administration due to increased central venous pressure. In patients with any hemodynamic disturbance, a pericardial drain is placed using ultrasound guidance (Fig. 7-10). Removing as little as 15 to 20 mL of blood will often temporarily stabilize the patient’s hemodynamic status, and alleviate subendocardial ischemia with associated lethal arrhythmias, and allow safe transport to the OR for sternotomy. Pericardiocentesis is successful in decompressing tamponade in approximately 80% of cases; the majority of failures are due to the presence of clotted blood within the pericardium. Patients with a SBP <60 mm Hg warrant resuscitative thoracotomy (RT) with opening of the pericardium for rapid decompression and to address the injury.
Figure 7-10.
Pericardiocentesis is indicated for patients with evidence of pericardial tamponade. A. Access to the pericardium is obtained through a subxiphoid approach, with the needle angled 45 degrees up from the chest wall and toward the left shoulder. B. Seldinger technique is used to place a pigtail catheter. Blood can be repeatedly aspirated with a syringe or the tubing may be attached to a gravity drain. Evacuation of unclotted pericardial blood prevents subendocardial ischemia and stabilizes the patient for transport to the operating room for sternotomy.
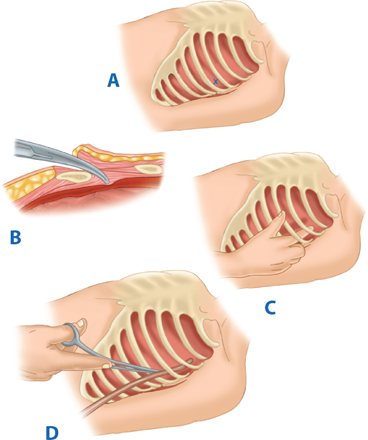
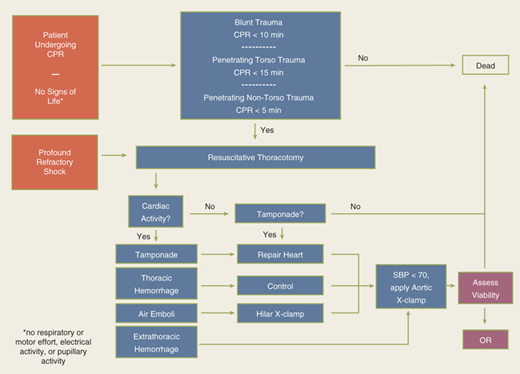
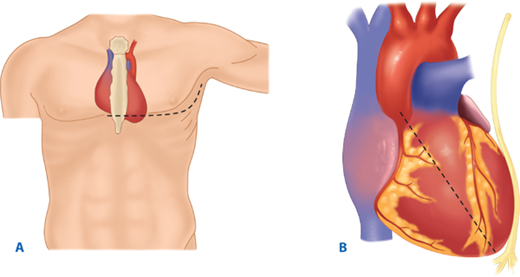
The utility of RT has been debated for decades. Current indications are based on 30 years of prospective data, supported by a recent multicenter prospective study (Table 7-2).17,18 RT is associated with the highest survival rate after isolated cardiac injury; 35% of patients presenting in shock and 20% without vital signs (i.e., no pulse or obtainable blood pressure) are salvaged after isolated penetrating injury to the heart. For all penetrating wounds, survival rate is 15%. Conversely, patient outcome is poor when RT is done for blunt trauma, with 2% survival among patients in shock and <1% survival among those with no vital signs. Thus, patients undergoing cardiopulmonary resuscitation upon arrival to the ED should undergo RT selectively based on injury and transport time (Fig. 7-11). RT is best accomplished using a generous left anterolateral thoracotomy, with the skin incision started to the right of the sternum (Fig. 7-12). A longitudinal pericardiotomy anterior to the phrenic nerve is used to release cardiac tamponade and permits access to the heart for cardiac repair and open cardiac massage. Cross-clamping of the aorta improves central circulation, augments cerebral and coronary blood flow, and limits further abdominal blood loss (Fig. 7-13). The patient must sustain a SBP of 70 mm Hg after RT and associated interventions to be considered resuscitatable, and hence transported to the OR.17,18
Indications Salvageable postinjury cardiac arrest: Patients sustaining witnessed penetrating trauma to the torso with <15 min of prehospital CPR Patients sustaining witnessed blunt trauma with <10 min of prehospital CPR Patients sustaining witnessed penetrating trauma to the neck or extremities with <5 min of prehospital CPR Persistent severe postinjury hypotension (SBP ≤60 mm Hg) due to: Cardiac tamponade Hemorrhage—intrathoracic, intra-abdominal, extremity, cervical Air embolism Contraindications Penetrating trauma: CPR >15 min and no signs of life (pupillary response, respiratory effort, motor activity) Blunt trauma: CPR >10 min and no signs of life or asystole without associated tamponade |
Figure 7-12.
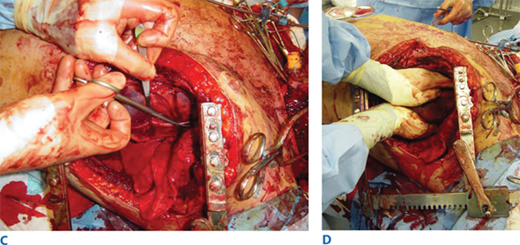
A. Resuscitative thoracotomy (RT) is performed through the fifth intercostal space using the anterolateral approach. B and C. The pericardium is opened anterior to the phrenic nerve, and the heart is rotated out for evaluation. D. Open cardiac massage should be performed with a hinged, clapping motion of the hands, with sequential closing from palms to fingers. The two-handed technique is strongly recommended because the one-handed massage technique poses the risk of myocardial perforation with the thumb.
The Glasgow coma scale (GCS) score should be determined for all injured patients (Table 7-3). It is calculated by adding the scores of the best motor response, best verbal response, and the best eye response. Scores range from 3 (the lowest) to 15 (normal). Scores of 13 to 15 indicate mild head injury, 9 to 12 moderate injury, and ≤8 severe injury. The GCS is a quantifiable determination of neurologic function that is useful for triage, treatment, and prognosis.
ADULTS | INFANTS/CHILDREN | ||
|---|---|---|---|
Eye opening | 4 | Spontaneous | Spontaneous |
3 | To voice | To voice | |
2 | To pain | To pain | |
1 | None | None | |
Verbal | 5 | Oriented | Alert, normal vocalization |
4 | Confused | Cries, but consolable | |
3 | Inappropriate words | Persistently irritable | |
2 | Incomprehensible words | Restless, agitated, moaning | |
1 | None | None | |
Motor response | 6 | Obeys commands | Spontaneous, purposeful |
5 | Localizes pain | Localizes pain | |
4 | Withdraws | Withdraws | |
3 | Abnormal flexion | Abnormal flexion | |
2 | Abnormal extension | Abnormal extension | |
1 | None | None |
Neurologic evaluation is critical before administration of neuromuscular blockade for intubation. Subtle changes in mental status can be caused by hypoxia, hypercarbia, or hypovolemia, or may be an early sign of increasing intracranial pressure. An abnormal mental status should prompt an immediate re-evaluation of the ABCs and consideration of central nervous system injury. Deterioration in mental status may be subtle and may not progress in a predictable fashion. For example, previously calm, cooperative patients may become anxious and combative as they become hypoxic. However, a patient who is agitated and combative from drugs or alcohol may become somnolent if hypovolemic shock develops. Patients with neurogenic shock are typified by hypotension with relative bradycardia, and are often first recognized due to paralysis, decreased rectal tone or priapism. Patients with high spinal cord disruption are at greatest risk for neurogenic shock due to physiologic disruption of sympathetic fibers; treatment consists of volume loading and a dopamine infusion which is both inotropic and chronotropic. Seriously injured patients must have all of their clothing removed to avoid overlooking limb- or life-threatening injuries.
Classic signs and symptoms of shock are tachycardia, hypotension, tachypnea, altered mental status, diaphoresis, and pallor(Table 7-4). In general, the quantity of acute blood loss correlates with physiologic abnormalities. For example, patients in class II shock are tachycardic but they do not exhibit a reduction in blood pressure until over 1500 mL of blood loss, or class III shock. Physical findings should be used as an aid in the evaluation of the patient’s response to treatment. The goal of fluid resuscitation is to re-establish tissue perfusion. Fluid resuscitation begins with a 2 L (adult) or 20 mL/kg (child) IV bolus of isotonic crystalloid, typically Ringer’s lactate. For persistent hypotension (SBP <90 mm Hg in an adult), the current trend is to activate a massive transfusion protocol (MTP) in which red blood cells (RBC) and fresh-frozen plasma (FFP) are administered early. The details of a MTP are discussed later. Patients who have a good response to fluid infusion (i.e., normalization of vital signs, clearing of the sensorium) and evidence of good peripheral perfusion (warm fingers and toes with normal capillary refill) are presumed to have adequate overall perfusion. Urine output is a quantitative, reliable indicator of organ perfusion. Adequate urine output is 0.5 mL/kg per hour in an adult, 1 mL/kg per hour in a child, and 2 mL/kg per hour in an infant <1 year of age. Because measurement of this resuscitation-related variable is time dependent, it is generally more useful in the OR and intensive care unit (ICU) setting, than in initial evaluation in the trauma bay.
CLASS I | CLASS II | CLASS III | CLASS IV | |
|---|---|---|---|---|
Blood loss (mL) | Up to 750 | 750–1500 | 1500–2000 | >2000 |
Blood loss (%BV) | Up to 15% | 15%–30% | 30%–40% | >40% |
Pulse rate | <100 | >100 | >120 | >140 |
Blood pressure | Normal | Normal | Decreased | Decreased |
Pulse pressure (mm Hg) | Normal or increased | Decreased | Decreased | Decreased |
Respiratory rate | 14–20 | >20–30 | 30–40 | >35 |
Urine output (mL/h) | >30 | >20–30 | 5–15 | Negligible |
CNS/mental status | Slightly anxious | Mildly anxious | Anxious and confused | Confused and lethargic |
There are several caveats to be considered when evaluating the injured patient for shock. Tachycardia is often the earliest sign of ongoing blood loss, but the critical issue is change over time. Furthermore, individuals in good physical condition with a resting pulse rate in the fifties may manifest a relative tachycardia in the nineties; although clinically significant, this does not meet the standard definition of tachycardia. Conversely, patients receiving cardiac medications such as beta blockers may not be capable of increasing their heart rate to compensate for hypovolemia. Bradycardia can occur with rapid severe blood loss13; this is an ominous sign, often heralding impending cardiovascular collapse. Other physiologic stresses, aside from hypovolemia, may produce tachycardia, such as hypoxia, pain, anxiety, and stimulant drugs (cocaine, amphetamines). As noted previously, decreased SBP is not a reliable early sign of hypovolemia, because blood loss must exceed 30% before hypotension occurs. Additionally, younger patients may maintain their SBP due to sympathetic tone despite severe intravascular deficits until they are on the verge of cardiac arrest. Pregnant patients have a progressive increase in circulating blood volume over gestation; therefore, they must lose a relatively larger volume of blood before manifesting signs and symptoms of hypovolemia (see Special Trauma Populations).
Based on the initial response to fluid resuscitation, hypovolemic injured patients can be separated into three broad categories: responders, transient responders, and nonresponders. Individuals who are stable or have a good response to the initial fluid therapy as evidenced by normalization of vital signs, mental status, and urine output are unlikely to have significant ongoing hemorrhage, and further diagnostic evaluation for occult injuries can proceed in an orderly fashion (see “Secondary Survey”). At the other end of the spectrum are patients classified as “nonresponders” who have persistent hypotension despite aggressive resuscitation. These patients mandate immediate identification of the source of hypotension with appropriate intervention to prevent a fatal outcome. Transient responders are those who respond initially to volume loading with improvement in vital signs, but then deteriorate hemodynamically again. This group of patients can be challenging to triage for definitive management.
Patients with ongoing hemodynamic instability, whether “nonresponders” or “transient responders,” require systematic evaluation and prompt intervention.The spectrum of disease in patients with persistent hypotension ranges from overwhelming multisystem injury to easily reversible problems such as a tension pneumothorax. One must first consider the four categories of shock that may be the underlying cause: hemorrhagic, cardiogenic, neurogenic, and septic. In patients with persistent hypotension and tachycardia, cardiogenic or hemorrhagic shock are the likely causes. Ultrasound evaluation of the pericardium, pleural cavities, and abdomen in combination with plain radiographs of the chest and pelvis will usually identify the source of hemorrhagic and/or cardiogenic shock. Evaluation of the CVP may further assist in distinguishing between these two categories. A patient with distended neck veins and a CVP of >15 cm H2O is likely to be in cardiogenic shock. The CVP may be falsely elevated, however, if the patient is agitated and straining, or fluid administration is overzealous; isolated readings must be interpreted with caution. A patient with flat neck veins and a CVP of <5 cm H2O is likely hypovolemic due to ongoing hemorrhage. Serial base deficit measurements are helpful; a persistent base arterial deficit of >8 mmol/L implies ongoing cellular shock.19,20 Serum lactate is also used to monitor the patient’s physiologic response to resuscitation.21 Evolving technology, such as near infrared spectroscopy, may provide noninvasive monitoring of oxygen delivery to tissue.22 Except for patients transferred from outside facilities >12 hours after injury, few patients present in septic shock in the trauma bay. Patients with neurogenic shock as a component of hemodynamic instability often are recognized during the disability section of the primary survey to have paralysis, but those patients chemically paralyzed before physical examination may be misdiagnosed.
The differential diagnosis of cardiogenic shock in trauma patients is: (a) tension pneumothorax, (b) pericardial tamponade, (c) blunt cardiac injury, (d) myocardial infarction, and (e) bronchovenous air embolism. Tension pneumothorax, the most frequent cause of cardiac failure, and pericardial tamponade have been discussed earlier. Although as many as one-third of patients sustaining significant blunt chest trauma experience some degree of blunt cardiac injury, few such injuries result in hemodynamic embarrassment. Patients with electrocardiographic (ECG) abnormalities or dysrhythmias require continuous ECG monitoring and antidysrrhythmic treatment as needed. Unless myocardial infarction is suspected, there is no role for routine serial measurement of cardiac enzyme levels—they lack specificity and do not predict significant dysrhythmias.23 In patients who have no identified injuries who are being considered for discharge from the ED, the combination of a normal EKG and troponin level at admission and 8 hours later, rules out significant blunt cardiac injury.24 The patient with hemodynamic instability requires appropriate resuscitation and may benefit from hemodynamic monitoring to optimize preload and guide inotropic support. Echocardiography (ECHO) is performed to exclude valvular or septal injuries, and the most common finding is right ventricular dyskinesia due to the anterior orientation of the right versus left ventricle. Transthoracic and transesophageal ECHO are now becoming routine in many surgical intensive care units (SICUs).25,26 Patients with refractory cardiogenic shock may occasionally require placement of an intra-aortic balloon pump to decrease myocardial work and enhance coronary perfusion. Acute myocardial infarction may be the cause of a motor vehicle collision or other trauma in older patients. Although optimal initial management includes treatment for the evolving infarction, such as lytic therapy and emergent angioplasty, these decisions must be individualized in accordance with the patient’s other injuries.
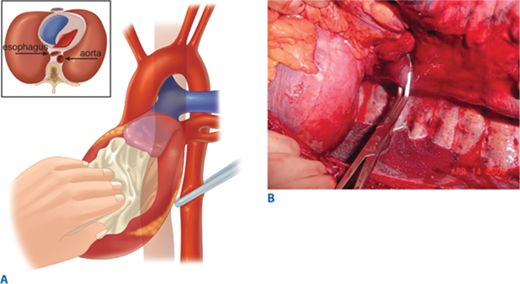
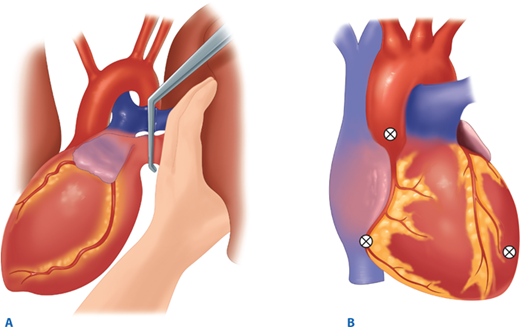
Air embolism is a frequently overlooked lethal complication of pulmonary injury. Air emboli can occur after blunt or penetrating trauma, where air from an injured bronchus enters an adjacent injured pulmonary vein (bronchovenous fistula) and returns air to the left heart. Air accumulation in the left ventricle impedes diastolic filling, and during systole air is pumped into the coronary arteries, disrupting coronary perfusion. The typical case is a patient with a penetrating thoracic injury who is hemodynamically stable but experiences cardiac arrest after being intubated and placed on positive pressure ventilation. The patient should immediately be placed in Trendelenburg’s position to trap the air in the apex of the left ventricle. Emergency thoracotomy is followed by cross-clamping of the pulmonary hilum on the side of the injury to prevent further introduction of air (Fig. 7-14). Air is aspirated from the apex of the left ventricle and then the aortic root with an 18-gauge needle and 50-mL syringe. Vigorous massage is used to force the air bubbles through the coronary arteries; if this is unsuccessful, a tuberculin syringe is used to aspirate air bubbles from the right coronary artery. Once circulation is restored, the patient should be kept in Trendelenburg’s position with the pulmonary hilum clamped until the pulmonary venous injury is controlled operatively.
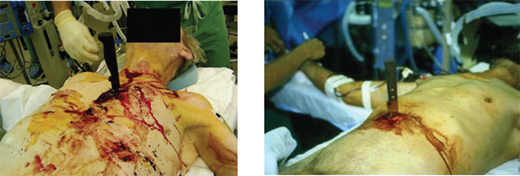
Persistent hypotension due to uncontrolled hemorrhage is associated with high mortality. A rapid search for the source or sources of hemorrhage includes visual inspection with knowledge of the injury mechanism, FAST, and chest and pelvic radiographs. During diagnostic evaluation, type O RBCs (O-negative for women of childbearing age) and thawed AB plasma should be administered at a ratio of 2:1. Type-specific RBCs should be administered as soon as available. The acute coagulopathy of trauma is now well recognized, and underscores the importance of pre-emptive blood component administration. The resurgent interest in viscoelastic hemostatic assays (thrombelastography [TEG] and thrombelastometry [ROTEM]) has facilitated the appropriate and timely use of clotting adjuncts, including the prompt recognition of fibrinolysis. In patients with clear indications for operation, essential films should be taken and the patient transported to the OR immediately. Such patients include those with blunt trauma and massive hemothorax, those with penetrating trauma and an initial chest tube output of >1 L, and those with abdominal trauma and ultrasound evidence of extensive hemoperitoneum. In patients with gunshot wounds to the chest or abdomen, a chest and abdominal film, with radiopaque markers at the wound sites, should be obtained to determine the trajectory of the bullet or location of a retained fragment. For example, a patient with a gunshot wound to the upper abdomen should have a chest radiograph to ensure that the bullet did not traverse the diaphragm causing intrathoracic injury. Similarly, a chest radiograph is important in a patient with a gunshot wound to the right chest to evaluate the left hemithorax. If a patient arrives with a penetrating weapon remaining in place, the weapon should not be removed in the ED, because it could be tamponading a lacerated blood vessel (Fig. 7-15). The surgeon should extract the offending instrument in the controlled environment of the OR, ideally once an incision has been made with adequate exposure. In situations where knives are embedded in the head or neck, preoperative imaging may be useful to anticipate arterial injuries.
In patients without clear operative indications and persistent hypotension, one should systematically evaluate the five potential sources of blood loss: scalp, chest, abdomen, pelvis, and extremities. Significant bleeding at the scene may be noted by paramedics, but its quantification is unreliable. Examination should seek active bleeding from a scalp laceration that may be readily controlled with clips or staples. Thoracoabdominal trauma should be evaluated with a combination of chest radiograph, FAST, and pelvic radiograph. If the FAST results are negative and no other source of hypotension is obvious, diagnostic peritoneal aspiration should be entertained.27 Extremity examination and radiographs should be used to search for associated fractures. Fracture-related blood loss, when additive, may be a potential source of the patient’s hemodynamic instability. Each rib fracture can produce 100 to 200 mL of blood loss; for tibial fractures, 300 to 500 mL; for femur fractures, 800 to 1000 mL; and for pelvic fractures >2000 mL. Although no single injury can account for the patient’s hemodynamic instability, the sum of the injuries may result in life-threatening blood loss. The diagnostic measures advocated earlier are those that can be easily performed in the trauma bay. Transport of a hypotensive patient out of the ED for computed tomographic (CT) scanning is hazardous; monitoring is compromised, and the environment is suboptimal for dealing with acute problems. The surgeon must accompany the patient and be prepared to abort the CT scan with diversion to the OR. This dilemma is becoming less common in many trauma centers where CT scanning is done in the ED.
The concept of hypotensive resuscitation in the ED remains controversial, and it is primarily relevant for patients with penetrating vascular injuries. Experimental work suggests that an endogenous sealing clot of an injured artery may be disrupted at an SBP of >90 mm Hg28; thus, many believe that this should be the preoperative blood pressure target for patients with potential torso arterial injuries. On the other hand, optimal management of traumatic brain injury (TBI) includes maintaining the SBP >100 mm Hg,29 and thus, hypotensive resuscitation is not appropriate for most blunt trauma patients.
Once the immediate threats to life have been addressed, a thorough history is obtained and the patient is examined in a systematic fashion. The patient and surrogates should be queried to obtain an AMPLE history (Allergies, Medications, Past illnesses or Pregnancy, Last meal, and Events related to the injury). The physical examination should be literally head to toe, with special attention to the patient’s back, axillae, and perineum, because injuries here are easily overlooked. All potentially seriously injured patients should undergo digital rectal examination to evaluate for sphincter tone, presence of blood, rectal perforation, or a high-riding prostate; this is particularly critical in patients with suspected spinal cord injury, pelvic fracture, or transpelvic gunshot wounds. Vaginal examination with a speculum should be performed in women with pelvic fractures to exclude an open fracture. Specific injuries, their associated signs and symptoms, diagnostic options, and treatments are discussed in detail later in this chapter.
Adjuncts to the physical examination include vital sign and CVP monitoring, ECG monitoring, nasogastric tube placement, Foley catheter placement, radiographs, hemoglobin, urinalysis, and base deficit measurements, and repeat FAST exam. A nasogastric tube should be inserted in all intubated patients to decrease the risk of gastric aspiration but may not be necessary in the awake patient. Nasogastric tube placement in patients with complex mid-facial fractures is contraindicated; rather, a tube should be placed orally if required. Nasogastric tube evaluation of stomach contents for blood may suggest occult gastroduodenal injury or the errant path of the nasogastric tube on a chest film may indicate a left diaphragm injury. A Foley catheter should be inserted in patients unable to void to decompress the bladder, obtain a urine specimen, and monitor urine output. Gross hematuria demands evaluation of the genitourinary system for injury. Foley catheter placement should be deferred until urologic evaluation in patients with signs of urethral injury: blood at the meatus, perineal or scrotal hematomas, or a high-riding prostate. Although policies vary at individual institutions, most agree patients in extremis with need for Foley catheter placement should undergo one attempt at catheterization; if the catheter does not pass easily, a percutaneous suprapubic cystostomy should be considered.
Selective radiography and laboratory tests are done early in the evaluation after the primary survey. For patients with severe blunt trauma, chest and pelvic radiographs should be obtained. Historically, a lateral cervical spine radiograph was also obtained, hence the reference to the big three films, but currently patients preferentially undergo CT scanning of the spine rather than plain film radiography. For patients with truncal gunshot wounds, anteroposterior and at times lateral radiographs of the chest and abdomen are warranted. It is important to mark the entrance and exit sites of penetrating wounds with ECG pads, metallic clips, or staples so that the trajectory of the missile can be estimated. Limited one-shot extremity radiographs also may be taken. In critically injured patients, blood samples for a routine trauma panel (type and cross-match, complete blood count, blood chemistries, coagulation studies, and arterial blood gas analysis) should be sent to the laboratory. For less severely injured patients only a complete blood count and urinalysis may be required. Because older patients may present in subclinical shock, even with minor injuries, routine analysis of an arterial blood gas in patients over the age of 55 should be considered. Repeat FAST is performed if there are any signs of abdominal injury or unexplained blood loss.
Many trauma patients cannot provide specific information about the mechanism of their injury. Emergency medical service personnel and police are trained to evaluate an injury scene and should be questioned while they are present in the ED. For automobile collisions, the speed of the vehicles involved, angle of impact, use of restraints, airbag deployment, condition of the steering wheel and windshield, amount of intrusion, ejection of the patient from the vehicle, and fate of other passengers should be ascertained. For other injury mechanisms, critical information includes such things as height of a fall, surface impact, helmet use, and weight of an object by which the patient was crushed. In patients sustaining gunshot wounds, velocity, caliber, distance, and presumed path of the bullet are important, if known. For patients with stab wounds, the length and type of object is helpful. Finally, some patients experience a combination of blunt and penetrating trauma. Do not assume that someone who was stabbed was not also assaulted; the patient may have a multitude of injuries and cannot be presumed to have only injuries associated with the more obvious penetrating mechanism. In short, these details of information are critical to the clinician to determine overall mechanism of injury and anticipate its associated injury patterns.
In general, more energy is transferred over a wider area during blunt trauma than from a penetrating wound. As a result, blunt trauma is associated with multiple widely distributed injuries, whereas in penetrating wounds the damage is localized to the path of the bullet or knife. In blunt trauma, organs that cannot yield to impact by elastic deformation are most likely to be injured, namely, the solid organs (liver, spleen, and kidneys). For penetrating trauma, organs with the largest surface area when viewed from the front are most prone to injury (small bowel, liver, and colon). Additionally, because bullets and knives usually follow straight lines, adjacent structures are commonly injured (e.g., the pancreas and duodenum).
Trauma surgeons often separate patients who have sustained blunt trauma into categories according to their risk for multiple injuries: those sustaining high energy transfer injuries and those sustaining low energy transfer injuries. Injuries involving high energy transfer include auto-pedestrian accidents, motor vehicle collisions in which the car’s change of velocity (ΔV) exceeds 20 mph or in which the patient has been ejected, motorcycle collisions, and falls from heights >20 ft.30 In fact, for motor vehicle accidents the variables strongly associated with life-threatening injuries, and hence reflective of the magnitude of the mechanism, are death of another occupant in the vehicle, extrication time of >20 minutes, ΔV >20 mph, lack of restraint use, and lateral impact.30 Low-energy trauma, such as being struck with a club or falling from a bicycle, usually does not result in widely distributed injuries. However, potentially lethal lacerations of internal organs can occur, because the net energy transfer to any given location may be substantial.
In blunt trauma, particular constellations of injury or injury patterns are associated with specific injury mechanisms. For example, when an unrestrained driver sustains a frontal impact, the head strikes the windshield, the chest and upper abdomen hit the steering column, and the legs or knees contact the dashboard. The resultant injuries can include facial fractures, cervical spine fractures, laceration of the thoracic aorta, myocardial contusion, injury to the spleen and liver, and fractures of the pelvis and lower extremities. When such patients are evaluated, the discovery of one of these injuries should prompt a search for the others. Collisions with side impact also carry the risk of cervical spine and thoracic trauma, diaphragm rupture, and crush injuries of the pelvic ring, but solid organ injury usually is limited to either the liver or spleen based on the direction of impact. Not surprisingly, any time a patient is ejected from the vehicle or thrown a significant distance from a motorcycle, the risk of any injury exists.
Penetrating injuries are classified according to the wounding agent (i.e., stab wound, gunshot wound, or shotgun wound). Gunshot wounds are subdivided further into high- and low-velocity injuries, because the speed of the bullet is much more important than its weight in determining kinetic energy. High-velocity gunshot wounds (bullet speed >2000 ft/s) are infrequent in the civilian setting. Shotgun injuries are divided into close-range (<20 feet) and long-range wounds. Close-range shotgun wounds are tantamount to high-velocity wounds because the entire energy of the load is delivered to a small area, often with devastating results. In contrast, long-range shotgun blasts result in a diffuse pellet pattern in which many pellets miss the victim, and those that do strike are dispersed and of comparatively low energy.
Based on mechanism, location of injuries identified on physical examination, screening radiographs, and the patient’s overall condition, additional diagnostic studies often are indicated. However, the seriously injured patient is in constant jeopardy when undergoing special diagnostic testing; therefore, the surgeon must be in attendance and must be prepared to alter plans as circumstances demand. Hemodynamic, respiratory, and mental status will determine the most appropriate course of action. With these issues in mind, additional diagnostic tests are discussed on an anatomic basis.
Evaluation of the head includes examination for injuries to the scalp, eyes, ears, nose, mouth, facial bones, and intracranial structures. Palpation of the head will identify scalp lacerations, which should be evaluated for depth, and depressed or open skull fractures. The eye examination includes not only pupillary size and reactivity, but also examination for visual acuity and for hemorrhage within the globe. Ocular entrapment, caused by orbital fractures with impingement on the ocular muscles, is evident when the patient cannot move his or her eyes through the entire range of motion. It is important to perform the eye examination early, because significant orbital swelling may prevent later evaluation. A lateral canthotomy may be needed to relieve periorbital pressure. The tympanic membrane is examined to identify hemotympanum, otorrhea, or rupture, which may signal an underlying head injury. Otorrhea, rhinorrhea, raccoon eyes, and Battle’s sign (ecchymosis behind the ear) suggest a basilar skull fracture. Although such fractures may not require treatment, there is an association with blunt cerebrovascular injuries, cranial nerve injuries, and risk of meningitis.
Anterior facial structures should be examined to rule out fractures. This entails palpating for bony step-off of the facial bones and instability of the midface (by grasping the upper palate and seeing if this moves separately from the patient’s head). A good question to ask awake patients is whether their bite feels normal to them; abnormal dental closure suggests malalignment of facial bones and a possibility for a mandible or maxillary fracture. Nasal fractures, which may be evident on direct inspection or palpation, typically bleed vigorously. This may result in the patient’s having airway compromise due to blood running down the posterior pharynx, or there may be vomiting provoked by swallowed blood. Nasal packing or balloon tamponade may be necessary to control bleeding. Examination of the oral cavity includes inspection for open fractures, loose or fractured teeth, and sublingual hematomas.
All patients with a significant closed head injury (GCS score <14) should undergo CT scanning of the head. Additionally, elderly patients or those patients on antiplatelet agents or anticoagulation should be imaged despite a GCS of 15.31,32 For penetrating injuries, plain skull films may be helpful in the trauma bay to determine the trajectory of injury in hemodynamically unstable patients who cannot be transported for CT scan. The presence of lateralizing findings (e.g., a unilateral dilated pupil unreactive to light, asymmetric movement of the extremities either spontaneously or in response to noxious stimuli, or unilateral Babinski’s reflex) suggests an intracranial mass lesion or major structural damage.
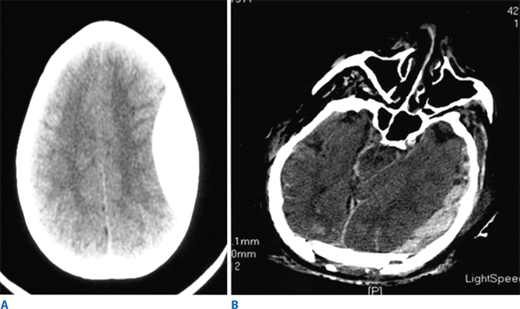
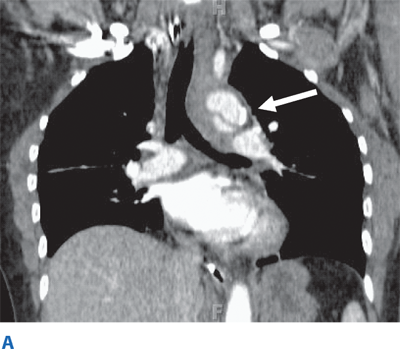
Such lesions include hematomas, contusions, hemorrhage into ventricular and subarachnoid spaces, and diffuse axonal injury (DAI). Epidural hematomas occur when blood accumulates between the skull and dura, and are caused by disruption of the middle meningeal artery or other small arteries in that potential space, typically after a skull fracture (Fig. 7-16). Subdural hematomas occur between the dura and cortex and are caused by venous disruption or laceration of the parenchyma of the brain. Due to associated parenchymal injury, subdural hematomas have a much worse prognosis than epidural collections. Hemorrhage into the subarachnoid space may cause vasospasm and further reduce cerebral blood flow. Intraparenchymal hematomas and contusions can occur anywhere within the brain. DAI results from high-speed deceleration injury and represents direct axonal damage from shear effects. CT scan may demonstrate blurring of the gray and white matter interface and multiple small punctate hemorrhages, but magnetic resonance imaging is a more accurate test. Although prognosis for these injuries is extremely variable, early evidence of DAI is associated with a poor outcome. Stroke syndromes should prompt a search for carotid or vertebral artery injury using multislice CT angiography (CTA) (Fig. 7-17).
Significant intracranial penetrating injuries usually are produced by bullets from handguns, but an array of other weapons or instruments can injure the cerebrum via the orbit or through the thinner temporal region of the skull. Although the diagnosis usually is obvious, in some instances wounds in the auditory canal, mouth, and nose can be elusive. Prognosis is variable, but virtually all supratentorial wounds that injure both hemispheres are fatal.
All blunt trauma patients should be assumed to have cervical spine injuries until proven otherwise. During cervical examination one must maintain cervical spine precautions and in-line stabilization. Due to the devastating consequences of quadriplegia, a diligent evaluation for occult cervical spine injuries is mandatory. In the awake patient, the presence of posterior midline pain or tenderness should provoke a thorough radiologic evaluation. Additionally, intubated patients, patients with distracting injuries, or another identified spine fracture should undergo CT imaging. A ligamentous injury may not be visible with standard imaging techniques.33 Flexion and extension views or MRI are obtained to further evaluate patients at risk or those with persistent symptoms, but generally are not done in the acute setting.
Spinal cord injuries can vary in severity. Complete injuries cause either quadriplegia or paraplegia, depending on the level of injury. These patients have a complete loss of motor function and sensation two or more levels below the bony injury. Patients with high spinal cord disruption are at risk for shock due to physiologic disruption of sympathetic fibers. Significant neurologic recovery is rare. However, there are several partial or incomplete spinal cord injury syndromes where the prognosis is better. Central cord syndrome typically occurs in older persons who experience hyperextension injuries. Motor function, pain, and temperature sensation are preserved in the lower extremities but diminished in the upper extremities. Some functional recovery usually occurs, but is often not a return to normal. Anterior cord syndrome is characterized by diminished motor function, pain, and temperature sensation below the level of the injury, but position sensing, vibratory sensation, and crude touch are maintained. Prognosis for recovery is poor. Brown-Séquard syndrome is usually the result of a penetrating injury in which one-half of the spinal cord is transected. This lesion is characterized by the ipsilateral loss of motor function, proprioception, and vibratory sensation, whereas pain and temperature sensation are lost on the contralateral side.
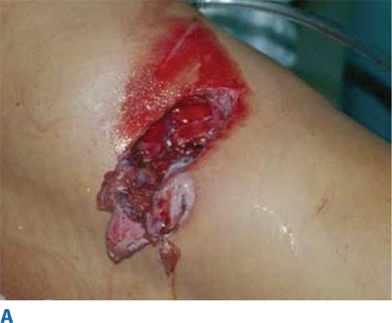
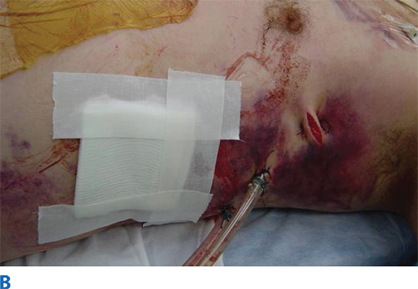
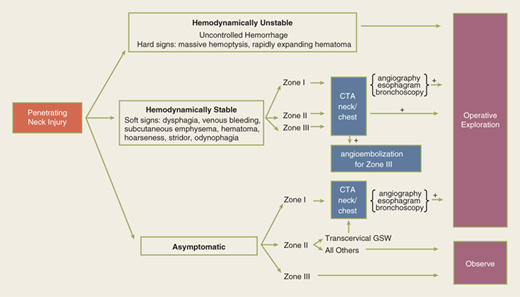
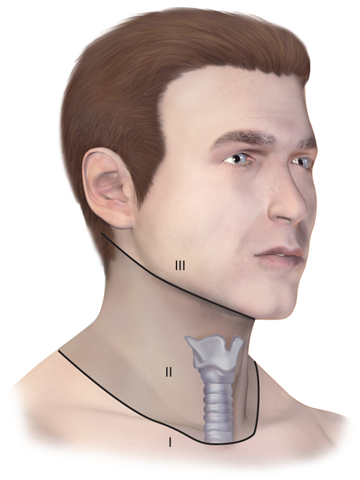
During the primary survey, identification of injuries to the neck with exsanguination, expanding hematomas, airway obstruction, or aerodigestive injuries is a priority. A more subtle injury that may not be identified is a fracture of the larynx due to blunt trauma. Signs and symptoms include hoarseness, subcutaneous emphysema (Fig. 7-18), and a palpable fracture. Penetrating injuries of the anterior neck that violate the platysma are potentially life-threatening because of the density of critical structures in this region. Although operative exploration is appropriate in some circumstances, selective nonoperative management has been proven safe (Fig. 7-19).34 Indications for immediate operative intervention for penetrating cervical injury include hemodynamic instability, significant external hemorrhage, or evidence of aerodigestive injury. The management algorithm for hemodynamically stable patients is based on the presenting symptoms and anatomic location of injury, with the neck being divided into three distinct zones (Fig. 7-20). Zone I is inferior to the clavicles encompassing the thoracic outlet structures, zone II is between the thoracic outlet and the angle of the mandible, and zone III is above the angle of the mandible. Due to technical difficulties of injury exposure and varying operative approaches, a precise preoperative diagnosis is desirable for symptomatic zone I and III injuries. Therefore, these patients should ideally undergo diagnostic imaging before operation if they remain hemodynamically stable. Management of patients is further divided into those who are symptomatic and those who are not (Fig 7-19). Specific symptoms or signs that should be identified include dysphagia, hoarseness, hematoma, venous bleeding, minor hemoptysis, and subcutaneous emphysema. Symptomatic patients should undergo CTA with further evaluation or operation based upon the imaging findings; less than 15% of penetrating cervical trauma requires neck exploration.35 Asymptomatic patients are typically observed for 6 to12 hours. The one caveat is asymptomatic patients with a transcervical gunshot wound; these patients should undergo CTA to determine the track of the bullet. CTA of the neck and chest determines trajectory of the injury tract; further studies are performed based on proximity to major structures.35 Such additional imaging includes angiography, soluble contrast esophagram followed by barium esophagram, esophagoscopy, or bronchoscopy. Angiographic diagnosis, particularly of zone III injuries, can then be managed by selective angioembolization.
Figure 7-20.
For the purpose of evaluating penetrating injuries, the neck is divided into three zones. Zone I is to the level of the clavicular heads and is also known as the thoracic outlet. Zone II is located between the clavicles and the angle of the mandible. Zone III is above the angle of the mandible.
Blunt trauma to the chest may involve the chest wall, thoracic spine, heart, lungs, thoracic aorta and great vessels, and rarely the esophagus. Most of these injuries can be evaluated by physical examination and chest radiography, with supplemental CT scanning based on initial findings. Any patient who undergoes an intervention in the ED—endotracheal intubation, central line placement, tube thoracostomy—needs a repeat chest radiograph to document the adequacy of the procedure. This is particularly true in patients undergoing tube thoracostomy for a pneumothorax or hemothorax. Patients with persistent pneumothorax, large air leaks after tube thoracostomy, or difficulty ventilating should undergo fiber-optic bronchoscopy to exclude a tracheobronchial injury or presence of a foreign body. Patients with hemothorax must have a chest radiograph documenting complete evacuation of the chest; a persistent hemothorax that is not drained by two chest tubes is termed a caked hemothorax and mandates immediate thoracotomy (Fig. 7-21).
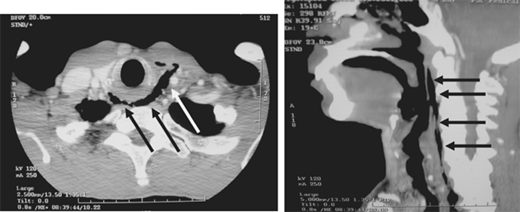
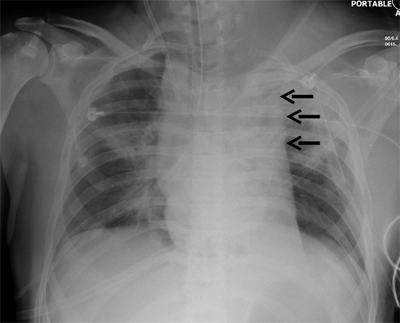
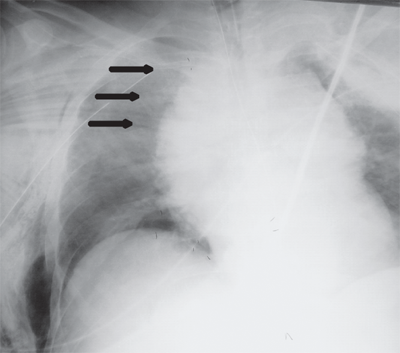
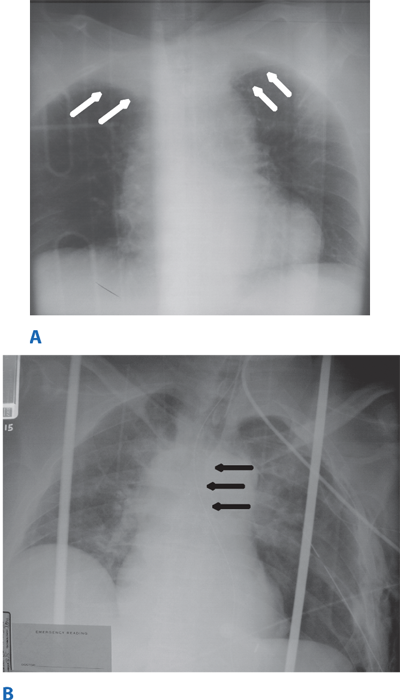
Occult thoracic vascular injury must be diligently sought due to the high mortality of a missed lesion. Widening of the mediastinum on initial anteroposterior chest radiograph, caused by a hematoma around an injured vessel that is contained by the mediastinal pleura, suggests an injury of the great vessels. The mediastinal abnormality may suggest the location of the arterial injury (i.e., left-sided hematomas are associated with descending torn aortas, whereas right-sided hematomas are commonly seen with innominate injuries) (Fig. 7-22). Posterior rib fractures, sternal fractures with laceration of small vessels, and mediastinal venous bleeding also can produce similar hematomas. Other chest radiographic findings suggestive of an aortic tear are summarized in Table 7-5 (Fig. 7-23). However, at least 7% of patients with a descending torn aorta have a normal chest radiograph.36 Therefore, screening spiral CT scanning is performed based on the mechanism of injury: high-energy deceleration motor vehicle collision with frontal or lateral impact (> 30 mph frontal impact and >23 mph lateral impact), motor vehicle collision with ejection, falls of >25 ft, or direct impact (horse kick to chest, snowmobile or ski collision with tree). 37,38 In >95% of patients who survive to reach the ED, the aortic injury occurs just distal to the left subclavian artery, where it is tethered by the ligamentum arteriosum (Fig. 7-24). In 2% to 5% of patients the injury occurs in the ascending aorta, in the transverse arch, or at the diaphragm. Reconstructions with multislice CTA obviate the need for invasive arteriography.37
1. Widened mediastinum |
2. Abnormal aortic contour |
3. Tracheal shift |
4. Nasogastric tube shift |
5. Left apical cap |
6. Left or right paraspinal stripe thickening |
7. Depression of the left main bronchus |
8. Obliteration of the aorticopulmonary window |
9. Left pulmonary hilar hematoma |
Figure 7-22.
Location of the hematoma within the mediastinal silhouette suggests the type of great vessel injury. A predominant hematoma on the left suggests the far more common descending torn aorta (A; arrows), whereas a hematoma on the right indicates a relatively unusual but life-threatening innominate artery injury (B; arrows).
For penetrating thoracic trauma, physical examination, plain posteroanterior and lateral chest radiographs with metallic markings of wounds, pericardial ultrasound, and CVP measurement will identify the majority of injuries. Injuries of the esophagus and trachea are exceptions. Bronchoscopy should be performed to evaluate the trachea in patients with a persistent air leak from the chest tube or mediastinal air. Because esophagoscopy can miss injuries following an apparent normal endoscopy, patients at risk should undergo soluble contrast esophagraphy followed by barium examination to look for extravasation of contrast to identify an injury.39 As with neck injuries, hemodynamically stable patients with transmediastinal gunshot wounds should undergo CT scanning to determine the path of the bullet; this identifies the vascular or visceral structures at risk for injury and directs angiography or endoscopy as appropriate. If there is a suspicion of a subclavian artery injury, brachial-brachial indices should be measured, but >60% of patients with an injury may not have a pulse deficit.40 Therefore, CTA should be performed based on injury proximity to intrathoracic vasculature. Finally, with wounds identified on the chest, penetrating trauma should not be presumed to be isolated to the thorax. Injury to contiguous body cavities (i.e., the abdomen and neck) must be excluded; plain radiographs are a rapid, effective screening modality.
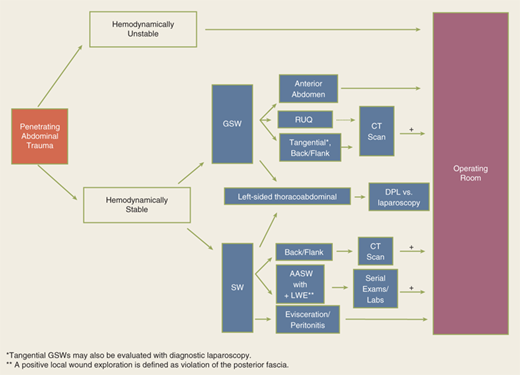
The abdomen is a diagnostic black box. Fortunately, with few exceptions, it is not necessary to determine in the emergency department which intra-abdominal organs are injured, only whether an exploratory laparotomy is necessary. However, physical examination of the abdomen can be unreliable in making this determination, and drugs, alcohol, and head and spinal cord injuries complicate clinical evaluation. The presence of abdominal rigidity and hemodynamic compromise is an undisputed indication for prompt surgical exploration. For the remainder of patients, a variety of diagnostic adjuncts are used to identify abdominal injury.
The diagnostic approach differs for penetrating trauma and blunt abdominal trauma. As a rule, minimal evaluation is required before laparotomy for gunshot or shotgun wounds that penetrate the peritoneal cavity, because over 90% of patients have significant internal injuries. Anterior truncal gunshot wounds between the fourth intercostal space and the pubic symphysis whose trajectory as determined by radiograph or wound location indicates peritoneal penetration should undergo laparotomy (Fig. 7-25). The exception is penetrating trauma isolated to the right upper quadrant; in hemodynamically stable patients with trajectory confined to the liver by CT scan, nonoperative observation may be reasonable.41 In obese patients, if the gunshot wound is thought to be tangential through the subcutaneous tissues, CT scan can delineate the track and exclude peritoneal violation. Laparoscopy is another option to assess peritoneal penetration for tangential wounds. If there is doubt, however, it is always safer to explore the abdomen. In the scenario of tangential high energy GSWs, however, it is possible to sustain a transmitted intraperitoneal hollow visceral injury due to a blast insult. Gunshot wounds to the back or flank are more difficult to evaluate because of the retroperitoneal location of the injured abdominal organs. Triple-contrast CT scan can delineate the trajectory of the bullet and identify peritoneal violation or retroperitoneal entry, but may not identify the specific injuries. In contrast to gunshot wounds, stab wounds that penetrate the peritoneal cavity are less likely to injure intra-abdominal organs. Anterior abdominal stab wounds (from costal margin to inguinal ligament and bilateral midaxillary lines) should be explored under local anesthesia in the ED to determine if the fascia has been violated. Injuries that do not penetrate the peritoneal cavity do not require further evaluation, and the patient may be discharged from the ED. Patients with fascial penetration must be further evaluated for intra-abdominal injury, because there is up to a 50% chance of requiring laparotomy. Debate remains over whether the optimal diagnostic approach is serial examination, diagnostic peritoneal lavage (DPL), or CT scanning; the most recent evidence supports serial examination and laboratory evaluation.42,43 Patients with stab wounds to the right upper quadrant can undergo CT scanning to determine trajectory and confinement to the liver for potential nonoperative care.41 Those with stab wounds to the flank and back should undergo triple-contrast CT to assess for the potential risk of retroperitoneal injuries of the colon, duodenum, and urinary tract.
Penetrating thoracoabdominal wounds may cause occult injury to the diaphragm. Patients with gunshot or stab wounds to the left lower chest should be evaluated with diagnostic laparoscopy or DPL to exclude diaphragmatic injury. For patients undergoing DPL evaluation, laboratory value cutoffs to rule out diaphragm injury are different from traditional values formerly used for abdominal stab wounds (see Table 7-6). An RBC count of >10,000/μL is considered a positive finding and an indication for abdominal evaluation; patients with a DPL RBC count between 1000/μL and 10,000/μL should undergo laparoscopy or thoracoscopy. Diagnostic laparoscopy may be preferred in patients with a positive chest radiograph (hemothorax or pneumothorax) or in those who would not tolerate a DPL.
ABDOMINAL TRAUMA | THORACOABDOMINAL STAB WOUNDS | |
|---|---|---|
Red blood cell count | >100,000/mL | >10,000/mL |
White blood cell count | >500/mL | >500/mL |
Amylase level | >19 IU/L | >19 IU/L |
Alkaline phosphatase level | >2 IU/L | >2 IU/L |
Bilirubin level | >0.01 mg/dL | >0.01 mg/dL |
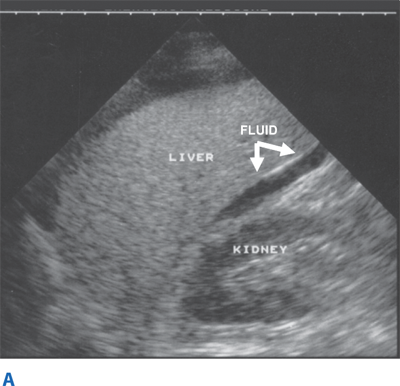
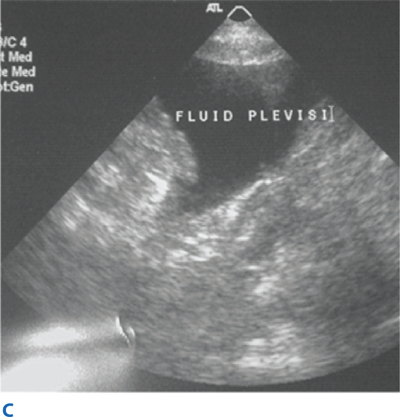
Blunt abdominal trauma is evaluated initially by FAST examination in most major trauma centers, and this has largely supplanted DPL (Fig. 7-26). FAST is not 100% sensitive, however, so diagnostic peritoneal aspiration is warranted in hemodynamically unstable patients without a defined source of blood loss to rule out abdominal hemorrhage.27 FAST is used to identify free intraperitoneal fluid (Fig. 7-27) in Morrison’s pouch, the left upper quadrant, and the pelvis. Although this method is exquisitely sensitive for detecting intraperitoneal fluid of >250 mL, it does not reliably determine the source of hemorrhage nor grade solid organ injuries.44 Patients with fluid on FAST examination, considered a “positive FAST,” who do not have immediate indications for laparotomy and are hemodynamically stable undergo CT scanning to quantify their injuries. Injury grading using the American Association for the Surgery of Trauma grading scale (Table 7-7) is an important component of nonoperative management of solid organ injuries. Additional findings that should be noted on CT scan in patients with solid organ injury include contrast extravasation (i.e., a “blush”), the amount of intra-abdominal hemorrhage, and presence of pseudoaneurysms (Fig. 7-28). CT also is indicated for hemodynamically stable patients for whom the physical examination is unreliable. Despite the increasing diagnostic accuracy of multidetector CT scanners, identification of intestinal injuries remains a limitation. Bowel injury is suggested by findings of thickened bowel wall, “streaking” in the mesentery, free fluid without associated solid organ injury, or free intraperitoneal air.45,46 Patients with free intra-abdominal fluid without solid organ injury are closely monitored for evolving signs of peritonitis; if patients have a significant closed head injury or cannot be serially examined, DPL should be performed to exclude bowel injury. If DPL is pursued, an infraumbilical approach is used (Fig. 7-29). After placement of the catheter, a 10-mL syringe is connected and the abdominal contents aspirated (termed a diagnostic peritoneal aspiration). The aspirate is considered to show positive findings if >10 mL of blood is aspirated. If <10 mL is withdrawn, a liter of normal saline is instilled. The effluent is withdrawn via siphoning and sent to the laboratory for RBC count, white blood cell (WBC) count, and determination of amylase, bilirubin, and alkaline phosphatase levels. Values representing positive findings are summarized in Table 7-6.
SUBCAPSULAR HEMATOMA | LACERATION | |
|---|---|---|
Liver Injury Grade | ||
Grade I | <10% of surface area | <1 cm in depth |
Grade II | 10%–50% of surface area | 1–3 cm |
Grade III | >50% of surface area or >10 cm in depth | >3 cm |
Grade IV | 25%–75% of a hepatic lobe | |
Grade V | >75% of a hepatic lobe | |
Grade VI | Hepatic avulsion | |
Splenic Injury Grade | ||
Grade I | <10% of surface area | <1 cm in depth |
Grade II | 10%–50% of surface area | 1–3 cm |
Grade III | >50% of surface area or >10 cm in depth | >3 cm |
Grade IV | >25% devascularization | Hilum |
Grade V | Shattered spleen Complete devascularization | |
Figure 7-28.
Computed tomographic images reveal critical information about solid organ injuries, such as associated contrast extravasation from a grade IV laceration of the spleen (A; arrows) and the amount of subcapsular hematoma in a grade III liver laceration (B; arrows).